Abstract
Endogenous retroviruses (ERVs) constitute approximately 8−10% of the human and mouse genome. Some autoimmune diseases are attributed to the altered expression of ERVs. In this study, we examined the ERV expression profiles in lymphoid tissues and analyzed their biological properties. Tissues (spleen, thymus, and lymph nodes [axillary, inguinal, and mesenteric]) from C57BL/6J mice were analyzed for differential murine ERV (MuERV) expression by RT-PCR examination of polymorphic U3 sequences. Each tissue had a unique profile of MuERV expression. A genomic map identifying 60 putative MuERVs was established using 22 unique U3s as probes and their biological properties (primer binding site, coding potential, transcription regulatory element, tropism, recombination event, and integration age) were characterized. Interestingly, 12 putative MuERVs retained intact coding potentials for all three polypeptides essential for virus assembly and replication. We suggest that MuERV expression is differentially regulated in conjunction with the transcriptional environment of individual lymphoid tissues.
Keywords: endogenous retrovirus, spleen, thymus, lymph node, coding potential, transcription, genome
Introduction
Infection of germline cells with retroviruses leads to their permanent colonization into the germline genome. These germline-integrated retroviruses are transmitted vertically to the offspring in a Mendelian fashion and are called endogenous retroviruses (ERVs), in contrast to exogenous retroviruses, which are acquired from external surroundings and transmitted horizontally. ERVs represent collections of retroviral proviruses introduced into the germline and accumulated throughout an entire set of generations.
ERVs and other forms of transposable repetitive elements make up ∼45% of the human and mouse genomes (Urnovitz and Murphy, 1996). Among them, ∼8% of the human genome and ∼10% of the mouse genome consist of ERVs (Griffiths, 2001; Lander et al., 2001; Waterston et al., 2002). Although the majority of ERVs are defective primarily due to deletions and point mutations leading to a disruption of the coding potential of the gag, pol, and/or env genes, some are characterized to be biologically active.
Most ERVs have the potential to modulate the expression of host genes near the integration site, for example, the transcriptional control of the amylase gene in the salivary gland (Samuelson, Phillips, and Swanberg, 1996). The HTDV (human teratocarcinoma-derived virus), a member of the human ERV (HERV)-K family, produces type C retroviral particles and retains the coding potential for retroviral polypeptides essential for virus assembly and replication (Knossl, Lower, and Lower, 1999; Lower et al., 1993). The HTDV U3 promoter is highly specific for the transcriptional environment in testicular tumor cells (Lower, Lower, and Kurth, 1996).
Furthermore, the pathogenic processes of certain autoimmune diseases, such as systemic lupus erythematosus, insulin-dependent diabetes mellitus, and multiple sclerosis, have been associated with altered expression of ERVs (Conrad et al., 1997; Deas et al., 1998; Wang et al., 2001). In particular, expression of the HERV-W encoded envelope glycoprotein, called syncytin, was increased in the brain of multiple sclerosis patients (Antony et al., 2004). Syncytin expression in astrocytes was responsible for neuroinflammation, resulting in demyelination and the death of oligodendrocytes. The proinflammatory properties of syncytin demonstrated a unique role of an ERV protein in a pathologic process in humans. In addition, recent studies from our laboratory provide evidence that burn-elicited stress signals can alter the transcriptional activities of certain murine ERVs (MuERVs) in distant organs of mice (Aziz, Hanna, and Jolicoeur, 1989; Cho, Adamson, and Greenhalgh, 2002; Cho and Greenhalgh, 2003). Interestingly, some of these burn-elicited MuERVs are structurally similar to the MAIDS (murine acquired immunodeficiency syndrome)-inducing virus.
It is likely that the unique transcriptional environment in each cell or tissue type directly influences the MuERV expression profile. The genome-wide distribution of MuERVs and their differential expression in various types of tissues and cells may be directly networked to a vast range of signaling events controlling normal physiologic as well as pathologic processes. Two main types of MuERVs, murine leukemia virus (MuLV) and mouse mammary tumor virus (MMTV), are reported to be involved in various pathophysiologic processes involving immune organs (Choi et al., 1987; King and Corley, 1990). In this study, we examined the MuERV expression profiles in various lymphoid tissues of mice and characterized their biological properties.
Results
Differential expression of MuERVs among various lymphoid tissues
We postulate that MuERV expression profiles vary depending on tissue type mainly due to the unique composition of the transcriptional environment within the diverse cell populations comprising each tissue. In this study, the MuERV expression profile in five different lymphoid tissues (spleen, thymus, and lymph nodes [axillary, inguinal, and mesenteric]) were examined in female C57BL/6J mice. The NCBI (National Center for Biotechnology Information) mouse genome database, which is derived from the C57BL/6J strain, was used for comparative analysis. RT-PCR analyses of the MuERV expression profiles were performed by amplifying the polymorphic U3 region within the 3’ long terminal repeats (LTRs). Electrophoretic analysis of amplified U3 products revealed a unique MuERV expression profile within each lymphoid tissue examined (Figure 1). There were variations in the length (ranging from ∼470 bp to ∼750 bp) as well as in the intensity of amplified MuERV U3s. Interestingly, the genomic MuERV profile (size and intensity of amplified U3s) was substantially different from the expression profiles of all lymphoid tissues examined. Only four different MuERV U3 fragments (labeled as I, II, III, and IV), which were present in all five tissues, were selected for a direct comparison of differential expression levels followed by downstream characterization of biological properties. Further investigation into the other MuERV U3 fragments, which were presumed to be derived from certain MuERVs expressed only in some tissues examined, may provide additional information in regard to differential expression of MuERVs in lymphoid tissues.
Figure 1.
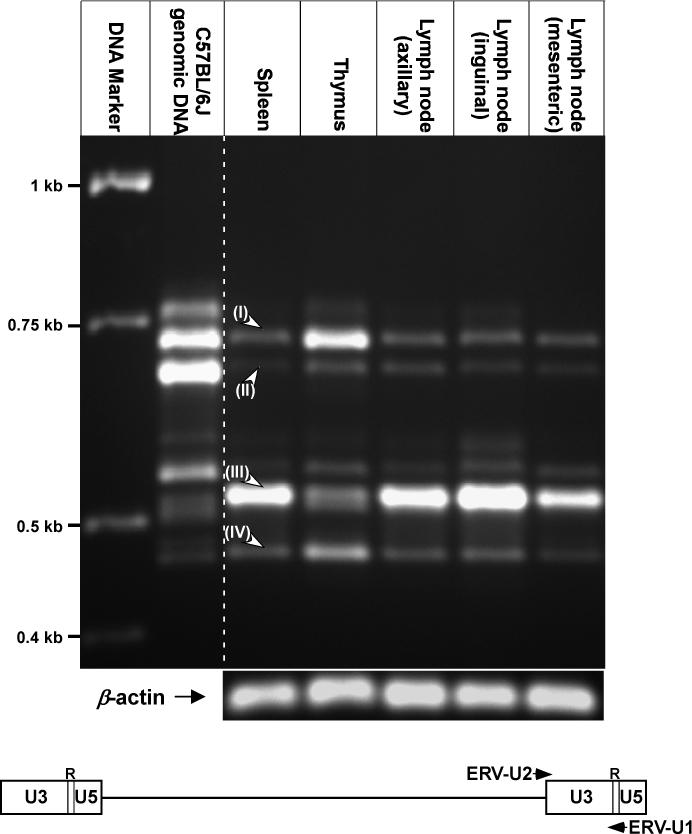
RT-PCR analyses of the MuERV expression profiles in various lymphoid tissues. Five different lymphoid tissues (spleen, thymus, lymph nodes [axillary, inguinal, and mesenteric]) from C57BL/6J mice were subjected to RT-PCR analysis of MuERV expression using the primer set (ERV-U1 and ERV-U2). Tissue-specific MuERV expression profile is evident. Four different fragments from each tissue, which were subjected to further analyses, are indicated (I, II, III, and IV). The genomic MuERV U3 profile serves as a reference. Bottom panel depicts locations of primers on a schematic MuERV.
Multiple alignment and phylogenetic analyses of differentially expressed MuERV U3 sequences
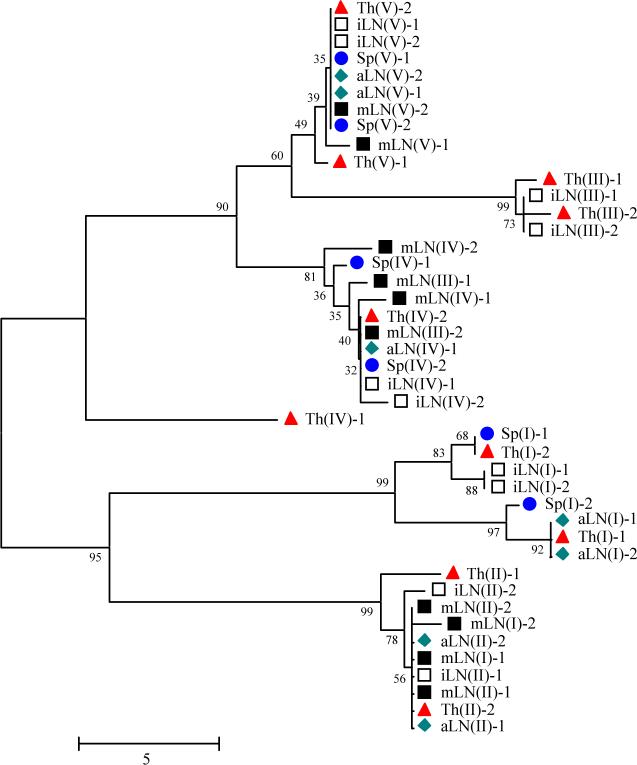
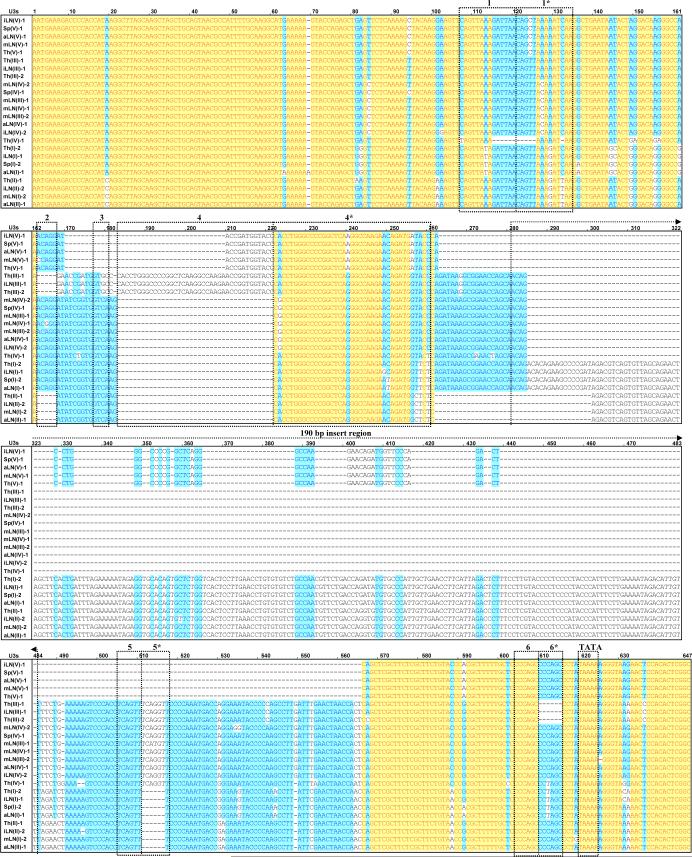
Cloning and sequencing analyses of four different U3 fragments (I, II, III, and IV) from all five lymphoid tissues yielded 43 MuERV U3 sequences which represent at least two sequences from each fragment (Figure 2A). These U3 sequences were subjected to multiple alignment and phylogenetic analyses. Since the amplified U3 regions include ∼117 bp upstream and downstream of the exact U3 sequence, these additional nucleotides were trimmed prior to the alignment analyses. The initial multiple alignment analysis yielded 22 unique U3s (24 U3s are shown to include at least one sequence from each fragment) (Figure 2B). The U3 sequences were apparently grouped into six main branches coinciding with the differences in their sizes (ranging from 346 bp to 601 bp) (Figure 2A). In addition, the Th(IV)-1 U3 sequence formed a unique branch, which is consistent with its unique size of 392 bp and absence of a direct repeat (1/1*) (Figure 2B) although it had high sequence similarities with the branches of Th(III)-1 and mLN(IV)-2. Multiple alignment of the 24 unique U3 sequences revealed that both the 5’-end and 3’-end were well-conserved and the middle region, which includes a single 190 bp insertion, was hypervariable (Figure 2B). It has been established that the cellular tropism of MuERVs can be determined by surveying specific sequence features (e.g., direct repeat, unique region) within the U3 promoter (Tomonaga and Coffin, 1998; Tomonaga and Coffin, 1999). Putative cellular tropisms of the unique U3 sequences were determined by the examination of four direct repeat regions (1/1*, 4/4*, 5/5*, and 6/6*), a single 190 bp insertion, and two unique regions (2 and 3) (Figure 2B and Table 1). There were 15 xenotropic and nine polytropic/modified polytropic U3 sequences.
Figure 2.
A. Phylogenetic analysis of differentially expressed MuERV U3 sequences. A phylogenetic tree of differentially expressed MuERV U3 sequences was established. The confidence values at the branch nodes represent the percentage support of a particular branching. Various colored shapes represent different lymphoid tissues. Sp (spleen), Th (thymus), aLN (axillary lymph node), iLN (inguinal lymph node), mLN (mesenteric lymph node). B. Multiple alignment analysis of differentially expressed MuERV U3 sequences. Differentially expressed MuERV U3 sequences were subjected to a multiple alignment analysis and yielded 22 unique U3 sequences. Yellow (100 % homology), white (low homology), blue (conserved sequences), and dash (absence of sequences). The locations of the direct repeats (1/1*, 4/4*, 5/5*, and 6/6*), unique regions (2 and 3), and TATA box are indicated in dotted boxes. An insertion of 190 bp is identified in the middle.
Table 1.
Tropism analysis of 22 unique MuERV U3 sequences isolated from five different lymphoid tissues. Dot indicates absence of a homology to reference sequences. Underscore represents the reference type closest to the individual U3 sequences examined. * Direct repeat region.
| U3s |
Direct Repeat/Unique Region |
Tropism | |||||
|---|---|---|---|---|---|---|---|
| 1/1* | 2 | 3 | 4/4* | 5/5* | 6/6* | ||
| iLN(V)-1 | X-II, X-III | X-I,X-II, X-IV, P-II, P-III, P-V | . | X-III | . | X-I, X-II, X-III, X-IV, Poly | X-III |
| Sp(V)-1 | X-II, X-III | X-I,X-II, X-IV, P-II, P-III, P-V | . | X-III | . | X-I, X-II, X-III, X-IV, Poly | X-III |
| aLN(V)-1 | X-II, X-III | X-I,X-II, X-IV, P-II, P-III, P-V | . | X-III | . | X-I, X-II, X-III, X-IV, Poly | X-III |
| mLN(V)-1 | X-II, X-III | . | . | X-III | . | X-I, X-II, X-III, X-IV, Poly | X-III |
| Th(V)-1 | X-II, X-III | X-I,X-II, X-IV, P-II, P-III, P-V | . | X-III | . | X-I, X-II, X-III, X-IV, Poly | X-III |
| Th(III)-1 | X-II, X-III | X-III, P-I, P-IV | . | X-II, X-IV | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III | X-II |
| iLN(III)-1 | X-II, X-III | X-III, P-I, P-IV | . | X-II, X-IV | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III | X-II |
| Th(III)-2 | X-II, X-III | X-III, P-I, P-IV | . | X-II, X-IV | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III | X-II |
| mLN(IV)-2 | X-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | X-II | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III, X-IV, Poly | X-II |
| Sp(IV)-1 | X-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | X-II | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III, X-IV, Poly | X-II |
| mLN(III)-1 | X-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | X-II | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III, X-IV, Poly | X-II |
| mLN(IV)-1 | X-III | . | Xeno/Poly/mPoly | X-II | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III, X-IV, Poly | X-II |
| mLN(III)-2 | X-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | X-II | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III, X-IV, Poly | X-II |
| aLN(IV)-1 | X-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | X-II | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III, X-IV, Poly | X-II |
| iLN(IV)-2 | X-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | X-II | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III, X-IV, Poly | X-II |
| Th(IV)-1 | . | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | P-IV | X-II, X-III, X-IV, P-I, P-IV | X-I, X-II, X-III, X-IV, Poly | P-IV |
| Th(I)-2 | P-II,P-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | P-II | P-I, P-II, P-III | P-II | P-II |
| iLN(I)-1 | P-II,P-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | P-II | P-I, P-II, P-III | P-II | P-II |
| Sp(I)-2 | P-II,P-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | P-II | P-I, P-II, P-III | P-II | P-II |
| aLN(I)-1 | P-II,P-III | X-I,X-II, X-IV, P-II, P-III, P-V | Xeno/Poly/mPoly | P-II | P-I, P-II, P-III | P-II | P-II |
| Th(II)-1 | P-I | X-III, P-I, P-IV | Xeno/Poly/mPoly | P-I | P-I, P-II, P-III | X-I, X-II, X-III, X-IV, Poly | P-I |
| iLN(II)-2 | P-I | X-III, P-I, P-IV | Xeno/Poly/mPoly | P-I | P-I, P-II, P-III | X-I, X-II, X-III, X-IV, Poly | P-I |
| mLN(I)-2 | P-I | X-III, P-I, P-IV | Xeno/Poly/mPoly | P-I | P-I, P-II, P-III | X-I, X-II, X-III, X-IV, Poly | P-I |
| aLN(II)-1 | P-I | X-III, P-I, P-IV | Xeno/Poly/mPoly | P-I | P-I, P-II, P-III | X-I, X-II, X-III, X-IV, Poly | P-I |
Profile of transcription regulatory elements on individual MuERV U3 promoters.
It is expected that the 3’ U3 sequences are almost identical to the 5’ U3 sequences, the latter which serve as promoters. To examine the transcription potential of the 22 unique MuERV U3 sequences (3’ U3s), the putative transcription regulatory elements were mapped on each U3 (Table 2). Only the transcription regulatory elements with a core similarity (compared to conserved sequences) of greater than 90% were selected. This mapping study yielded 71 transcription regulatory elements, which included binding sites for the glucocorticoid receptor and NF-κB. Although some elements were shared by the majority of the U3 sequences examined, other elements were unique to certain U3 promoters. For instance, a glucocorticoid response element (GRE), binding site for glucocorticoid receptor (highlighted with *), was present only in the iLN(II)-2 U3 promoter. A binding site for signal transducers and activators of transcription 3 (STAT3) (highlighted with *) was mapped only in the Th(IV)-1 U3 promoter. It will be of interest to test whether the putative GRE responds to stimulation by glucocorticoids in vitro and in vivo. On the other hand, a binding site for NF-κB (highlighted with *), a key transcription factor involved in inflammatory response, was identified in five different U3 promoters (Th(IV)-1, Th(I)-2, iLN(I)-1, Sp(I)-2, and aLN(I)-1). The unique profiles of transcription regulatory elements on each U3 promoter suggest their differential transcription potentials in a given transcriptional environment.
Table 2.
Transcription regulatory elements in 22 unique MuERV U3 promoter sequences isolated from five different lymphoid tissues. The number in the box indicates frequency of a transcriptional regulatory element within a given U3 promoter sequence, and the gray box indicates no occurrence of the specific transcriptional regulatory element.
 |
Genomic localization of putative MuERVs using the U3 sequences as a probe and characterization of their biological properties
Genomic localization of putative MuERVs
In order to investigate the genome-wide distribution of MuERVs harboring the U3 sequences identified in various lymphoid tissues, the NCBI mouse genome database was probed with individual U3 sequences. Subsequently, the putative MuERVs with a homology of greater than 98% relative to the U3 probe and approximately 5 kb to 9 kb in size were selected for further analyses (Table 3). The defective MuERVs with substantial deletions in pol and env genes tend to be approximately 5 kb and the full-length MuERVs are about 9 kb. Genomic probing using the 22 unique U3 sequences revealed 60 defective or full-length MuERVs dispersed throughout the entire genome in both strands except for on chromosomes 17 and Y. Chromosomal location, including proviral size and strand orientation, of these putative MuERVs are summarized in Table 3.
Table 3.
In silico cloning and biological properties of putative MuERVs differentially expressed in lymphoid tissues. Gray indicates putative full-length MuERVs. Two putative MuERVs mapped by only one U3 probe are highlighted in purple. Absence of direct repeat sequences is indicated with “/” between two sequences. Yellow indicates putative MuERVs with a cytosine insertion. Blue indicates putative MuERVs with ATG→ GTG mutation at the RT start site. Green indicates that the gag was considered intact despite one amino acid deletion within p12. PBS (primer binding site): P (tRNAproline) and Q (tRNA glutamine). ND (not determined). Coding potential: intact (+), partial (P), and defective (−). NA (lacking a proper start codon).
| Putative MuERV | Source U3 | Ch | Location & Orientation | Size (bp) | PBS | Direct Repeat | Integration Age (Mutation Rate) | Open Reading Frame | ||
|---|---|---|---|---|---|---|---|---|---|---|
| gag (a.a.) | pol (a.a.) | env (a.a.) | ||||||||
| aLN(II)-1d 100% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 1 | NC_000067.4 184093795−184102775 (+) | 8981 | Q | TTTG | <1.1036(<0.1435%) | (+) 565 | (p) 1072 | (+) 641 |
| aLN(II)-1d 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 1 | NC_000067.4 193634228−193643208 (+) | 8981 | Q | GTTG | <1.1036(<0.1435%) | (+) 536 | (p) 1028 | (−) 196 |
| mLN(I)-2 99% | mLN(I)-2, aLN(II)-1, iLN(II)-2, Th(II)-1 | 1 | NC_000067.4 166060482−166066227 (+) | 5746 | Q | CAAG | <1.1020(0.1433%) | (+) 536 | (−) NA | (−) NA |
| aLN(I)-1a 100% | aLN(I)-1, Sp(I)-2, Th(I)-2, iLN(I)-1, iLN(I)-2 | 1 | NC_000067.4 133470113−133479166 (+) | 9054 | Q | ACAC | 2.0567(0.2673%) | (−) 85 | (+) 1088 | (+) 632 |
| aLN(II)-1n 99% | aLN(II)-1, Th(II)-1, iLN(II)-2, mLN(I)-2 | 2 | NC_000068.5 57038778−57029798 (−) | 8981 | Q | GTGT | <1.1036(<0.1435%) | (+) 536 | (+) 1091 | (+) 641 |
| Th(I)-2a 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 2 | NC_000068.5 15947290−15938249 (−) | 9042 | Q | ATTG | <1.0381(<0.1350%) | (−) 162 | (+) 1088 | (+) 632 |
| Th(I)-2b 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 3 | NC_000069.4 67184007−67191723 (+) | 7717 | Q | ACTT | <1.0367(<0.1348%) | (+) 536 | (−) 70 | (−) 390 |
| Th(I)-2c 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 3 | NC_000069.4 152260526−152269568 (+) | 9043 | Q | ATGT | <1.0367(<0.1348%) | (+) 536 | (+) 1088 | (+) 632 |
| aLN(II)-1d 98% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 4 | NC_000070.4 101370070−101361089 (−) | 8982 | Q | CACC | <1.1036(<0.1435%) | (+) 536 | (p) 886 | (+) 641 |
| aLN(II)-1m 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 4 | NC_000070.4 107652948−107643966 (−) | 8983 | Q | AAAC | 1.102(0.1432%) | (+) 536 | (+) 1091 | (+) 641 |
| Th(I)-2b 98% | Th(I)-2, iLN(I)-1, iLN(I)-2 | 4 | NC_000070.4 33126327−33118967 (−) | 7362 | Q | CCAA/CCTG | ND | (−) 162 | (p) 886 | (−) 19 |
| Th(I)-2d 99% | Th(I)-2, Sp(I)-2, iLN(I)-1, iLN(I)-2, aLN(I)-1 | 4 | NC_000070.4 15169957−15163352 (−) | 6606 | Q | CAGG | 1.038(0.1349%) | (−) 34 | (−) 189 | (−) 19 |
| aLN(IV)-1a 100% | aLN(IV)-1, Sp(IV)-1, mLN(IV)-1, mLN(IV)-2, mLN(III)-2, mLN(III)-1 | 4 | NC_000070.4 133431467−133436778 (+) | 5312 | Q | AACA | <1.4063(0.1828%) | (+) 536 | (−) NA | (−) 48 |
| Sp(V)-1b 100% | Sp(V)-1, Th(V)-1, mLN(V)-1 | 4 | NC_000070.4 132368033−132373699 (+) | 5667 | Q | CCTT | <1.5795(0.2053%) | (−) 56 | (−) 63 | (−) NA |
| Th(IV)-1a 100% | Th(IV)-1 | 5 | NC_000071.4 23221069−23212403 (−) | 8667 | P | CTGG | <1.4432(<0.1876%) | (+) 537 | (−) 1081 | (+) 645 |
| aLN(II)-1a 100% | aLN(II)-1, iLN(II)-2, Th(II)-1, mLN(I)-2 | 5 | NC_000071.4 144923366−144932346 (+) | 8981 | Q | AGGG | <1.1036(0.1435%) | (−) 34 | (+) 1091 | (+) 641 |
| aLN(II)-1a 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 5 | NC_000071.4 44422608−44431589 (+) | 8982 | Q | CCAC | <1.1036(0.1435%) | (−) 189 | (+) 1091 | (+) 641 |
| aLN(II)-1b 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-3 | 5 | NC_000071.4 78005291−78014272 (+) | 8982 | Q | CACC | <1.1036(0.1435%) | (+) 536 | (+) 1091 | (+) 641 |
| Th(I)-2d 98% | Th(I)-2, ILN(I)-1, iLN(I)-2 | 5 | NC_000071.4 122453464−122460825 (+) | 7362 | Q | GATG | <1.0381(0.1350%) | (−) 34 | (p) 722 | (−) 19 |
| Th(I)-2e 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 5 | NC_000071.4 24740764−24749735 (+) | 8972 | Q | ATAC | <1.0381(0.1350%) | (+) 536 | (p) 1025 | (p) 452 |
| Th(I)-2f 99% | Th(I)-2, Sp(I)-2, iLN(I)-1, iLN(I)-2 | 5 | NC_000071.4 43496189−43505229 (+) | 9041 | Q | ATAT/TTAT | ND | (+) 536 | (+) 1088 | (+) 632 |
| Th(I)-2g 99% | Th(I)-2, iLN(I)-1, iLN(I)-2, Sp(I)-2, aLN(I)-1 | 5 | NC_000071.4 110148316−110140851 (−) | 7466 | Q | ATAG | <1.0381(0.1350%) | (p) 143 | (p) 1025 | (−) NA |
| Th(I)-2k 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 6 | NC_000072.4 73223659−73216301 (−) | 7359 | Q | ACAA/ACAC | ND | (+) 536 | (−) 244 | (−) 19 |
| aLN(II)-1i 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 7 | NC_000073.4 6682978−6673997 (−) | 8982 | Q | ATGA | 2.2072(0.2869%) | (p) 469 | (−) 104 | (−) NA |
| aLN(II)-1j 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 7 | NC_000073.4 29323967−29332665 (+) | 8699 | Q | ATTT | <1.1036(<0.1435%) | (+) 536 | (+) 1091 | (−) 99 |
| aLN(II)-1k 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 7 | NC_000073.4 30397400−30406380 (+) | 8981 | Q | CATG | <1.1036(<0.1435%) | (+) 536 | (p) 1072 | (−) NA |
| aLN(II)-1l 99% | aLN(II)-1, mLN(I)-2, Th(II)-1, iLN(II)-2 | 7 | NC_000073.4 116223258−116228931 (+) | 5674 | Q | CTAA | <1.1020(0.1433%) | (p) 509 | (−) NA | (−) 72 |
| Th(I)-2j 99% | Th(I)-2, Sp(I)-2, iLN(I)-1, iLN(I)-2, aLN(I)-1 | 7 | NC_000073.4 64005512−64014552 (+) | 9041 | Q | CCTG | <1.0381(0.1350%) | (+) 536 | (+) 1091 | (+) 632 |
| iLN(III)-1c 98% | iLN(III)-1, Th(III)-1, Th(III)-2 | 8 | NC_000074.4 44819280−44810547 (−) | 8734 | Q | GGTC/GTCT | ND | (+) 536 | (p) 1084 | (+) 643 |
| iLN(III)-1d 98% | iLN(III)-1, Th(III)-1, Th(III)-2 | 8 | NC_000074.4 88022347−88015960 (−) | 6388 | Q | GTAT | <1.3401(0.1742%) | (+) 536 | (−) NA | (−) NA |
| Th(I)-2i 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 8 | NC_000074.4 126050806−126058167 (+) | 7362 | Q | GGAA/GGTG | ND | (+) 536 | (−) 637 | (−) 19 |
| Sp(V)-1a 100% | Sp(V)-1, Th(V)-1, mLN(V)-1 | 8 | NC_000074.4 93776396−93782063 (+) | 5668 | Q | ATAT | <1.5795(0.2053%) | (−) 56 | (−) 63 | (−) NA |
| Th(II)-1a 100% | Th(II)-1, aLN(II)-1, iLN(II)-2, mLN(I)-2 | 8 | NC_000074.4 122783017−122775542 (−) | 7476 | Q | AGGT | <1.1036(<0.1435%) | (−) 162 | (−) NA | (+) 641 |
| iLN(III)-1a 98% | iLN(III)-1 | 9 | NC_000075.4 62237180−62245938 (+) | 8759 | Q | CTGG | ND | (+) 536 | (p) 1084 | (+) 641 |
| iLN(III)-1b 99% | iLN(III)-1, Th(III)-1, Th(III)-2 | 9 | NC_000075.4 41663263−41656480 (−) | 6784 | Q | GGAA/GGGG | ND | (−) NA | (p) 1084 | (+) 644 |
| aLN(II)-1p 99% | aLN(II)-1, iLN(II)-2, Th(II)-1, mLN(I)-2 | 10 | NC_000076.4 8241458−8234325 (−) | 7134 | Q | GTGC | <1.1036(<0.1435%) | (−) 189 | (−) 182 | (−) 36 |
| aLN(II)-1q 99% | aLN(II)-1, Th(II)-1, iLN(II)-2, mLN(I)-2 | 10 | NC_000076.4 41125063−41134043 (+) | 8981 | Q | GATG | <1.1036(<0.1435%) | (+) 536 | (+) 1091 | (+) 641 |
| Th(I)-2l 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 10 | NC_000076.4 4628826−4619788 (−) | 9039 | Q | ACAG/TTAG | ND | (−) 162 | (−) 179 | (−) 45 |
| Th(I)-2p 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 10 | NC_000076.4 22423694−22432738 (+) | 9045 | Q | CTGC/TTGC | ND | (+) 536 | (+) 1088 | (+) 632 |
| aLN(II)-1c 100% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 11 | NC_000077.4 6658394−6649425 (−) | 8970 | Q | GTTC | <1.1036(<0.1435%) | (+) 536 | (−) 182 | (+) 641 |
| aLN(II)-1c 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 11 | NC_000077.4 8820301−8829280 (+) | 8980 | Q | ATAG | <1.1036(<0.1435%) | (+) 536 | (+) 1091 | (+) 641 |
| Th(I)-2m 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 11 | NC_000077.4 60402203−60394844 (−) | 7360 | Q | ACAC | <1.0381(0.1350%) | (−) 85 | (−) NA | (−) 19 |
| Th(I)-2n 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 11 | NC_000077.4 76365003−76374047 (+) | 9045 | Q | AGGG/TTGG | ND | (+) 536 | (+) 1088 | (+) 632 |
| Th(I)-2o 99% | Th(I)-2, Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 11 | NC_000077.4 86698511−86707551 (+) | 9041 | Q | ACAC | <1.0381(0.1350%) | (+) 536 | (+) 1091 | (+) 632 |
| Sp(I)-2a 98% | Sp(I)-2, aLN(I)-1, iLN(I)-1, iLN(I)-2 | 11 | NC_000077.4 102899753−102908794 (+) | 9042 | Q | GAAAC | 1.038(0.1349%) | (p) 235 | (p) 988 | (+) 632 |
| Th(II)-1r 99% | Th(II)-1, aLN(II)-1, iLN(II)-2, mLN(I)-2 | 11 | NC_000077.4 88713056−88706270 (−) | 6787 | Q | GGAG | <1.1036(<0.1435%) | (+) 535 | (−) 358 | (+) 641 |
| aLN(II)-1t 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 12 | NC_000078.4 20945382−20951142 (+) | 5761 | Q | CTGT | <1.1036(<0.1435%) | (−) 205 | (−) NA | (−) NA |
| Th(I)-2h 99% | Th(I)-2, Sp(I)-2, iLN(I)-1, iLN(I)-2, aLN(I)-1 | 12 | NC_000078.4 70465411−70474451 (+) | 9041 | Q | AGAC | <1.0381(0.1350%) | (p) 235 | (+) 1091 | (+) 632 |
| aLN(II)-1o 99% | aLN(II)-1, iLN(II)-2, Th(II)-1, mLN(I)-2 | 13 | NC_000079.4 100095412−100086432 (−) | 8981 | Q | GTGG | <1.1036(<0.1435%) | (+) 536 | (p) 1069 | (+) 641 |
| Th(I)-2a 100% | Th(I)-2, iLN(I)-1, iLN(I)-2, Sp(I)-2, aLN(I)-1 | 13 | NC_000079.4 21819875−21827661 (+) | 7787 | Q | CTAC | <1.0381(0.1350%) | (+) 536 | (+) 1088 | (−) 160 |
| aLN(IV)-1a 99% | aLN(IV)-1, Sp(IV)-1, mLN(IV)-1, mLN(IV)-2, mLN(III)-2, mLN(III)-1 | 13 | NC_000079.4 68392677−68383991 (−) | 8687 | Q | GTAC | <1.4063(0.1828%) | (+) 536 | (p) 1084 | (+) 644 |
| iLN(III)-1 99% | iLN(III)-1, Th(III)-1, Th(III)-2 | 14 | NC_000080.4 53484571−53492393 (+) | 7823 | Q | GTAT | 1.3401(0.1742%) | (−) NA | (p) 1084 | (+) 643 |
| aLN(II)-1h 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 15 | NC_000081.4 76394257−76387072 (−) | 7186 | Q | AAACAAACAAAC | <1.1036(<0.1435%) | (−) 162 | (−) NA | (+) 641 |
| aLN(II)-1c 98% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 16 | NC_000082.4 76026704−76017723 (−) | 8982 | Q | CTGG | <1.1036(<0.1435%) | (+) 536 | (p) 886 | (−) 45 |
| aLN(II)-1g 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 16 | NC_000082.4 93594977−93585997 (−) | 8981 | Q | GCCT | <1.1036(<0.1435%) | (−) 34 | (+) 1091 | (+) 641 |
| aLN(II)-1b 100% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 18 | NC_000084.4 82827979−82836858 (+) | 8880 | Q | CCTG | <1.1036(<0.1435%) | (−) 164 | (+) 1091 | (+) 641 |
| iLN(III)-1b 98% | iLN(III)-1, Th(III)-1, Th(III)-2 | 19 | NC_000085.4 60988696−60979970 (−) | 8727 | Q | CTTG | <1.3401(0.1742%) | (+) 536 | (p) 1084 | (+) 640 |
| aLN(II)-1s 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | 19 | NC_000085.4 38440166−38447227 (+) | 7062 | Q | CTTC | <1.1036(<0.1435%) | (−) 34 | (p) 883 | (−) NA |
| aLN(II)-1e 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | X | NC_000086.5 14631057−14639937 (+) | 8981 | Q | ATAC | <1.1036(<0.1435%) | (+) 536 | (p) 998 | (+) 641 |
| aLN(II)-1f 99% | aLN(II)-1, iLN(II)-2, mLN(I)-2, Th(II)-1 | X | NC_000086.5 51501607−51510587 (+) | 8981 | Q | TGAA/GAGT | ND | (+) 536 | (+) 1091 | (+) 641 |
Open reading frame (ORF)
The structures of the putative MuERVs identified in this study were determined by comparison to full-length murine leukemia virus (MuLV) reference sequences (GenBank accession nos. AF033811 and DQ241301). ORF analyses revealed that 12 of the 60 putative MuERVs were full-length and had intact coding potentials for all three retroviral polypeptides (gag, pol, and env) (highlighted in gray, Table 3). Six putative MuERVs (highlighted in yellow) had an insertion of a nucleotide (cytosine) within various poly C regions resulting in a defective gag polypeptide due to an insertional frame shift. The locations (nucleotide number from the 5’-end of the provirus) of the insertions within these MuERVs are indicated in parentheses: Th(I)-2a 99% (1,700), Th(I)-2b 98% (1,701), aLN(II)-1a 99% (1,738), Th(II)-1a 100% (1,657), aLN(II)-1p 99% (1,738), and aLN(II)-1h 99% (1,657). On the other hand, putative MuERV Th(I)-2g 99% had a deletion of a cytosine at 1,657 resulting in a frameshift of the gag polypeptide. It is possible that the insertion or deletion of a single cytosine in the poly C regions may be attributable to a sequencing error in the NCBI database. In addition, “ATG→GTG” mutations were observed in 26 putative MuERVs (highlighted in blue, Table 3) at the first start codon (ATG) of the reverse transcriptase (RT) region within the pol gene, resulting in a loss of three amino acids from the start of the RT protein. The putative MuERV Th(II)-1r 99% contains a green box under the gag column because the gag ORF was deemed complete, despite the one amino acid deletion within the p12 region.
Primer binding site (PBS)
Analyses of PBSs for all 60 putative MuERVs revealed that 59 proviruses contained a glutamine (Q) tRNA binding site, while the Th(IV)-1a 100% putative MuERV contained a proline (P) tRNA binding site (Table 3).
Recombination event and integration age
Only 6 out of 60 putative MuERVs had LTR mutations (5’ LTR sequence compared to 3’ LTR sequence) ranging from 0.1349% to 0.2869% (Table 3). The putative iLN(III)-1a 98% MuERV had a deletion of 39 nucleotides in the middle of the 5’ LTR in comparison to its 3’ counterpart and the mutation rate was not calculated. The integration age of these MuERVs was calculated (ranging from 1.038 million years [MYr] to 2.2072 MYr) based on a formula of “0.13% mutation rate between two flanking LTRs/one MYr” (Sverdlov, 2000). To determine whether there were genetic rearrangements by recombination during the lifespan of the putative MuERVs, we examined the presence or absence of a direct repeat sequence flanking both ends of the proviral sequences. Direct repeats are formed during initial integration of proviruses and any downstream recombination events result in two unique sequences instead. There were 10 putative MuERVs with unique sequences flanking the integration sites, suggesting recombination events, and the rest had direct repeats of four nucleotides (49 putative MuERVs) or 12 nucleotides (one putative MuERV: aLN(II)-1h 99%) (Table 3).
Tropism analysis by restriction fragment length polymorphism (RFLP)
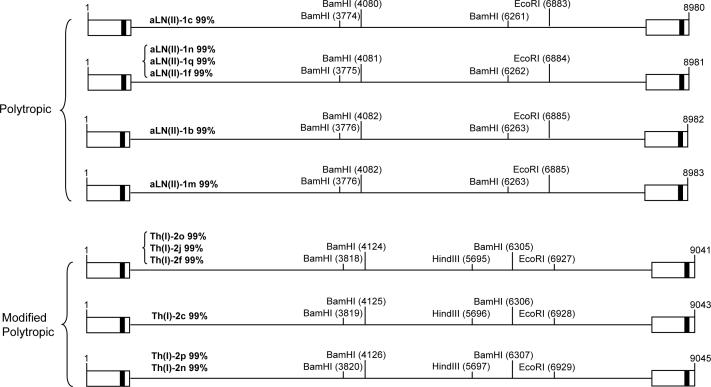
Cellular tropisms of 12 full-length putative MuERVs with intact coding potentials were determined by in silico RFLP using three restriction enzymes (BamHI, EcoRI, and HindIII) (Stoye and Coffin, 1988). It revealed six polytropic and six modified polytropic putative MuERVs (Figure 3). It needs to be noted that although the putative tropism traits described in this study are likely to be correct, it may be necessary to determine each MuERV's tropism by an in vitro infection study.
Figure 3.
RFLP tropism analysis of the putative full-length MuERVs with intact coding potentials. Cellular tropisms of 12 putative full-length MuERVs were determined by in silico RFLP analysis using three restriction enzymes (BamHI, EcoRI, and HindIII). Each restriction site is indicated in parenthesis on schematic MuERVs.
Discussion
The findings from this study demonstrated that certain MuERVs were actively transcribed and their expression profiles were specific for each lymphoid tissue examined. Further studies may be necessary to determine the MuERV expression profile in various subsets of cells within each lymphoid tissue. Individual lymphoid tissues and cell types are presumed to have unique characteristics specific to their transcriptional environment, such as transcription factor pool (Schon et al., 2001; van Opijnen et al., 2004). Considering the tissue and cell type-specific transcription factor pool, in conjunction with the unique set of transcription regulatory elements within each MuERV U3 promoter, the findings of differential MuERV expression in various lymphoid tissues in this study are readily predictable. Moreover, the transcription factor profile within each tissue or cell type is affected by its own pathophysiologic status and the surrounding environment, such as systemic immune modulation and carcinogenesis (Boral, Okenquist, and Lenz, 1989).
Due to the polymorphic nature of the MuERV U3 sequences, each U3 promoter is likely to harbor a unique transcription potential, primarily determined by its profile of binding sites for transcription factors. It suggests that changes in the transcription environment due to stress (e.g., injury, infection) will lead to differential expression of certain MuERVs in different tissues. The viral gene products and viral replication itself associated with the altered MuERV expression may participate in a range of pathophysiologic activities in a tissue- and cell type-specific manner.
In silico cloning of the putative MuERVs in the genome of the C57BL/6J strain using the U3 sequences as a probe allowed us to characterize their biological properties such as coding potential, replication competency, and tropism. The results from this study demonstrated that some of the putative MuERVs are full-length with intact ORFs for the gag, pol, and env genes and are presumably replication-competent. Further functional characterization of these putative MuERVs is essential to understanding their roles in normal physiology and pathology of individual lymphoid tissues and cells. In particular, the putative MuERVs retaining intact coding potentials for gag, pol, and/or env may be cloned from the C57BL/6J genome into an expression vector for functional analyses in vitro (e.g., cell tropism, viral gene expression, cytokine production, cytotoxicity) and in vivo (e.g., infection of virions into mice followed by examination of their effects on the immune system). In addition, the biological properties of individual gene products, primarily gag and env proteins, from each putative MuERV may be investigated in vitro using overexpression as well as knock-out protocols.
Retroviral U3 promoters are capable of controlling the expression of host genes adjacent to the integration sites through certain transcription regulatory elements, such as enhancers and negative regulatory elements (Trusko, Hoffman, and George, 1989; Yu et al., 2005). It will be of interest to investigate whether the U3 promoters of putative MuERVs identified in this study play a role in the transcriptional activities of neighboring host genes.
It may be reasonable to postulate that MuERVs, distributed throughout the genome, play crucial and differential roles in normal physiologic and pathologic processes in various lymphoid tissues and cells. The specificity of MuERV expression is primarily dependent upon the polymorphic U3 promoter sequences and the specific transcriptional environment in each tissue/cell type. Understanding the biological characteristics of tissue-specific MuERVs and their relationship with neighboring genes will broaden insight into their roles in a range of pathophysiologic events pertaining to individual lymphoid tissues and cells.
Materials and Methods
Animals
Female C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) were housed according to the guidelines of the National Institutes of Health. The Animal Use and Care Administrative Advisory Committee of the University of California, Davis, approved the experimental protocol. Three mice were sacrificed by cervical dislocation for lymphoid tissue collection without any pretreatments.
RT-PCR analysis
Total RNA isolation and cDNA synthesis were performed based on protocols described previously (Cho et al., 2000). Briefly, total RNA was extracted using a RNeasy kit (Qiagen, Valencia, CA). Total RNA (100 ng) from each tissue sample was subjected to reverse transcription. The sequence of the oligo-dT primer was as follows: 5’-GGC CAC GCG TCG ACT AGT ACT TTT TTT TTT TTT TTT T-3’. Primers, ERV-U1 (5’-CGG GCG ACT CAG TCT ATC GG-3’) and ERV-U2 (5’-CAG TAT CAC CAA CTC AAA TC-3’) were used to amplify the U3 regions of nonecotropic MuERVs. The primers for β-actin amplification were 5’-CCA ACT GGG ACG TGG AA-3’ and 5’-GTA GAT GGG CAC AGT GTG GG-3’. The comparability between samples was determined by both the electrophoresis of an equal amount (500 ng) of total RNA and the RT-PCR amplification of β-actin from each sample.
Cloning of MuERV U3 sequences
PCR products were gel purified using the QIAquick Gel Extraction kit (Qiagen) and cloned into the pGEMT-Easy vector (Promega, Madison, WI). Plasmid DNAs for sequencing analysis were prepared using a miniplasmid kit from Qiagen. Sequencing was performed at Davis Sequencing Inc. (Davis, CA).
Multiple alignment and phylogenetic tree analysis
The resulting 43 MuERV U3 sequences were aligned using Vector NTI Advance 10 (Invitrogen, Carlsbad, CA) program to identify unique U3 sequences. A phylogentic tree was obtained using the neighbor-joining protocol within the MEGA3 program (Kumar, Tamura, and Nei, 2004; Saitou and Nei, 1987). Bootstrap evaluation of the branching pattern was performed with 100 replications.
Tropism analysis
The putative tropism of unique U3 sequences was determined by comparison to the reference sequences (direct repeats, unique region) first reported by Tomonaga et al. (Tomonaga and Coffin, 1998; Tomonaga and Coffin, 1999). A total of four direct repeats (1/1*, 4/4*, 5/5*, and 6/6*), a single 190 bp insertion, and two unique sequences (2 and 3) were utilized for the tropism analysis.
Analysis of transcription regulatory elements
The 22 unique U3 sequences were analyzed for transcription regulatory elements using the MatInspector program (Genomatix, Munich, Germany). The core similarity was set to 0.9 and the matrix similarity was optimized within the vertebrate matrix group. The U3 sequences are organized in Table 2 to match the order within the multiple alignment and phylogenetic tree.
In silico mapping and cloning of putative MuERVs
Putative MuERV sequences were identified by probing the NCBI mouse genome database using the 22 unique U3 sequences with the NCBI Megablast program. The key reference for identifying viral sequences of interest was a 5−9 kb sequence region flanked by LTRs, which harbor U3 sequences. The following parameters were used with the Megablast program: in the options section “NC_000067:NC_000087” was entered into the “limited by entrez query” field. The filters were set to none while the percent identity, match, mismatch scores were set to 98, 1, and -3, respectively.
MuERV provirus nomenclature
The putative MuERV proviruses were named after the U3 probes/sequences used in Megablast (e.g., Th(I)-2), followed by the percentage homology of the blast hits (e.g., Th(I)-2 99%). The letters following the U3 probe/sequence (e.g., Th(I)-2a 99%) are to differentiate multiple hits found using the U3 probe with similar blast percentage hits.
ORF analysis
The ORFs of each putative MuERV were analyzed using the ORF search feature within Vector NTI (Invitrogen). The parameters were set at a minimum of 50 codons with “ATG” as the start codon, and each candidate ORF was translated. The amino acid translations were then compared using Vector NTI AlignX (Invitrogen) to the following MuLV references retrieved from NCBI (GenBank accession nos. M17327, AY219567.2, AA037285, DQ241301, and AF033811). The criteria for defining the intactness of each proviral gene depended on the p12 region of gag, RT (reverse transcriptase) of pol and SU (surface domain) of env. Proviral genes were deemed intact (+) if the aforementioned sequences were intact and the remaining amino acid sequence of each respective gene matched one of the reference sequences, while allowing for missense mutations. Proviral genes were classified as partial (p) if the defining sequences were intact but the remaining proviral gene sequences were defective. Defective (−) proviral gene sequences contained amino acid deletions and premature stop codons in addition to improper start codons leading to defective defining coding sequences.
Integration age and recombination event
5’ and 3’ LTR sequences of the putative MuERVs were compared using the Vector NTI AlignX program (Invitrogen). The integration age was calculated based on a formula of “0.13% mutation rate between two flanking LTRs per one MYr”. In case there is only a single nucleotide difference between two flanking LTRs, its integration age is recorded as less than the estimated age in consideration of potential error during cloning and sequencing. To examine the genomic rearrangement between MuERVs as well as with other parts of the genome, a short stretch of sequences (4 bp to 12 bp) flanking each MuERV was surveyed for a direct repeat, which is formed during the initial proviral integration.
PBS analysis
A stretch of 18 bp, located immediately downstream of the 5’ U5 region, was examined to determine PBSs for the putative MuERVs. The conserved PBS sequences for tRNAProline (P) and tRNAGlutamine (Q) were used as references (Harada, Peters, and Dahlberg, 1979; Nikbakht et al., 1985).
RFLP tropism analysis of full-length MuERVs
Tropism of the putative full-length MuERVs was determined by in silico RFLP analysis using three restriction enzymes, BamHI, EcoRI, and HindIII, using Vector NTI (Invitrogen). The RFLP data of each putative MuERV were compared to the reference profiles for each tropism (ecotropic, xenotropic, polytropic, and modified polytropic) reported previously (Stoye and Coffin, 1988).
Acknowledgement
This study was supported by grants from Shriners of North America (No. 8680 to KC) and National Institutes of Health (R01 GM071360 to KC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antony JM, van Marle G, Opii W, Butterfield DA, Mallet F, Yong VW, Wallace JL, Deacon RM, Warren K, Power C. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci. 2004;7(10):1088–95. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- Aziz DC, Hanna Z, Jolicoeur P. Severe immunodeficiency disease induced by a defective murine leukaemia virus. Nature. 1989;338(6215):505–8. doi: 10.1038/338505a0. [DOI] [PubMed] [Google Scholar]
- Boral AL, Okenquist SA, Lenz J. Identification of the SL3−3 virus enhancer core as a T-lymphoma cell-specific element. J Virol. 1989;63(1):76–84. doi: 10.1128/jvi.63.1.76-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Adamson LK, Greenhalgh DG. Induction of murine AIDS virus-related sequences after burn injury. J Surg Res. 2002;104(1):53–62. doi: 10.1006/jsre.2002.6410. [DOI] [PubMed] [Google Scholar]
- Cho K, Greenhalgh D. Injury-associated induction of two novel and replication-defective murine retroviral RNAs in the liver of mice. Virus Res. 2003;93(2):189–98. doi: 10.1016/s0168-1702(03)00097-2. [DOI] [PubMed] [Google Scholar]
- Cho K, Zipkin RI, Adamson LK, McMurtry AL, Griffey SM, Greenhalgh DG. Differential regulation of c-jun expression in liver and lung of mice after thermal injury. Shock. 2000;14(2):182–6. doi: 10.1097/00024382-200014020-00018. [DOI] [PubMed] [Google Scholar]
- Choi YW, Henrard D, Lee I, Ross SR. The mouse mammary tumor virus long terminal repeat directs expression in epithelial and lymphoid cells of different tissues in transgenic mice. J Virol. 1987;61(10):3013–9. doi: 10.1128/jvi.61.10.3013-3019.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad B, Weissmahr RN, Boni J, Arcari R, Schupbach J, Mach B. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell. 1997;90(2):303–13. doi: 10.1016/s0092-8674(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Deas JE, Liu LG, Thompson JJ, Sander DM, Soble SS, Garry RF, Gallaher WR. Reactivity of sera from systemic lupus erythematosus and Sjogren's syndrome patients with peptides derived from human immunodeficiency virus p24 capsid antigen. Clin Diagn Lab Immunol. 1998;5(2):181–5. doi: 10.1128/cdli.5.2.181-185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths DJ. Endogenous retroviruses in the human genome sequence. Genome Biol. 2001;2(6):REVIEWS1017. doi: 10.1186/gb-2001-2-6-reviews1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F, Peters GG, Dahlberg JE. The primer tRNA for Moloney murine leukemia virus DNA synthesis. Nucleotide sequence and aminoacylation of tRNAPro. J Biol Chem. 1979;254(21):10979–85. [PubMed] [Google Scholar]
- King LB, Corley RB. Lipopolysaccharide and dexamethasone induce mouse mammary tumor proviral gene expression and differentiation in B lymphocytes through distinct regulatory pathways. Mol Cell Biol. 1990;10(8):4211–20. doi: 10.1128/mcb.10.8.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knossl M, Lower R, Lower J. Expression of the human endogenous retrovirus HTDV/HERV-K is enhanced by cellular transcription factor YY1. J Virol. 1999;73(2):1254–61. doi: 10.1128/jvi.73.2.1254-1261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5(2):150–63. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blocker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, Szustakowki J, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lower R, Boller K, Hasenmaier B, Korbmacher C, Muller-Lantzsch N, Lower J, Kurth R. Identification of human endogenous retroviruses with complex mRNA expression and particle formation. Proc Natl Acad Sci U S A. 1993;90(10):4480–4. doi: 10.1073/pnas.90.10.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lower R, Lower J, Kurth R. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc Natl Acad Sci U S A. 1996;93(11):5177–84. doi: 10.1073/pnas.93.11.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikbakht KN, Ou CY, Boone LR, Glover PL, Yang WK. Nucleotide sequence analysis of endogenous murine leukemia virus-related proviral clones reveals primer-binding sites for glutamine tRNA. J Virol. 1985;54(3):889–93. doi: 10.1128/jvi.54.3.889-893.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Samuelson LC, Phillips RS, Swanberg LJ. Amylase gene structures in primates: retroposon insertions and promoter evolution. Mol Biol Evol. 1996;13(6):767–79. doi: 10.1093/oxfordjournals.molbev.a025637. [DOI] [PubMed] [Google Scholar]
- Schon U, Seifarth W, Baust C, Hohenadl C, Erfle V, Leib-Mosch C. Cell type-specific expression and promoter activity of human endogenous retroviral long terminal repeats. Virology. 2001;279(1):280–91. doi: 10.1006/viro.2000.0712. [DOI] [PubMed] [Google Scholar]
- Stoye JP, Coffin JM. Polymorphism of murine endogenous proviruses revealed by using virus class-specific oligonucleotide probes. J Virol. 1988;62(1):168–75. doi: 10.1128/jvi.62.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverdlov ED. Retroviruses and primate evolution. Bioessays. 2000;22(2):161–71. doi: 10.1002/(SICI)1521-1878(200002)22:2<161::AID-BIES7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Tomonaga K, Coffin JM. Structure and distribution of endogenous nonecotropic murine leukemia viruses in wild mice. J Virol. 1998;72(10):8289–300. doi: 10.1128/jvi.72.10.8289-8300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga K, Coffin JM. Structures of endogenous nonecotropic murine leukemia virus (MLV) long terminal repeats in wild mice: implication for evolution of MLVs. J Virol. 1999;73(5):4327–40. doi: 10.1128/jvi.73.5.4327-4340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusko SP, Hoffman EK, George DL. Transcriptional activation of cKi-ras proto-oncogene resulting from retroviral promoter insertion. Nucleic Acids Res. 1989;17(22):9259–65. doi: 10.1093/nar/17.22.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urnovitz HB, Murphy WH. Human endogenous retroviruses: nature, occurrence, and clinical implications in human disease. Clin Microbiol Rev. 1996;9(1):72–99. doi: 10.1128/cmr.9.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Opijnen T, Jeeninga RE, Boerlijst MC, Pollakis GP, Zetterberg V, Salminen M, Berkhout B. Human immunodeficiency virus type 1 subtypes have a distinct long terminal repeat that determines the replication rate in a host-cell-specific manner. J Virol. 2004;78(7):3675–83. doi: 10.1128/JVI.78.7.3675-3683.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pelisson I, Melana SM, Go V, Holland JF, Pogo BG. MMTV-like env gene sequences in human breast cancer. Arch Virol. 2001;146(1):171–80. doi: 10.1007/s007050170201. [DOI] [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520–62. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Yu X, Zhu X, Pi W, Ling J, Ko L, Takeda Y, Tuan D. The long terminal repeat (LTR) of ERV-9 human endogenous retrovirus binds to NF-Y in the assembly of an active LTR enhancer complex NF-Y/MZF1/GATA-2. J Biol Chem. 2005;280(42):35184–94. doi: 10.1074/jbc.M508138200. [DOI] [PubMed] [Google Scholar]