Abstract
T-cell antigen receptor triggering mechanisms and lipid rafts are of broad interest, but are also controversial topics. Here, we review some recent progress in these two research fields, which has been accomplished mostly in live cells and with the use of advanced technologies. We then discuss the potential relationship between membrane-domain organization and T-cell antigen receptor-triggering mechanisms. On the basis of the relevant experimental observations, we argue that the key to achieving a better understanding of both processes is the ability to monitor the molecular dynamics and interactions taking place in the membrane of T cells at a spatial scale of tens to hundreds of nanometres, with a subsecond-to-second temporal resolution.
Keywords: lipid rafts, live cells, membrane domains, signal transduction, T-cell receptor
Introduction
The T-cell antigen receptor (TCR) is a recognition module that is composed of an αβ-heterodimer or a γδ-heterodimer, and a signal-transducing module containing CD3ɛ, CD3δ, CD3γ and CD3ζ polypeptides. The recognition of peptides bound to the major histocompatibility complex (MHC) by the TCR leads to the transduction of signals across the plasma membrane, which is a process referred to as TCR triggering, and to the induction of cellular responses involved in T-lymphocyte effector function (Davis et al, 1998; Weiss & Littman, 1994). An early and essential signalling event that occurs during TCR triggering is the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs), which are located in the cytoplasmic domains of the CD3 subunits, by the SRC kinase LCK. This phosphorylation results in the recruitment and activation of ζ-chain-associated protein kinase 70 kDa (ZAP70), which in turn couples TCR phosphorylation to downstream signalling cascades by phosphorylating crucial intermediates such as the adaptor linker for activation of T cells (LAT; Germain & Stefanova, 1999; He et al, 2005). Despite the identification of key molecules that participate in the SRC-kinase-mediated phosphorylation of TCR/CD3, a central unanswered question is how ligand engagement of TCR/CD3 at the cell surface brings about the phosphorylation of CD3 ITAMs by LCK, which is located on the membrane inner leaflet. In this context, a role for lipid rafts in TCR triggering has recently been studied and hotly debated. Here, we present the latest advances in the study of lipid rafts in native cell membranes, and discuss the potential relationship between membrane-domain organization and TCR-triggering mechanisms.
Lipid-dependent domains in intact cell membranes
The lateral inhomogeneity of biological membranes is now widely recognized (Edidin, 2003; Marguet et al, 2006), but the determinants of their local organization remain poorly understood. Among the proposed mechanisms, lipid-mediated microdomains—the so-called ‘lipid rafts' (Simons & Ikonen, 1997)—have been subject to extensive investigation by various sophisticated approaches. Lipid rafts are defined by preferential interactions between sphingolipids and cholesterol, which promote the formation of membrane domains that, compared with the rest of the membrane, are probably in a more ordered state (Brown & London, 1998). Owing to the huge range of cell-membrane components and the small size of rafts (<200 nm), one would expect nanodomains to be heterogeneous. To our understanding, the controversies about the lipid-raft model mostly reflect the difficulties faced when directly studying these complex membrane organizations in vivo. Initially defined as TX-100 detergent-resistant membranes (DRMs), rafts have more recently been investigated in intact cell membranes through methods with variable lengths and temporal resolutions, and different intentions—from those measuring direct molecular interactions (for example, fluorescence resonance energy transfer (FRET)-based techniques; Vogel et al, 2006) to those reporting the dynamics of molecular complexes (for example, diffusion-based observations; Marguet et al, 2006). Each of these methods has specific advantages as well as limitations. For instance, a positive FRET result reports a tight molecular interaction between donor–acceptor, whereas a simple measurement of a diffusion coefficient is mostly dominated by the overall membrane viscosity and is not sensitive enough to probe such a donor–acceptor interaction. New technological developments in imaging are promising for resolving the spatial distribution of membrane components; those based on the photoactivatable/photo-switchable fluorescent probes allow nanometre accuracy for the localization of activated molecules (Moerner, 2006), whereas those based on stimulated emission depletion significantly reduce the focal volume of observation below the diffraction limit (Donnert et al, 2006).
Evidence for a heterogeneous lipid-dependent distribution of membrane components. The characterization of the two-dimensional distribution of membrane components has been difficult on a submicrometre scale. FRET is the favoured approach owing to its strong dependence on the closeness of molecules. The fact that different conventional FRET-based experiments have failed to produce consensual conclusions might reflect the complexity of both the detection efficiency and the data treatment when analysing highly dynamic and transient molecular associations (Kenworthy & Edidin, 1998; Meyer et al, 2006; Sharma et al, 2004; Zacharias et al, 2002). Recent studies, combined with advanced experimental methodologies and theoretical modelling, have allowed the clear identification of glycophosphatidylinositol (GPI)-anchored protein-containing or membrane receptor-containing membrane clusters and/or organizations in the plasma membrane of living cells (Meyer et al, 2006; Sharma et al, 2004). These membrane organizations are cholesterol sensitive and extremely small (<10 nm).
Immunoelectron microscopy has provided a good alternative approach for exploring this problem. The examination of intact two-dimensional sheets of the plasma membrane, which are removed from adherent cells and placed directly onto electron microscopy (EM) grids, has revealed the presence of cholesterol-dependent and -independent nanodomains in the inner-membrane organization, which could have an important role in signal transmission inside cells (Prior et al, 2003; Tian et al, 2007). Sample preparation, using quick-frozen and freeze-fractured specimens, minimizes the possibility of artefacts, and has allowed the clear identification of clusters of gangliosides GM1 and GM3 at the surface of mouse fibroblasts (Fujita et al, 2007). These EM observations are based on robust statistical analysis tools that allow accurate quantification. Moreover, double-labelling experiments have provided evidence for the presence of heterogeneous microdomains.
Evidence for a condensed membrane in live cells. As postulated by the lipid-raft theory, and in accordance with observations made on model membranes, it is predicted that raft domains have a more ordered state. This hypothesis has been tested by labelling cells with laurdan, which is a fluorescent probe that detects the polarity of its environment and, hence, the extent of water penetration into the hydrophobic part of a lipid bilayer. Because laurdan orientation varies in disordered and ordered lipid bilayers, it has been possible to quantify the relative level of local organization of the plasma membrane in living cells by calculating a generalized polarization index. Although limited in spatial resolution, this approach has provided clear evidence of the in vivo existence of more-ordered areas covering 10–15% of the cell surface (Gaus et al, 2003). More importantly, the percentage of ordered areas varies during signalling processes (see below; Gaus et al, 2005).
Evidence for dynamic lateral confinement in live cells. Great advances in the identification of diffusion heterogeneities have been made possible by methods of tracking individual molecules, which provide excellent spatial precision and allow access to the different modes of motion (Destainville & Salome, 2006; Fujiwara et al, 2002; Kusumi et al, 2005; Wieser et al, 2007). The methods involved in obtaining an appropriate temporal resolution that allows the characterization of small and dynamic structures have been clearly assessed when determining high-speed single-particle tracking experiments. By recording images at a frequency of ∼40,000 frames/s, the time dependency of different compartmentalization structures has been identified (Fujiwara et al, 2002).
Alternatively, diffusion-coefficient measurements can be evaluated by fluorescence-recovery after photobleaching from areas of various sizes rather than just one area, and this provides additional spatio-temporal information. Pioneered in a seminal work (Yechiel & Edidin, 1987), this idea has made possible the detection of membrane microdomains. In the absence of corrals or domains, membrane components display a free-like lateral diffusion, whereas their presence leads to a deviation from the expected linear relationship between the average diffusion time and the surface of observation. Although markedly informative (Niv et al, 2002; Salome et al, 1998), this strategy was not widely used until it was recently revisited with fluorescence-correlation spectroscopy-based approaches (Lenne et al, 2006; Wawrezinieck et al, 2005; Wenger et al, 2007). Fluorescence-correlation spectroscopy measurements provide high temporal resolution, excellent statistical accuracy and single-molecule sensitivity. In addition, this approach introduces minimal perturbations to the experimental subject, as low concentrations of probes and low light excitation are required.
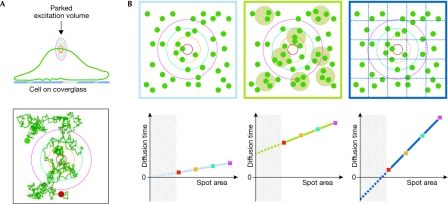
As shown in Fig 1, if Brownian motion occurs, there must be a strict linear relationship between the average time (τ) that the fluorescent molecules stay within a delimited surface and the size of this area. By contrast, different domain organizations would influence the path explored by a diffusing molecule and slow its spread. With such an analytical strategy, we clearly established that nanometre-scale domains (<100 nm) are observed in the plasma membrane of live cells at 37 °C. GPI-anchored proteins and sphingolipid analogues dynamically partition into or form these domains, depending on whether such domains consist of pre-existing organizations or self-promoting assemblies, with a timescale of tens to hundreds of milliseconds. Moreover, these membrane nanodomains are sensitive to either sphingomyelinase or cholesterol oxidase treatment. Therefore, the structural characteristics of these nanodomains fall within those expected of lipid rafts (Lenne et al, 2006; Wenger et al, 2007). Despite the limitations outlined above for each technique (Sidebar A; Marguet et al, 2006), there are now numerous observations supporting the raft concept of membrane organization. These different methodological approaches have also revealed an organization hierarchy at different spatial scales for lipid-dependent domains in intact cell membranes (Fig 2).
Figure 1.
The principle of identifying membrane organizations by measuring the lateral diffusion at different spatial scales. (A) Diffusion in the cell membrane can be spatially resolved by fluorescence-correlation spectroscopy (FCS) measurements at different spot areas of excitation. The lower panel illustrates the relationship between the average time (τ) taken for a single molecule to travel within a delimited surface and the size of this area. (B) Different confinement structures would limit the spreading within circles of different scales for a given molecule. In the free-like diffusion mode, fluorescent molecules show Brownian motion without constraint (left panel). As plotted from the measurements at different spatial scales, the FCS diffusion line (light blue) intercepts the time axis at the origin. In the presence of permeable isolated domains (as the result of dynamic partition processes), the molecules can diffuse in and out of the domains, but are transiently trapped (middle panel). This gives rise to a positive deviation of the FCS diffusion line (green) on the time axis. In the presence of adjacent domains, such as those created by actin-based cytoskeleton barriers (right panel), the probability of jumping from one domain to the adjacent one would slow the spreading of the molecule. This gives rise to a negative deviation of the FCS diffusion line (dark blue) on the time axis. The FCS cannot be determined experimentally below the optical diffraction limit (crosshatched area).
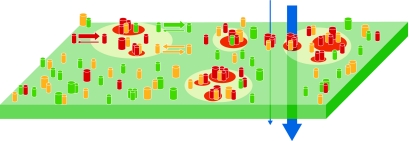
Figure 2.
Formation of specific molecular assemblies promoted by dynamic nanodomain partitioning for efficient receptor signalling. By promoting temporary lateral confinement, raft nanodomains might contribute to the formation of molecular assemblies, especially those based on weak protein–protein or protein–lipid interactions that might otherwise be insufficient for such a formation in a disordered membrane environment. In this way, nanodomains could have an important role in organizing specific components of the receptor signal machinery for speedy and efficient triggering on ligand engagement. The thickness of the vertical arrows indicates the efficiency of signal triggering.
TCR triggering and membrane domains
Spatio-temporal scales of TCR triggering. An accurate determination of TCR triggering kinetics has so far been unattainable owing to methodological limitations. However, by controlling antigen presentation with photoreleasable peptides, it has been possible to gain high temporal resolution and to analyse the kinetics of TCR triggering after the uncaging process (Huse et al, 2007). In this study, substantial phosphorylation of the LAT adaptor was observed within 4 s, indicating that TCR triggering should have occurred within a shorter timeframe. In addition, within seconds of cell contact, active molecular aggregation and reorganization processes occur in the T-cell membrane area in which the TCR interacts with the peptide–MHC (pMHC) complex. This was investigated using total internal reflection-fluorescence microscopy (TIRF), and by following the interaction of the T cell with a planar lipid bilayer containing GPI–pMHC and GPI–intercellular adhesion molecule 1 (ICAM1; Campi et al, 2005; Yokosuka et al, 2005). This technique has allowed the detection and characterization of TCR microcluster formation on T-cell contact with the planar bilayer (Campi et al, 2005; Mossman et al, 2005; Varma et al, 2006; Yokosuka et al, 2005). In the pMHC-stimulated T cells, after maximum spreading (for 1–2 min), TCR microclusters begin to undergo centripetal transport, resulting in the formation of a large central supramolecular activation cluster (cSMAC) of 2–3 μm in diameter. The process of cSMAC formation—the key event in establishing the immunological synapse—is accomplished 5–15 min after cell contact. Both the formation and the centripetal transport of the TCR microclusters depend on the actin cytoskeleton. Importantly, TCR microclusters display intense signalling activity soon after their formation, which gradually decreases as they are transported towards the cSMAC, and becomes weaker in the cSMAC itself (Mossman et al, 2005; Varma et al, 2006). Therefore, it is likely that TCR microclusters contribute positively to TCR signalling, even though their exact role remains to be determined. Conversely, it is clear that both the formation of the cSMAC and the immunological synapse are consequences of TCR triggering, and not the sites of TCR signal initiation.
Experimental evidence for the involvement of lipid-dependent membrane domains in TCR triggering. One popular model has proposed that, on ligation, TCR/CD3 is translocated into specific membrane microdomains (that is, lipid rafts) where it becomes phosphorylated by LCK (for a review, see He et al, 2005). This model is largely based on the association of TCR/CD3 with TX-100 DRMs, which is indicative, rather than being conclusive, of a tendency to partition into a more-ordered membrane environment. Therefore, it is important to establish a new paradigm for the involvement of membrane domains in TCR signalling based on direct observations of raft nanodomains in live cells (Lenne et al, 2006; Wenger et al, 2007), as well as previously published data.
By comparing these findings with the relationship observed between the palmitoylation and nanodomain partitioning of FAS (Chakrabandhu et al, 2007), we have considered that key elements involved in TCR signal initiation might also actively interact with raft nanodomains. Indeed, similarly to FAS, both CD8 and CD4 co-receptors are palmitoylated at the juxtamembrane region, which also increases their association with DRMs (He et al, 2005). It is therefore likely that these two co-receptors of the TCR partition dynamically into nanodomains. This could, in turn, promote further partitioning of the TCR–CD3 complex into nanodomains as a result of a constitutive association between TCR–CD3 and CD8 or CD4 (Doucey et al, 2003). Moreover, our recent observations on the nanodomain-targeting ability of the triple-acylation module (amino-terminus myristoylation and double palmitoylations) suggest that LCK and FYN partition into such membrane organizations (H.-T.H. & D.M., unpublished data). Results from functional studies on palmitoylation mutants support the functional importance of CD8, CD4 and LCK nanodomain associations in TCR triggering. The CD8αβ heterodimer has been shown to have an increased efficiency compared with the CD8αα homodimer in enhancing the activation of the TCR–CD3 complex. This is thought to be due to the high efficiency of CD8β palmitoylation (Arcaro et al, 2000, 2001). Similarly, mutation of the palmitoylation site compromised the ability of CD4 to enhance TCR-triggered tyrosine phosphorylation in Jurkat cells (Fragoso et al, 2003). LCK contains three acylation sites within its amino-terminal unique domain, including a myristoylation site at Gly 2, and two palmitoylation sites at Cys 3 and Cys 5. A non-acylated transmembrane LCK chimeric molecule failed to phosphorylate CD3 and to activate downstream cascades on receptor ligation in Jurkat cells (Kabouridis et al, 1997). Feeding cells with a less-hydrophobic heteroatom-substituted analogue of palmitic acid, 13-oxypalmitic acid (13-OP), inhibited the palmitoylation of LCK—but not its membrane association—as well as TCR-stimulated tyrosine phosphorylation (Hawash et al, 2002).
It has been shown that the TCR avidity for pMHC complexes is much higher in activated than non-activated CD8+ murine T lymphocytes, depending not only on the CD8 co-receptor but also on the membrane content of sphingolipids and cholesterol (Fahmy et al, 2001, 2002). Indeed, the hydrolysis of sphingomyelin in, or the depletion of cholesterol from, activated cells reduces the avidity of the TCR. Conversely, the addition of cholesterol to resting T cells enhances the avidity of the TCR. A recent study showed that cholesterol oxidation also negatively regulates the CD8 binding affinity for the MHC in primary murine T cells (Huang et al, 2007). Finally, cholesterol oxidation has also been found to inhibit TCR-induced tyrosine phosphorylation, including that of CD3 in a murine CD4+ T-cell line (Drevot et al, 2002).
Mechanisms by which lipid rafts might contribute to TCR triggering. At which step of TCR triggering could rafts have a role? In our opinion, they might be involved in TCR activation both before and on ligand engagement (Fig 2).
Raft nanodomains are presumably heterogeneous and their limited size means that only a few molecular species could simultaneously be found inside (for example, the size of the TCR–CD3 complex is 10–20 nm; Malissen, 2003; Schamel et al, 2005). The formation of molecular complexes further impinges on the composition of each nanodomain. So, there is no reason to consider that a TCR-containing or LCK-containing nanodomain should be enriched in GM1 ganglioside or lipidated protein probes (Douglass & Vale, 2005; Glebov & Nichols, 2004), even though the latter also partition into nanodomains. We propose that before TCR triggering, nanodomains promote lateral confinement and might contribute to the formation of molecular complexes based on weak protein–protein or protein–lipid interactions, which could otherwise be insufficient for such a formation in a disordered membrane environment. Conversely, the dynamic and transient nature of nanodomains would make the molecular complexes short-lived and/or only partly signalling-competent, thereby preventing effective TCR activation in the absence of ligand binding. Our proposal is consistent with the recent finding that both individual TCRs and TCR clusters of variable sizes could be detected in the plasma membrane of unstimulated T cells (Schamel et al, 2005; Varma et al, 2006), with the former showing sensitivity to the cholesterol level (Schamel et al, 2005). Furthermore, constitutive cis-association between TCR and the CD4 or CD8 co-receptors has been observed in different systems, with the involvement of non-ionic membrane interactions (Doucey et al, 2003).
The binding of the TCR to the pMHC complex, in combination with that of CD4 (or CD8) to the nonpolymorphic domain of the MHC, initiates TCR triggering. By facilitating lateral interactions between TCRs, and between the TCR and its co-receptors, rafts could contribute to lateral molecular aggregation during TCR triggering. Such a role would be crucial when the triggers consist of the concomitant binding of agonists and endogenous ligands that display extremely low affinities for the TCR (as in the pseudodimer model; Krogsgaard et al, 2005). In addition, as one of their prominent structural potentials, raft membrane domains can undergo coalescence to form larger and sometimes optically resolvable platforms and/or condensed membranes. This would occur particularly on crosslinking of the domain components. The presence of higher-ordered, condensed membranes at the site of TCR activation and in the immunological synapse has recently been reported (Gaus et al, 2005; Tavano et al, 2006). Therefore, kinetic considerations indicate that the formation of TCR microclusters is in parallel to a partial increase in order within the same membrane area. Interestingly, both events seem to be dependent on an intact actin cytoskeleton. Moreover, the early TCR microclusters (Varma et al, 2006), and presumably the ordered lipid bilayers, are excluded from CD45, thereby facilitating the proposed kinetic-segregation mechanism (Davis & van der Merwe, 2006). Interestingly, it was reported that the presence of acidic lipids could provoke a conformational change in the cytoplasmic segment of CD3ζ, which would modulate its susceptibility to phosphorylation and contribute to TCR triggering (Aivazian & Stern, 2000). Eventually, the TCR microclusters move towards the centre of the contact area, at which time they fuse to form larger clusters. By 5–15 min, these larger clusters become interconnected and immobilized at the centre of the contact area, giving rise to a cSMAC (>0.5–1.0 μm in size). The functional role of the cSMAC in TCR triggering is controversial. Some recent studies have indicated an involvement in the internalization of TCR–CD3 for recycling and/or degradation after triggering (Lee et al, 2003).
Conclusion
Lipid-dependent membrane domains are probably involved at various stages of TCR triggering. Instead of the often-evoked model of TCR ‘translocation' from non-raft to raft membrane compartments, we propose that lipid rafts contribute to TCR triggering both before and during ligand engagement. In this proposed model, rafts would work as ‘selective glue' rather than a ‘special organelle' by promoting temporary molecular confinement. Such confinement would be particularly important for efficient TCR activation, which is mediated mainly by low-energy-based specific protein–protein or protein–lipid interactions. The TCR signal initiation machine is therefore a highly dynamic membrane structure with great sensitivity and plasticity, which allows T lymphocytes to react readily and appropriately to a vast antigen repertoire.
Sidebar A | Current methods for investigating membrane dynamics.
Fluorescence-recovery after photobleaching (FRAP)
Principle and observable measures:
Level and rate of fluorescence recovery with time following the photobleaching of a limited area of observation
Translational diffusion
Mobile fraction
Comments:
Well established for FRAP in spot mode
Ensemble average method
Weakly sensitive to separate subpopulations with different diffusion characteristics
Observation area usually larger than the diffraction limit
Fluorescence-correlation spectroscopy (FCS) and image-correlation spectroscopy (ICS)
Principle and observable measures:
Fluorescence fluctuations induced by low numbers of diffusing fluorescent molecules into a limited area of observation
Molecular concentration
Translational diffusion of multiple subpopulations
Molecular clustering
Count rate per molecule
Comments:
Averaging of thousands of single-molecule diffusion events within short acquisition times
Low probe concentration (nanomolar range)
Highly sensitive to clustering (photon-counting histogram analysis)
Single and multiple colours
Standard approach weakly sensitive to slow-diffusing molecules
Observation area usually close to the diffraction limit
Single-particle tracking (SPT), single-dye tracing (SDT) and SPT photoactivated localization microscopy (PALM)
Principle and observable measures:
Scattered light from a single bead particle or fluorescence signal from a single emitter
Trajectories of individual particles or fluorescent molecules
Translational diffusion in a different cell area
Access to different mode of motion
Multiple molecular tracking
Comments:
Excellent spatial precision (dependent on the signal/noise ratio)
Temporal resolution limited by the frequency of image acquisition
Spatio-temporal identification of diffusion heterogeneities
Experimental bias favouring slow diffusing particles or molecules
Statistical quality strongly related to the length of the trajectories
Valence of the tagged molecules often weakly determined
Adapted from a table in Marguet et al, 2006.
Hai-Tao He

Didier Marguet
Acknowledgments
This work was supported by institutional grants from the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Centre National de la Recherche Scientifique (CNRS), and by specific grants from the Agence Nationale de la Recherche (ANR), the Association pour la Recherche sur le Cancer (ARC), the National Cancer Institute (INCa) and the CNRS. We thank E. Witty (AngloScribe) for initial editing of the English.
References
- Aivazian D, Stern LJ (2000) Phosphorylation of T cell receptor ζ is regulated by a lipid dependent folding transition. Nat Struct Biol 7: 1023–1026 [DOI] [PubMed] [Google Scholar]
- Arcaro A, Gregoire C, Boucheron N, Stotz S, Palmer E, Malissen B, Luescher IF (2000) Essential role of CD8 palmitoylation in CD8 coreceptor function. J Immunol 165: 2068–2076 [DOI] [PubMed] [Google Scholar]
- Arcaro A et al. (2001) CD8β endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes. J Exp Med 194: 1485–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E (1998) Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164: 103–114 [DOI] [PubMed] [Google Scholar]
- Campi G, Varma R, Dustin ML (2005) Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med 202: 1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabandhu K, Herincs Z, Huault S, Dost B, Peng L, Conchonaud F, Marguet D, He HT, Hueber AO (2007) Palmitoylation is required for efficient Fas cell death signaling. EMBO J 26: 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y (1998) Ligand recognition by αβ T cell receptors. Annu Rev Immunol 16: 523–544 [DOI] [PubMed] [Google Scholar]
- Davis SJ, van der Merwe PA (2006) The kinetic-segregation model: TCR triggering and beyond. Nat Immunol 7: 803–809 [DOI] [PubMed] [Google Scholar]
- Destainville N, Salome L (2006) Quantification and correction of systematic errors due to detector time-averaging in single-molecule tracking experiments. Biophys J 90: L17–L19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnert G, Keller J, Medda R, Andrei MA, Rizzoli SO, Luhrmann R, Jahn R, Eggeling C, Hell SW (2006) Macromolecular-scale resolution in biological fluorescence microscopy. Proc Natl Acad Sci USA 103: 11440–11445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucey MA, Goffin L, Naeher D, Michielin O, Baumgartner P, Guillaume P, Palmer E, Luescher IF (2003) CD3δ establishes a functional link between the T cell receptor and CD8. J Biol Chem 278: 3257–3264 [DOI] [PubMed] [Google Scholar]
- Douglass AD, Vale RD (2005) Single-molecule microscopy reveals plasma membrane microdomains created by protein–protein networks that exclude or trap signaling molecules in T cells. Cell 121: 937–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevot P, Langlet C, Guo XJ, Bernard AM, Colard O, Chauvin JP, Lasserre R, He HT (2002) TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J 21: 1899–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M (2003) The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct 32: 257–283 [DOI] [PubMed] [Google Scholar]
- Fahmy TM, Bieler JG, Edidin M, Schneck JP (2001) Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity 14: 135–143 [PubMed] [Google Scholar]
- Fahmy TM, Bieler JG, Schneck JP (2002) Probing T cell membrane organization using dimeric MHC–Ig complexes. J Immunol Methods 268: 93–106 [DOI] [PubMed] [Google Scholar]
- Fragoso R, Ren D, Zhang X, Su MW, Burakoff SJ, Jin YJ (2003) Lipid raft distribution of CD4 depends on its palmitoylation and association with Lck, and evidence for CD4-induced lipid raft aggregation as an additional mechanism to enhance CD3 signaling. J Immunol 170: 913–921 [DOI] [PubMed] [Google Scholar]
- Fujita A, Cheng J, Hirakawa M, Furukawa K, Kusunoki S, Fujimoto T (2007) Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol Biol Cell 18: 2112–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A (2002) Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol 157: 1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaus K, Gratton E, Kable EP, Jones AS, Gelissen I, Kritharides L, Jessup W (2003) Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc Natl Acad Sci USA 100: 15554–15559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaus K, Chklovskaia E, Fazekas de St Groth B, Jessup W, Harder T (2005) Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol 171: 121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN, Stefanova I (1999) The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol 17: 467–522 [DOI] [PubMed] [Google Scholar]
- Glebov OO, Nichols BJ (2004) Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol 6: 238–243 [DOI] [PubMed] [Google Scholar]
- Hawash IY, Hu XE, Adal A, Cassady JM, Geahlen RL, Harrison ML (2002) The oxygen-substituted palmitic acid analogue, 13-oxypalmitic acid, inhibits Lck localization to lipid rafts and T cell signaling. Biochim Biophys Acta 1589: 140–150 [DOI] [PubMed] [Google Scholar]
- He HT, Lellouch A, Marguet D (2005) Lipid rafts and the initiation of T cell receptor signaling. Semin Immunol 17: 23–33 [DOI] [PubMed] [Google Scholar]
- Huang J, Edwards LJ, Evavold BD, Zhu C (2007) Kinetics of MHC–CD8 interaction at the T cell membrane. J Immunol 179: 7653–7662 [DOI] [PubMed] [Google Scholar]
- Huse M, Klein LO, Girvin AT, Faraj JM, Li QJ, Kuhns MS, Davis MM (2007) Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity 27: 76–88 [DOI] [PubMed] [Google Scholar]
- Kabouridis PS, Magee AI, Ley SC (1997) S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J 16: 4983–4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy AK, Edidin M (1998) Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 Å using imaging fluorescence resonance energy transfer. J Cell Biol 142: 69–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM (2005) Agonist/endogenous peptide–MHC heterodimers drive T cell activation and sensitivity. Nature 434: 238–243 [DOI] [PubMed] [Google Scholar]
- Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T (2005) Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct 34: 351–378 [DOI] [PubMed] [Google Scholar]
- Lee KH et al. (2003) The immunological synapse balances T cell receptor signaling and degradation. Science 302: 1218–1222 [DOI] [PubMed] [Google Scholar]
- Lenne PF, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo XJ, Rigneault H, He HT, Marguet D (2006) Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J 25: 3245–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen B (2003) An evolutionary and structural perspective on T cell antigen receptor function. Immunol Rev 191: 7–27 [DOI] [PubMed] [Google Scholar]
- Marguet D, Lenne PF, Rigneault H, He HT (2006) Dynamics in the plasma membrane: how to combine fluidity and order. EMBO J 25: 3446–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BH, Segura JM, Martinez KL, Hovius R, George N, Johnsson K, Vogel H (2006) FRET imaging reveals that functional neurokinin-1 receptors are monomeric and reside in membrane microdomains of live cells. Proc Natl Acad Sci USA 103: 2138–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerner WE (2006) Single-molecule mountains yield nanoscale cell images. Nat Methods 3: 781–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman KD, Campi G, Groves JT, Dustin ML (2005) Altered TCR signaling from geometrically repatterned immunological synapses. Science 310: 1191–1193 [DOI] [PubMed] [Google Scholar]
- Niv H, Gutman O, Kloog Y, Henis YI (2002) Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J Cell Biol 157: 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior IA, Muncke C, Parton RG, Hancock JF (2003) Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol 160: 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome L, Cazeils JL, Lopez A, Tocanne JF (1998) Characterization of membrane domains by FRAP experiments at variable observation areas. Eur Biophys J 27: 391–402 [DOI] [PubMed] [Google Scholar]
- Schamel WW, Arechaga I, Risueno RM, van Santen HM, Cabezas P, Risco C, Valpuesta JM, Alarcon B (2005) Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med 202: 493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Varma R, Sarasij RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S (2004) Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 116: 577–589 [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387: 569–572 [DOI] [PubMed] [Google Scholar]
- Tavano R, Contento RL, Baranda SJ, Soligo M, Tuosto L, Manes S, Viola A (2006) CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunological synapse. Nat Cell Biol 8: 1270–1276 [DOI] [PubMed] [Google Scholar]
- Tian T, Harding A, Inder K, Plowman S, Parton RG, Hancock JF (2007) Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat Cell Biol 9: 905–914 [DOI] [PubMed] [Google Scholar]
- Varma R, Campi G, Yokosuka T, Saito T, Dustin ML (2006) T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity 25: 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SS, Thaler C, Koushik SV (2006) Fanciful FRET. Sci STKE 2006(331): re2. [DOI] [PubMed] [Google Scholar]
- Wawrezinieck L, Rigneault H, Marguet D, Lenne PF (2005) Fluorescence correlation spectroscopy diffusion laws to probe the submicron cell membrane organization. Biophys J 89: 4029–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Littman DR (1994) Signal transduction by lymphocyte antigen receptors. Cell 76: 263–274 [DOI] [PubMed] [Google Scholar]
- Wenger J, Conchonaud F, Dintinger J, Wawrezinieck L, Ebbesen TW, Rigneault H, Marguet D, Lenne PF (2007) Diffusion analysis within single nanometric apertures reveals the ultrafine cell membrane organization. Biophys J 92: 913–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser S, Moertelmaier M, Fuertbauer E, Stockinger H, Schutz GJ (2007) (Un)confined diffusion of CD59 in the plasma membrane determined by high-resolution single molecule microscopy. Biophys J 92: 3719–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiel E, Edidin M (1987) Micrometer-scale domains in fibroblast plasma membranes. J Cell Biol 105: 755–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T (2005) Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol 6: 1253–1262 [DOI] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY (2002) Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296: 913–916 [DOI] [PubMed] [Google Scholar]