Abstract
The National Institute for Medical Research, London, England, was requested by the WHO Expert Committee on Biological Standardization to arrange a collaborative study of the serum pool they had obtained, to determine its suitability to serve as an international reference preparation of rheumatoid arthritis serum. A batch of this serum was assayed by 11 laboratories in 7 countries against 30 test preparations. On the basis of the results obtained, the serum has been established as the International Reference Preparation of Rheumatoid Arthritis Serum and the International Unit of Rheumatoid Arthritis Serum has been defined as the activity contained in 0.171 mg of the international reference preparation.
A description is also given of the British reference preparation of rabbit antibody to sheep red blood cells (amboceptor) and this material was also tested in the collaborative study.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
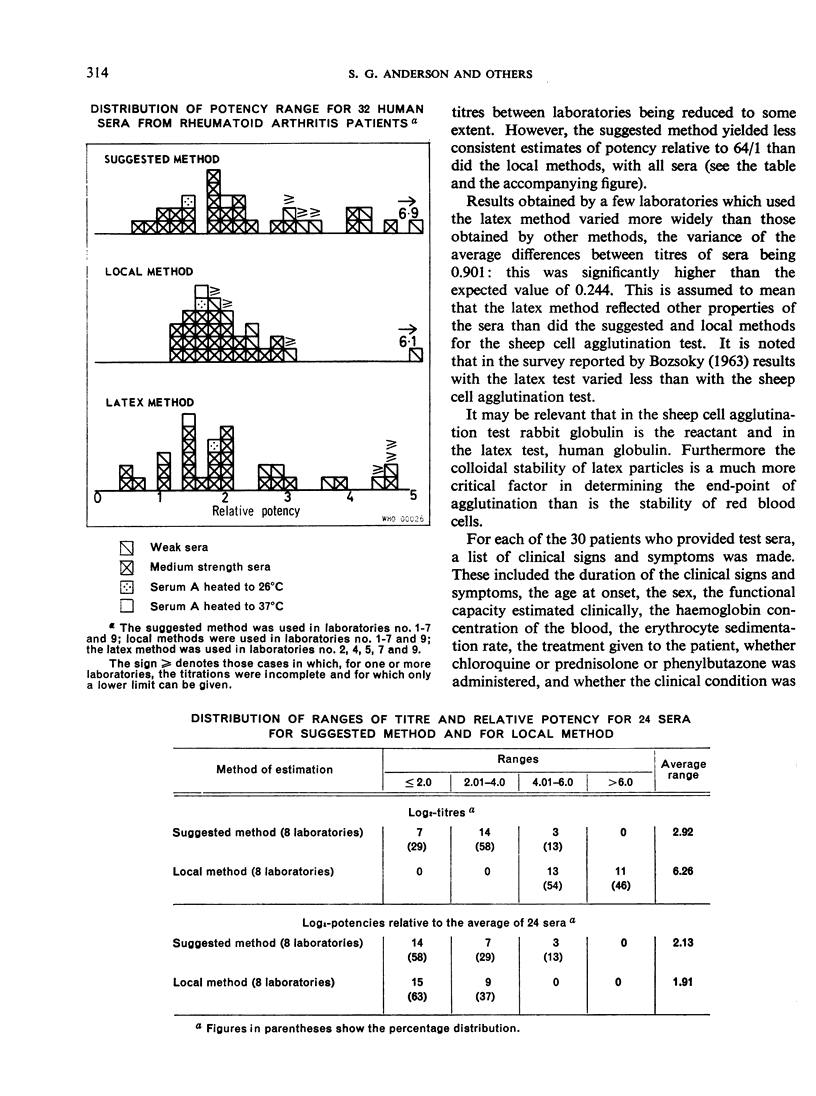
- BOZSOKY S. THE PROBLEM OF STANDARDIZATION IN RHEUMATOID ARTHRITIS SEROLOGY. Arthritis Rheum. 1963 Oct;6:641–649. doi: 10.1002/art.1780060508. [DOI] [PubMed] [Google Scholar]
- JERNE N. K., PERRY W. L. The stability of biological standards. Bull World Health Organ. 1956;14(1):167–182. [PMC free article] [PubMed] [Google Scholar]
- NASOU J. P., KAYHOE D. E., BOZICEVICH J. The F-II-bentonite flocculation test (F-II-BFT) for rheumatoid arthritis. A comparison of results from six laboratories. Tech Bull Regist Med Technol. 1963 Jun;33:97–100. [PubMed] [Google Scholar]


