Abstract
Until about 10 years ago heparin was prepared from ox lungs and was regarded as a homogeneous polymer (of a sulfated mucopolysaccharide) with a relatively constant biological activity of about 100 IU/mg. The mucus and mucosa of the intestine of hog, sheep and ox have now become the main industrial source of bulk starting material. Preparations from this new source have much higher specific biological activity and have been shown to consist of mixtures of components with a number of different chemical and physical characteristics. It had been variously reported that preparations of heparins from the 2 sources gave significantly different estimates when bioassayed by the 2 different pharmacopoeial assay methods which are in wide use. If this is in fact the case these discrepancies could cause trouble in commerce, national control and clinical usage.
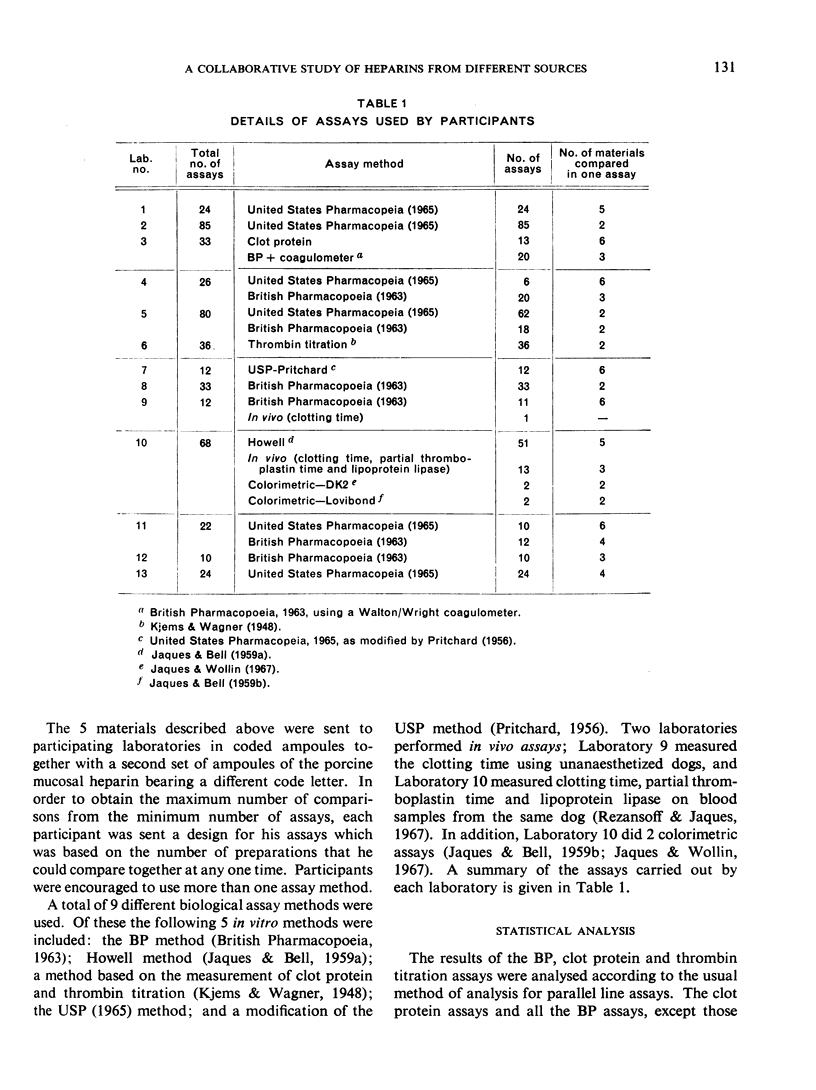
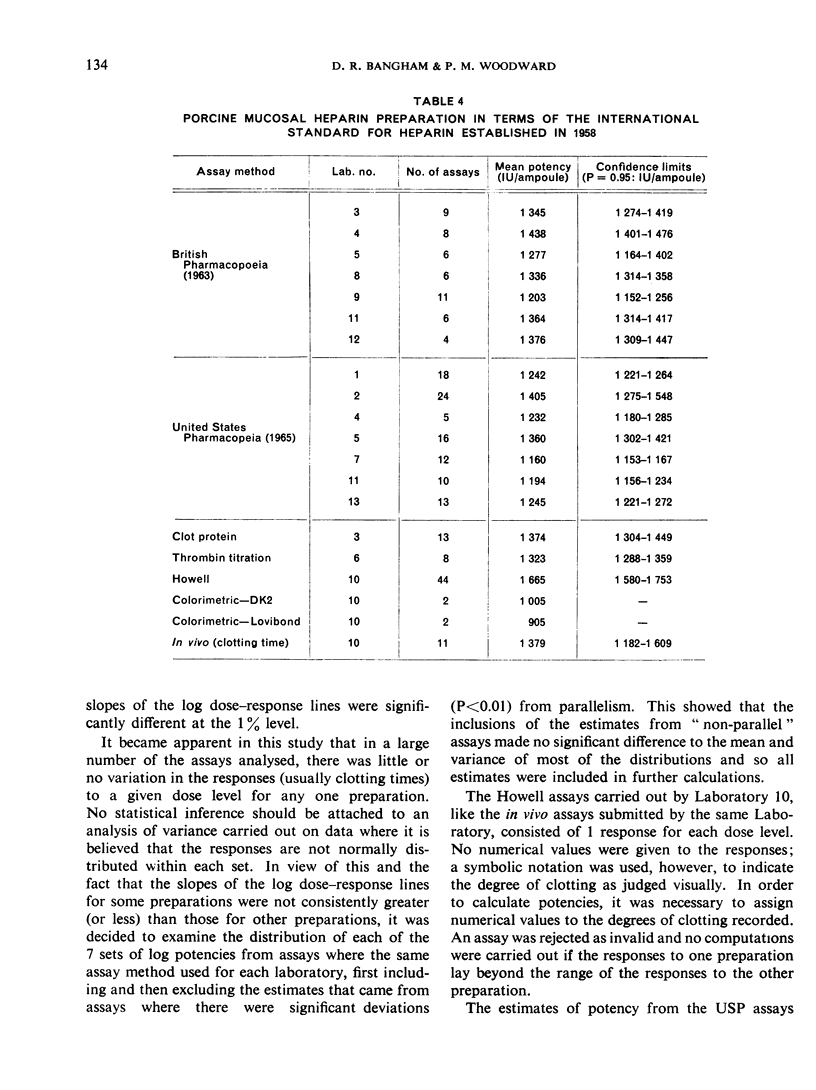
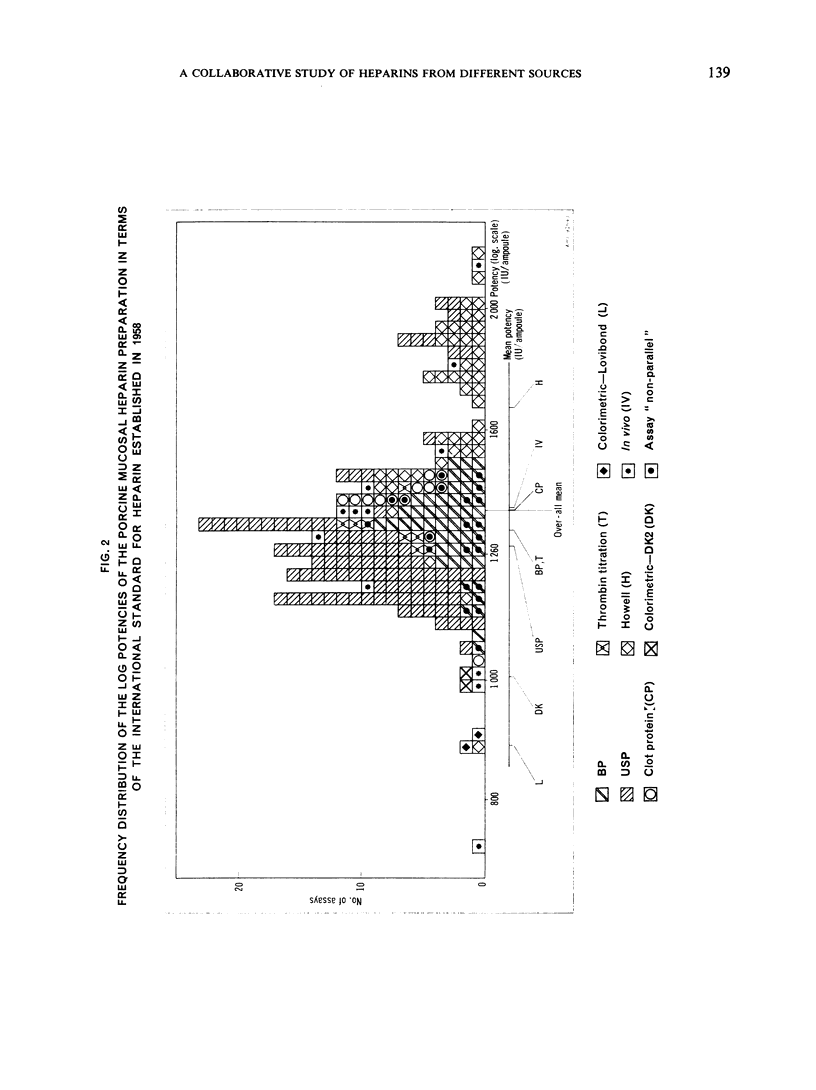
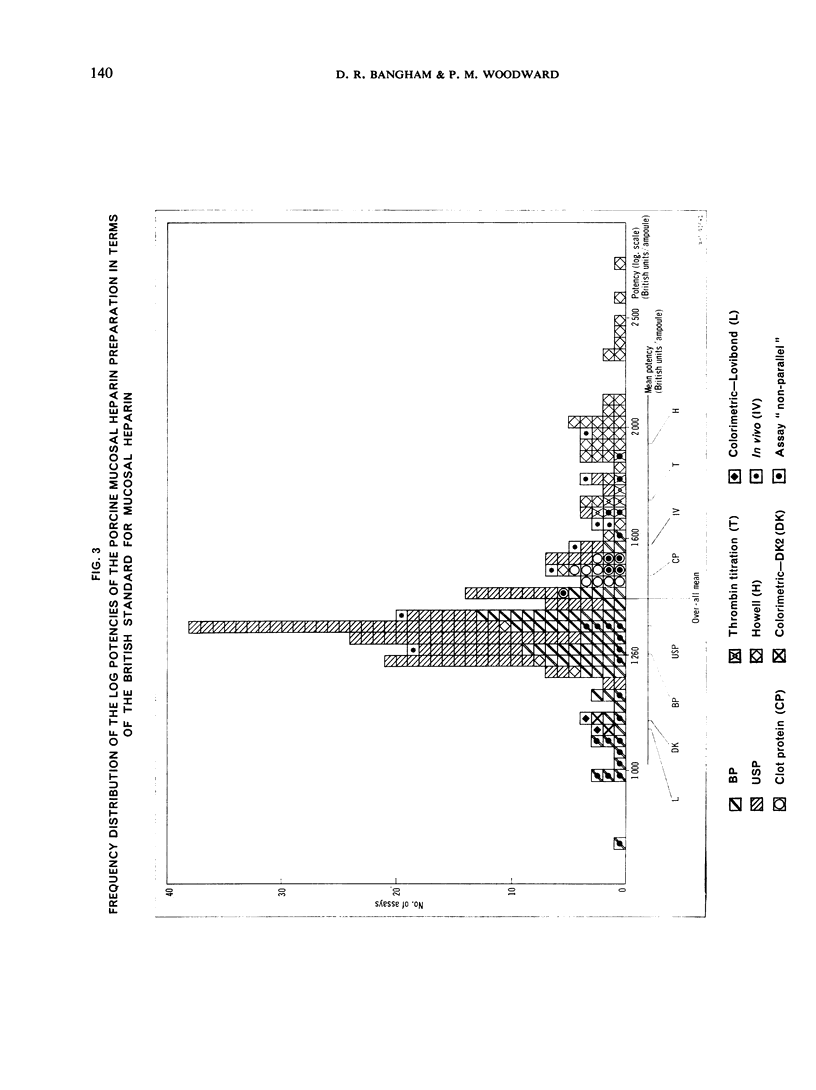
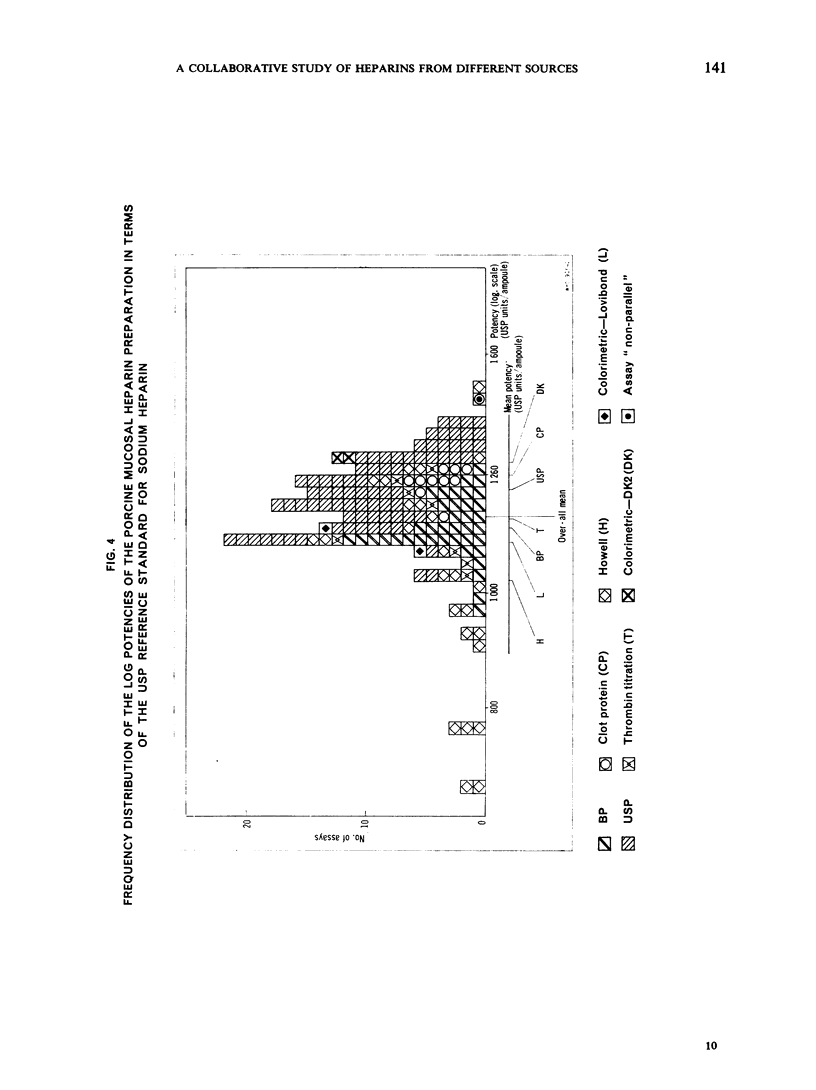
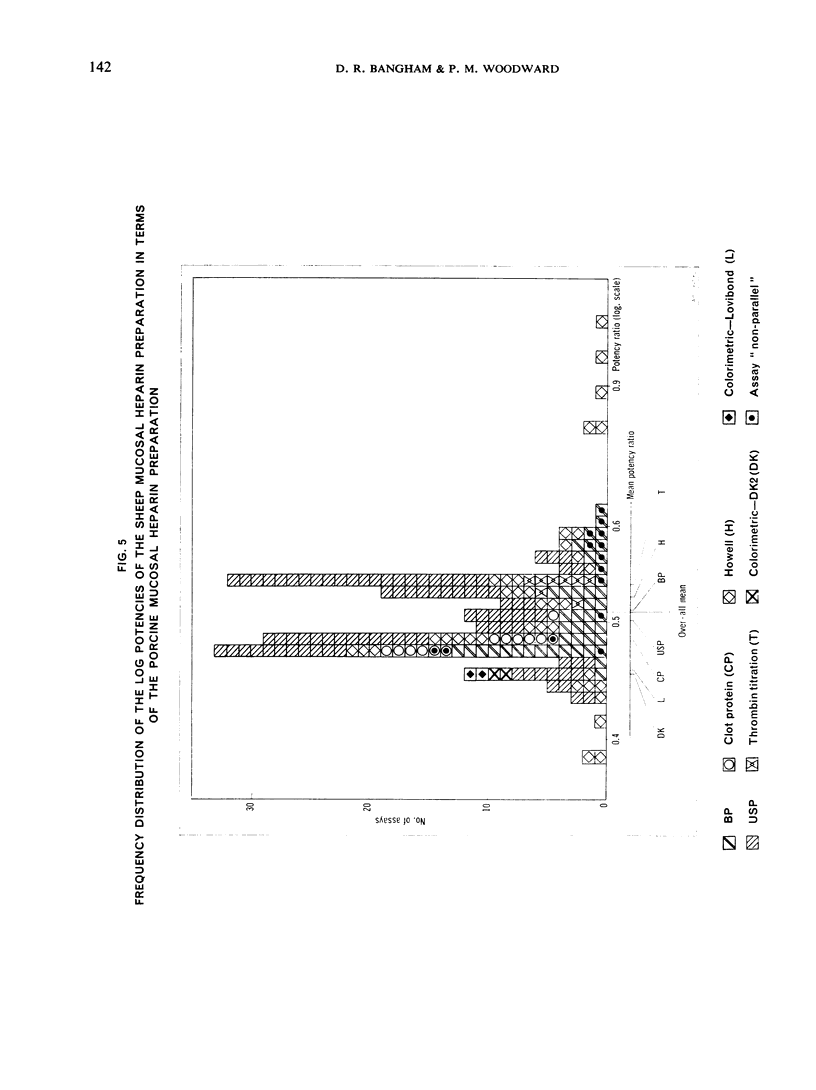
This paper describes an international collaborative study to investigate this and other problems in the assay of heparin. In this study preparations of heparin made from the intestinal mucosa of each of 3 species (hog, sheep and ox) were biossayed with the International Standard for Heparin (from ox lung) established in 1958. Two of these preparations were national standards.
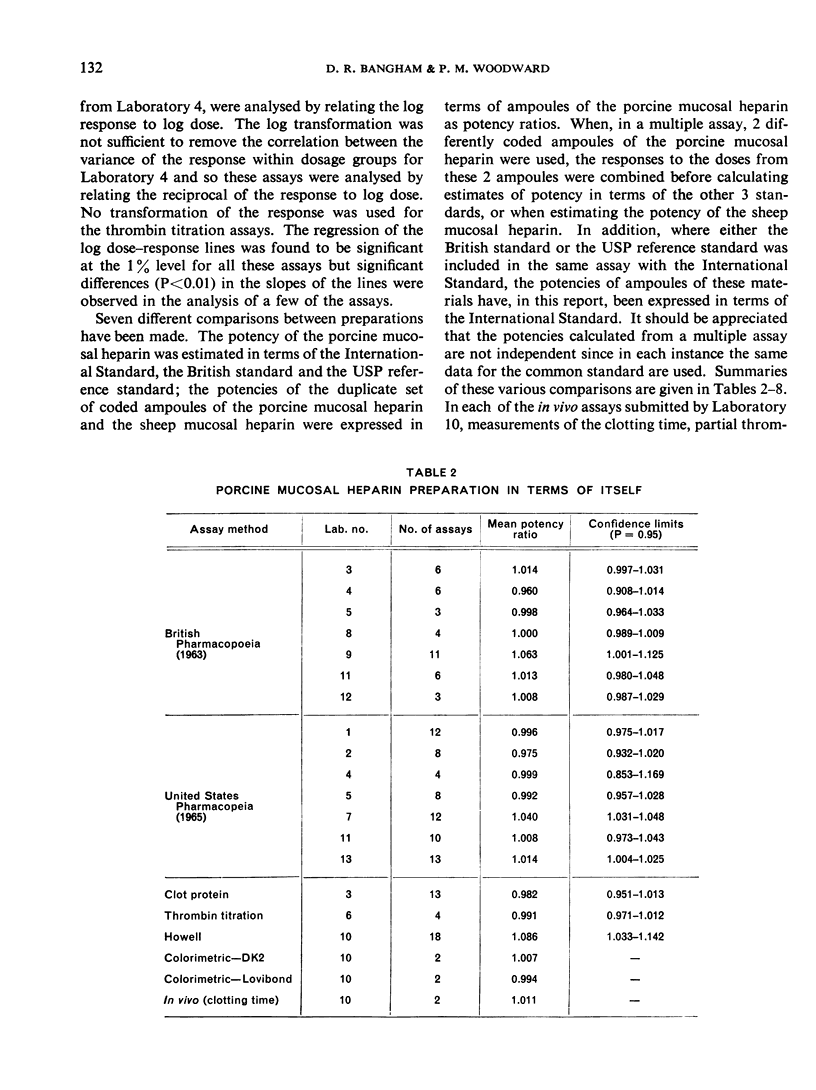
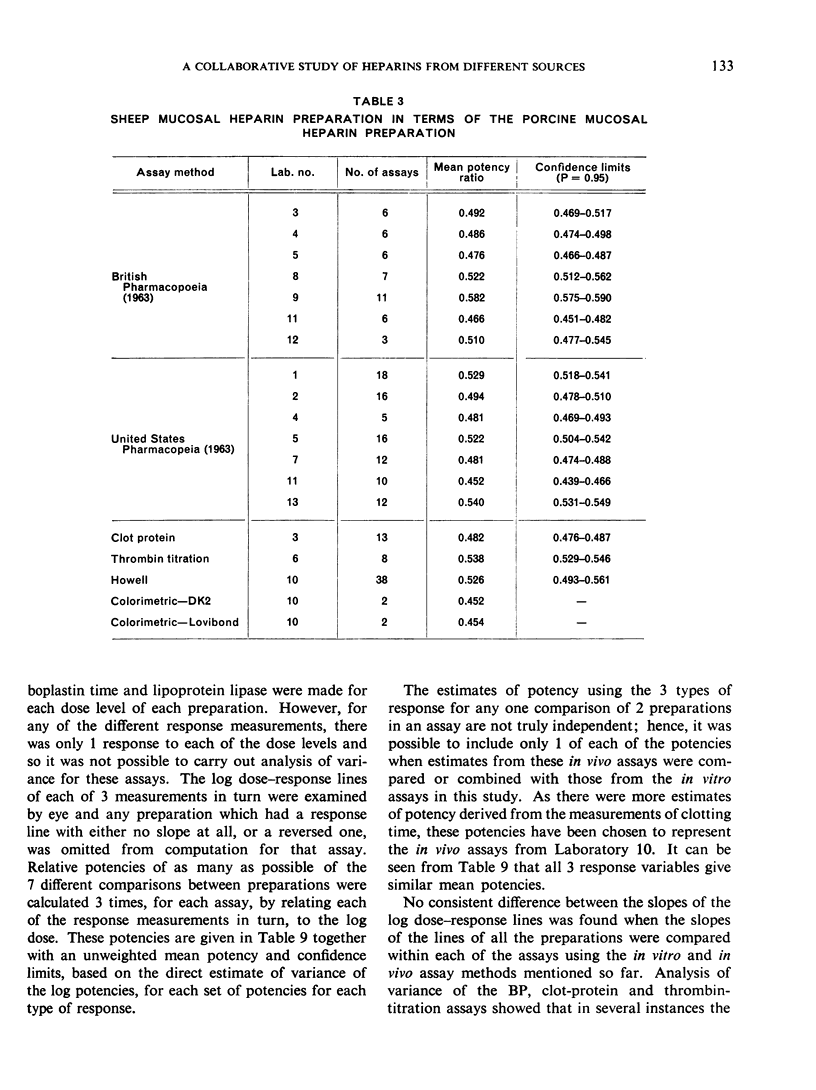
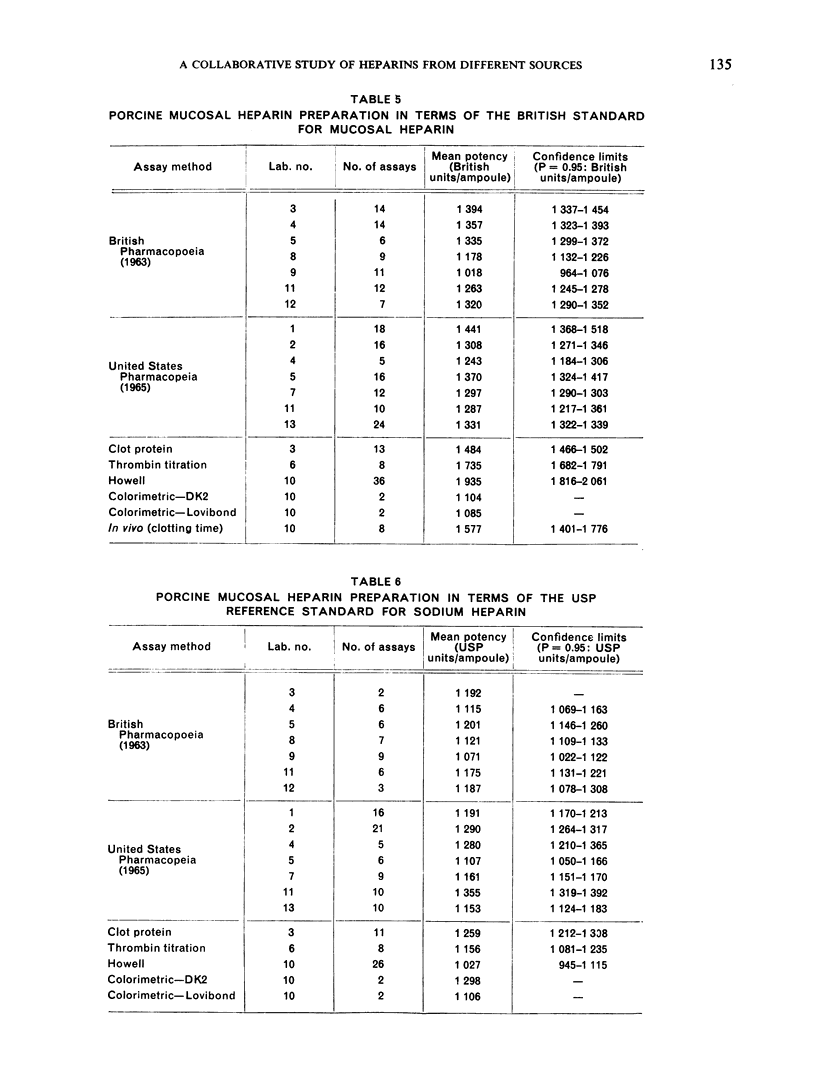
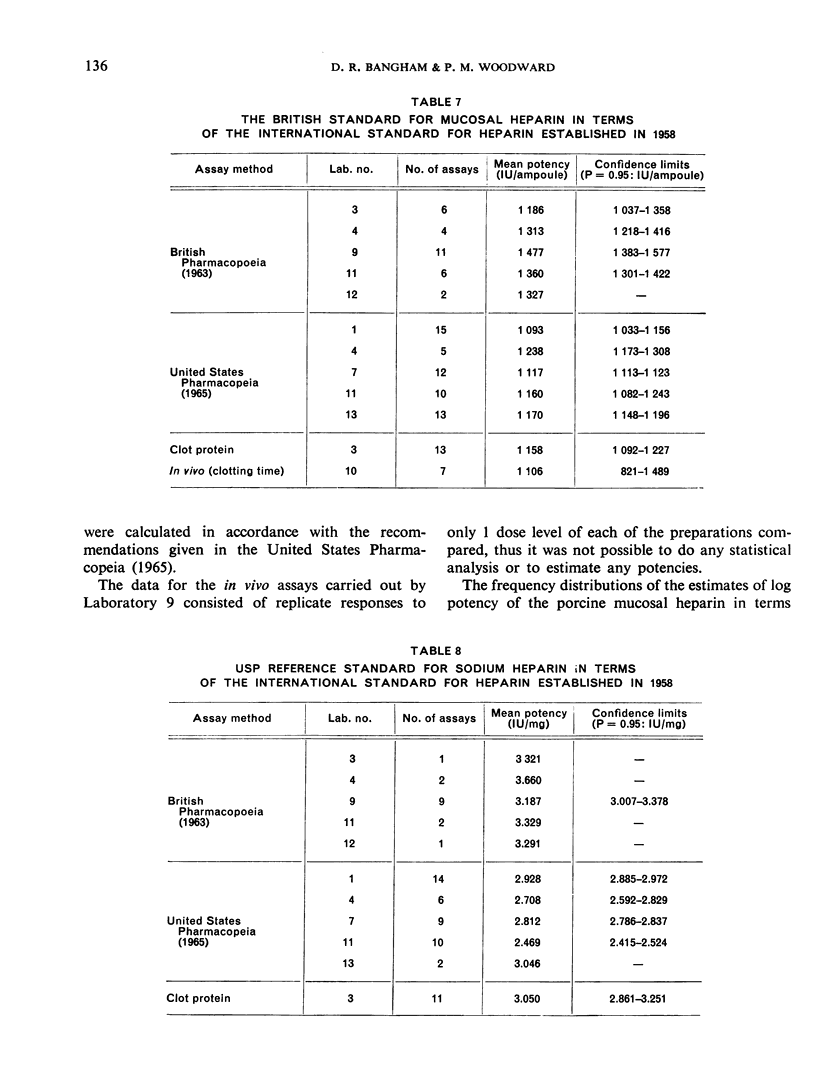
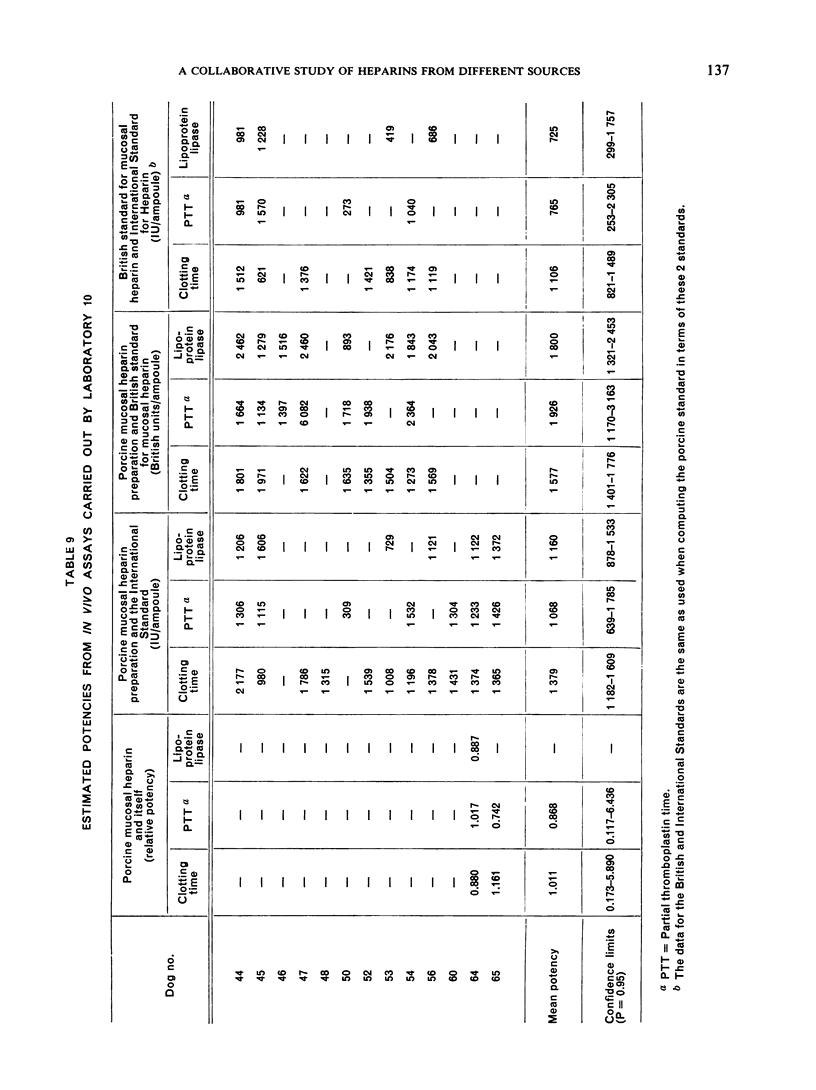
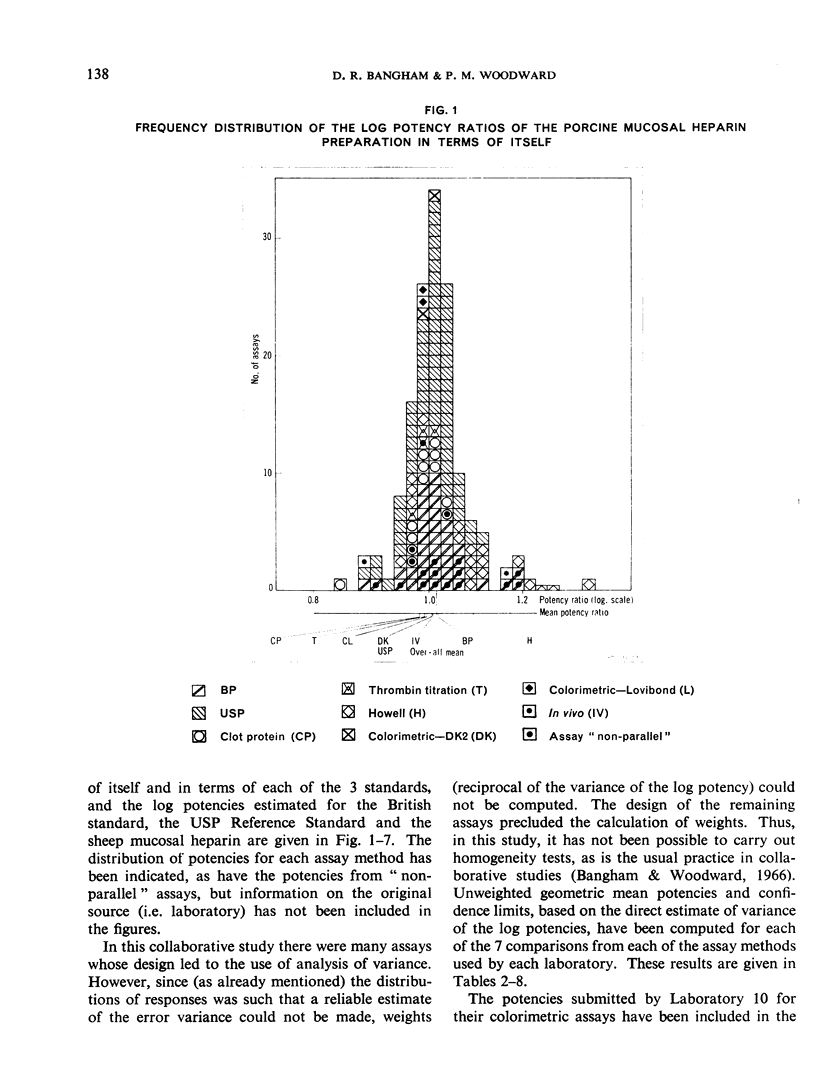
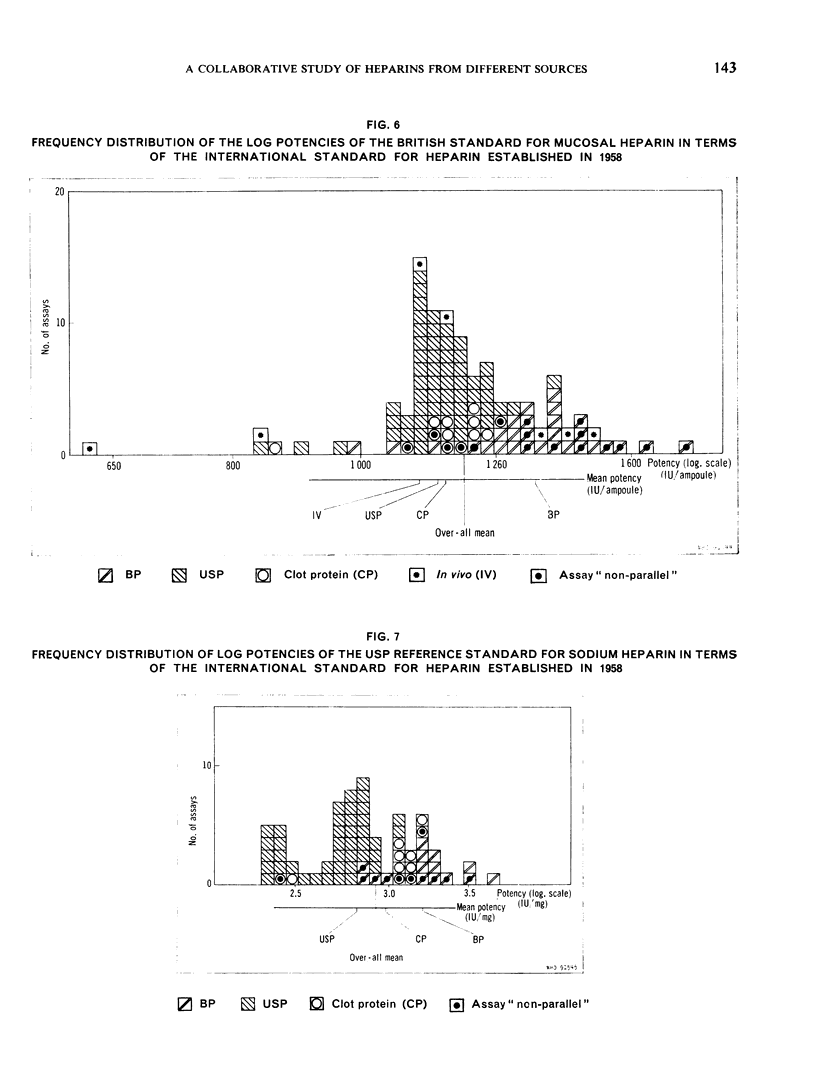
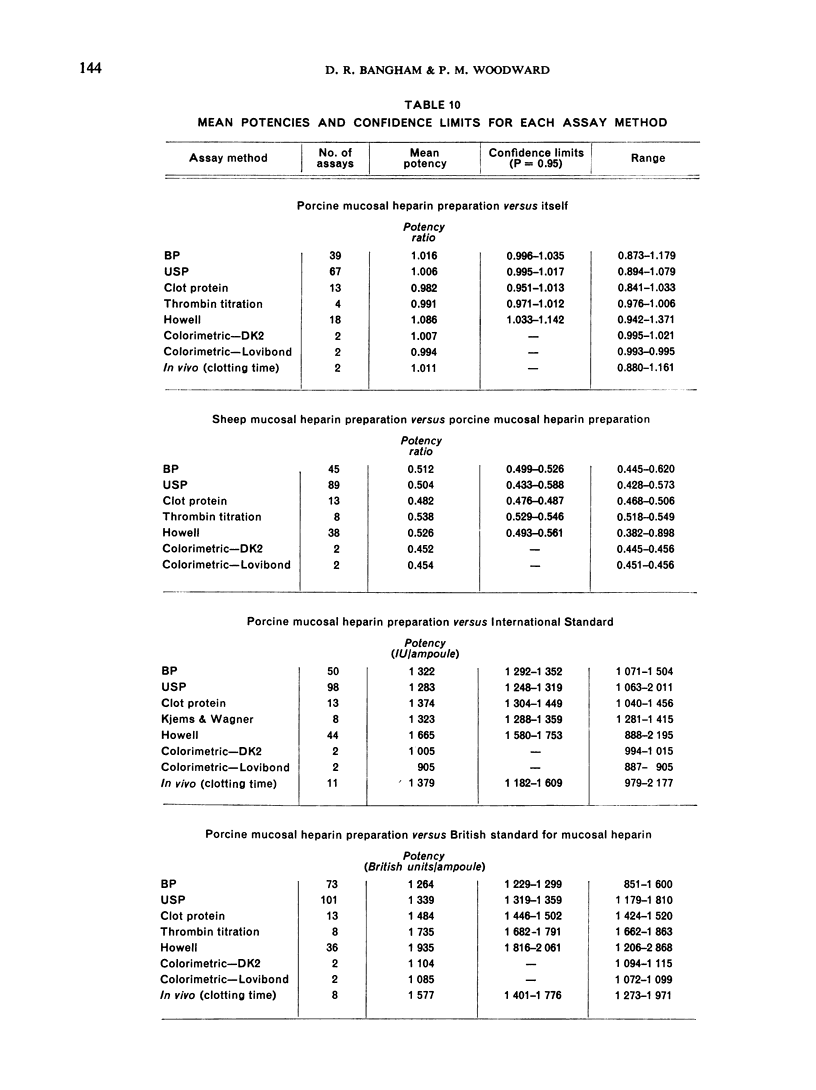
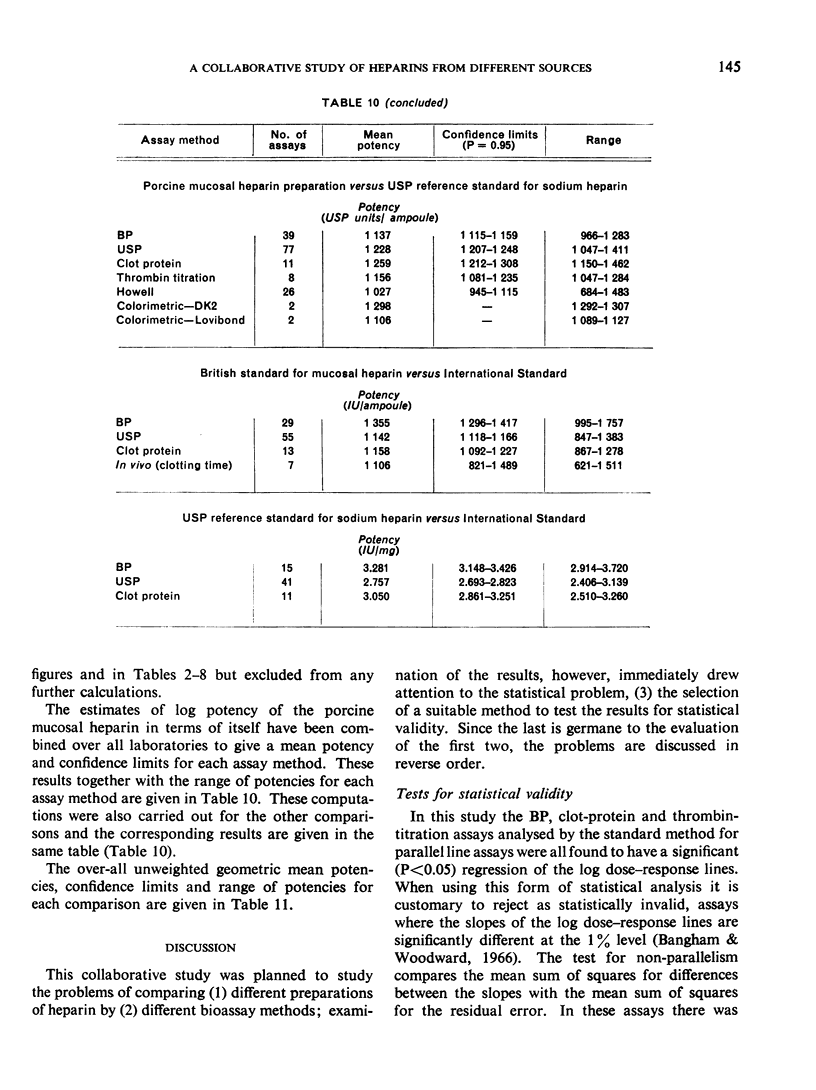
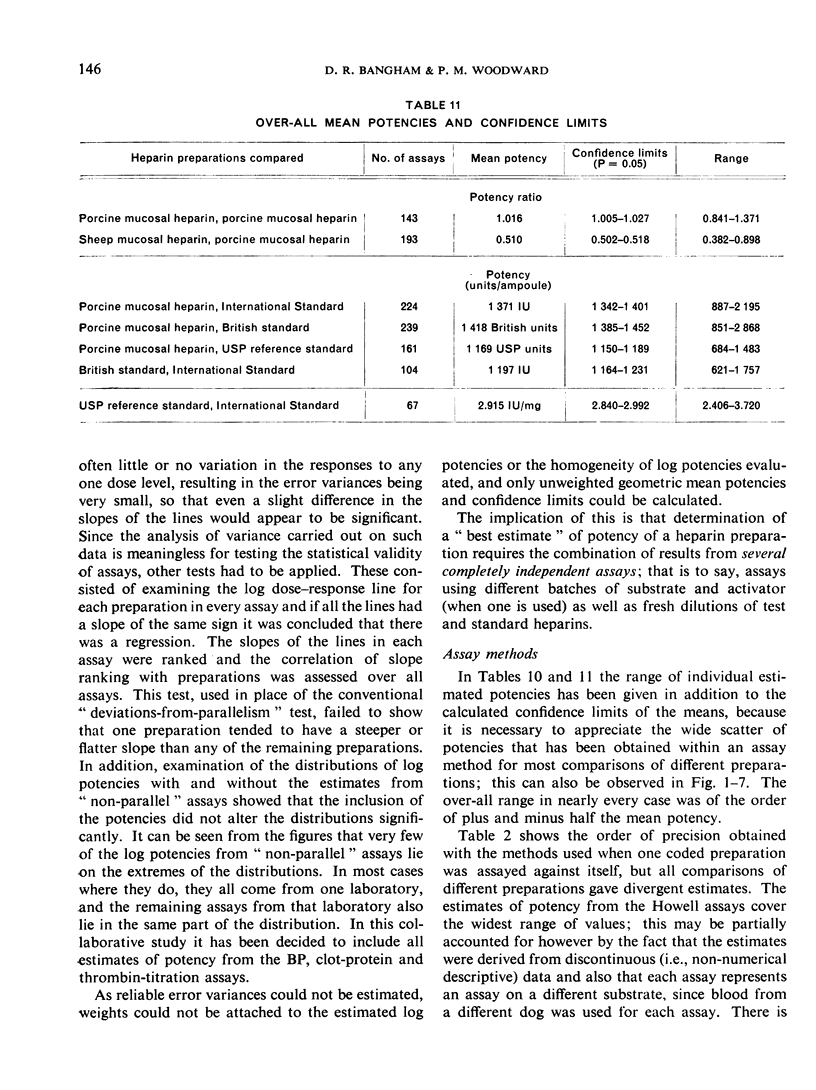
The results showed differences between the estimated relative potencies of heparins of lung and mucosal origin, but they were not so consistent as to necessitate a separate international standard for each, although the imprecision of the assays may have concealed significant heterogeneity arising from real differences between the preparations. A further problem brought to light in this study was that, with the 2 pharmacopoeial assay methods in wide use, simple replicate assays using the same substrate gave estimates so precise that an analysis of variance on them was meaningless, whereas estimates between laboratories or with different substrate batches could vary by as much as 40%-50%.
It is suggested that estimates of the biological activity of heparin preparations should be based on several independent assays using different batches of substrate.
The biological assay methods currently used were devised some 15-20 years ago and, in the light of modern knowledge of mechanisms of blood clotting, are regarded as unsatisfactory in a number of respects. It is hoped that research into the mechanism of action of heparin will lead to improved methods of assay.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM D. R., MUSSETT M. V. The Second International Standard for heparin. Bull World Health Organ. 1959;20:1201–1208. [PMC free article] [PubMed] [Google Scholar]
- Bangham D. R., Woodward P. M. The second international standard for serum gonadotrophin. Bull World Health Organ. 1966;35(5):761–773. [PMC free article] [PubMed] [Google Scholar]
- Jaques L. B., Wollin A. A modified method for the colorimetric determination of heparin. Can J Physiol Pharmacol. 1967 Sep;45(5):787–794. doi: 10.1139/y67-093. [DOI] [PubMed] [Google Scholar]
- PRITCHARD J. The assay of heparin. J Pharm Pharmacol. 1956 Jul;8(7):523–529. doi: 10.1111/j.2042-7158.1956.tb12185.x. [DOI] [PubMed] [Google Scholar]
- Rezansoff W. E., Jaques L. B. An investigation of the assay of heparin based on its activity in vivo. Thromb Diath Haemorrh. 1967 Aug 15;18(1-2):150–160. [PubMed] [Google Scholar]
- Walton P. L., Ricketts C. R., Bangham D. R. Heterogeneity of heparin. Br J Haematol. 1966 May;12(3):310–325. doi: 10.1111/j.1365-2141.1966.tb05637.x. [DOI] [PubMed] [Google Scholar]


