Abstract
Tests to determine the reactogenicity and immunogenicity of a live influenza vaccine when administered by mouth showed that the new method caused no reactions in adults and children and was adequately immunogenic, the results being in no way inferior to those obtained by intranasal administration. These results provide a basis for the wider use of this simpler and more convenient method of administration.
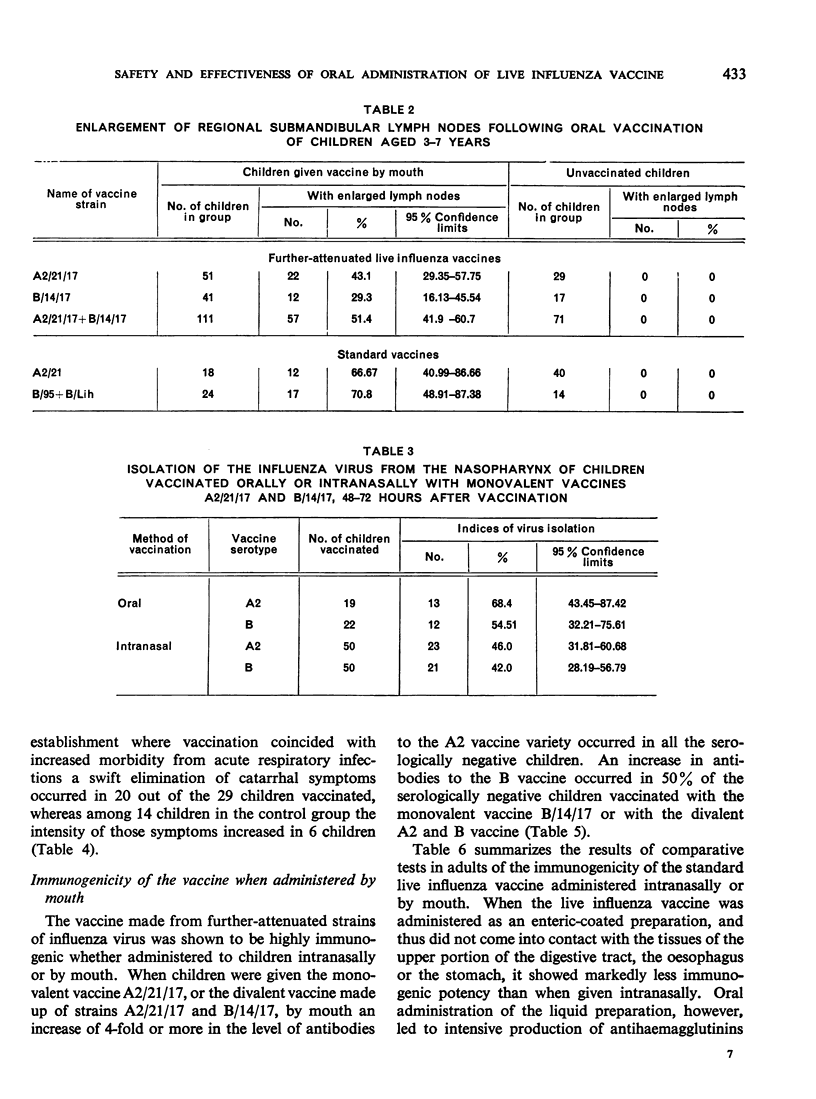
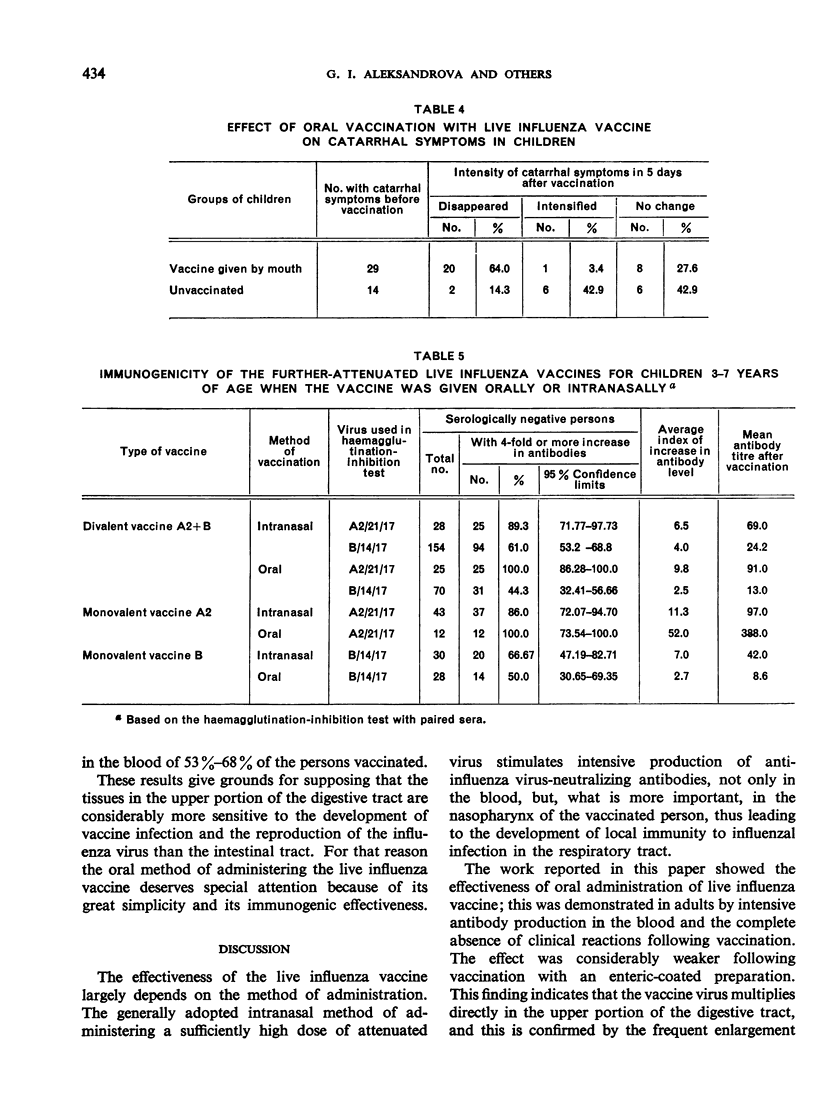
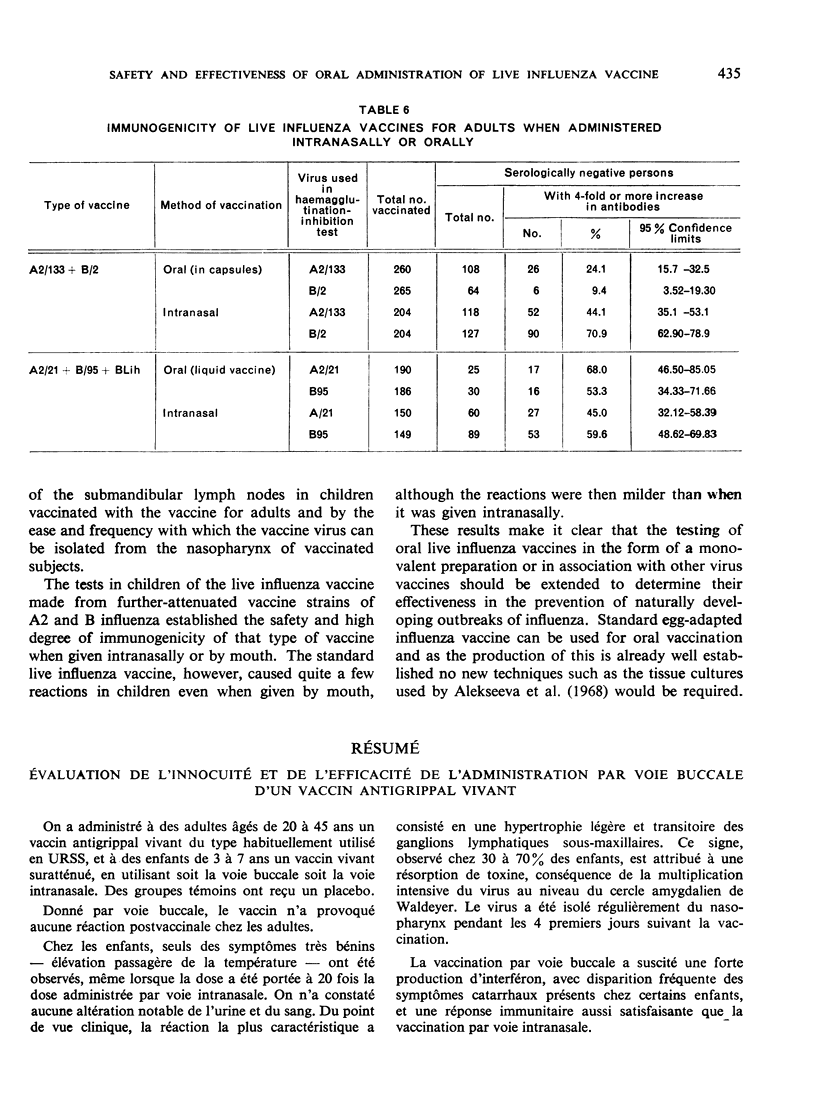
Oral administration of the live influenza vaccine was followed by reproduction of the vaccine virus. The rapid elimination of catarrhal symptoms existing before vaccination indicates intensive production of interferon in the nasopharynx of the vaccinated persons.
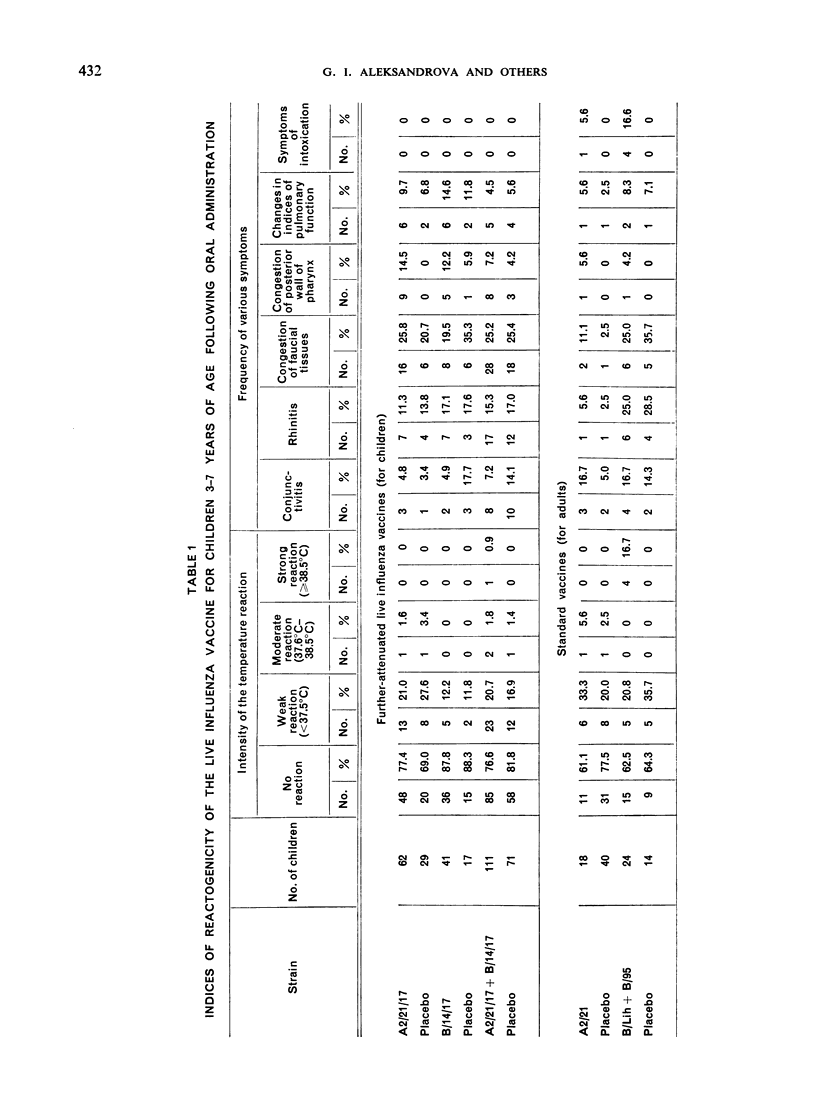
The standard influenza vaccine for adults induced strong reactions in children, even when the less dangerous oral route of vaccination was used. For oral vaccination of children it is thus essential to use the variant of the live influenza vaccine made from further-attenuated, cold-adapted vaccine strains of influenza virus. This vaccine is quite safe and effective whether administered intranasally or by mouth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chanock R. M., Ludwig W., Heubner R. J., Cate T. R., Chu L. W. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue cultures. I. Safety and lack of oncogenicity and tests for potency in volunteers. JAMA. 1966 Feb 7;195(6):445–452. [PubMed] [Google Scholar]


