Abstract
Emulsified inactivated influenza vaccines have been in use for some 18 years and the goal of enhanced serological response lasting 2 years or more has been attained. The safety of the method in relation to immediate pyrogenic reactions has been demonstrated and no carcinogenic effects are known to have occurred in man. However, the problem of delayed local reactions after the injection of mineral-oil vaccines has not been solved. British experience of adverse reactions to commercial adjuvant influenza vaccine is quoted.
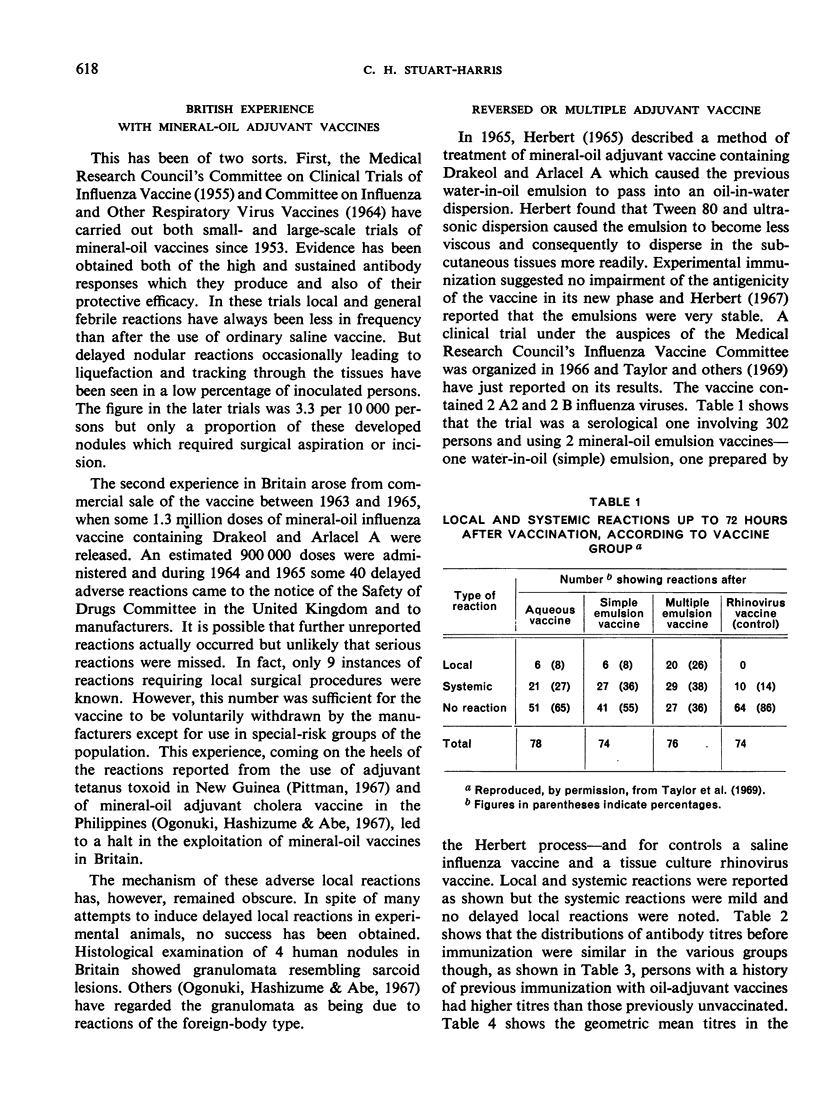
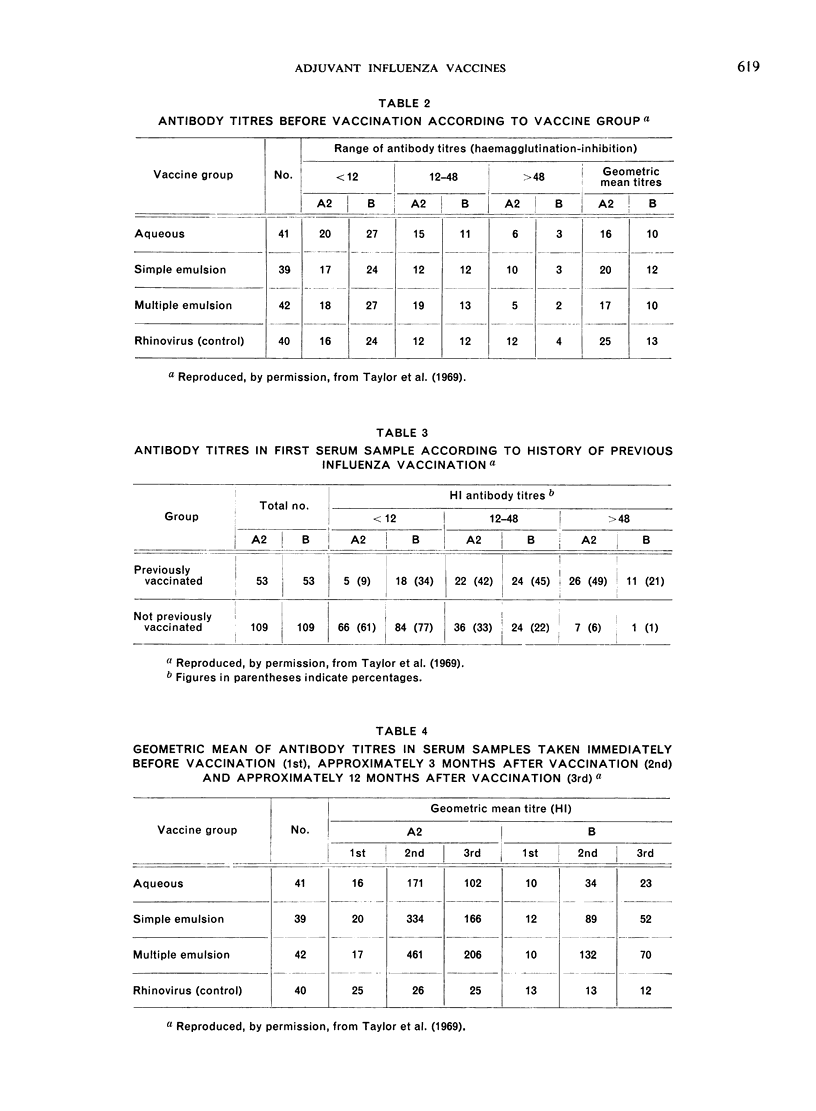
New methods for obtaining adjuvant action without the risk of local abscess formation are needed both for inactivated whole virus and for split haemagglutinin vaccines. Reversal of water-in-mineral-oil emulsion to oil-in-water emulsion reduces viscosity and permits diffusion of the depot injection. A trial in Britain has shown equally good adjuvant properties of the reversed emulsion incorporating influenza virus vaccine so far as serological response is concerned, although it has not yet been conducted on a scale that would allow of adequate evaluation of the likelihood of delayed local reactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEEBE G. W., SIMON A. H., VIVONA S. FOLLOW-UP STUDY ON ARMY PERSONNEL WHO RECEIVED ADJUVANT INFLUENZA VIRUS VACCINE 1951-1953. Am J Med Sci. 1964 Apr;247:385–406. doi: 10.1097/00000441-196404000-00001. [DOI] [PubMed] [Google Scholar]
- Davenport F. M., Hennessy A. V., Askin F. B. Lack of adjuvant effect of A1PO4 on purified influenza virus hemagglutinins in man. J Immunol. 1968 May;100(5):1139–1140. [PubMed] [Google Scholar]
- Davenport F. M. Seventeen years' experience with mineral oil adjuvant influenza virus vaccines. Ann Allergy. 1968 Jun;26(6):288–292. [PubMed] [Google Scholar]
- Forsyth J. R., Wilson T. E. Aqueous and oil-adjuvant influenza vaccines and isoimmunization to group A substance. J Hyg (Lond) 1968 Mar;66(1):1–6. doi: 10.1017/s0022172400040882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert W. J. Multiple emulsions. A new form of mineral-oil antigen adjuvant. Lancet. 1965 Oct 16;2(7416):771–771. doi: 10.1016/s0140-6736(65)90816-0. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN R., MANTEL N., HUMPHREY W., Jr Ascites production in 17 mouse strains. Proc Soc Exp Biol Med. 1961 May;107:163–165. doi: 10.3181/00379727-107-26566. [DOI] [PubMed] [Google Scholar]
- Medical Research Council THE PRICE AND UNIT VALUE OF INSULIN. Br Med J. 1923 Dec 22;2(3286):1232–1232. [PMC free article] [PubMed] [Google Scholar]
- POTTER M., BOYCE C. R. Induction of plasma-cell neoplasms in strain BALB/c mice with mineral oil and mineral oil adjuvants. Nature. 1962 Mar 17;193:1086–1087. doi: 10.1038/1931086a0. [DOI] [PubMed] [Google Scholar]
- Rask-Nielsen R., Ebbesen P. Reticular neoplasms induced in DBA-2 and CBA mice by intraperitoneal injections of mineral oil. J Natl Cancer Inst. 1965 Jul;35(1):83–94. doi: 10.1093/jnci/35.1.83. [DOI] [PubMed] [Google Scholar]
- SPRINGER G. F., SCHUSTER R. STIMULATION OF ISOHEMOLYSINS AND ISOHEMAGGLUTININS BY INFLUENZA VIRUS PREPARATIONS. Vox Sang. 1964 Sep-Oct;9:589–598. doi: 10.1111/j.1423-0410.1964.tb03329.x. [DOI] [PubMed] [Google Scholar]
- SPRINGER G. F., TRITEL H. Blood group A active substances in embryonated chicken eggs and their relation to egggrown virus. Science. 1962 Nov 9;138(3541):687–688. doi: 10.1126/science.138.3541.687. [DOI] [PubMed] [Google Scholar]
- Taylor P. J., Miller C. L., Pollock T. M., Perkins F. T., Westwood M. A. Antibody response and reactions to aqueous influenza vaccine, simple emulsion vaccine and multiple emulsion vaccine. A report to the Medical Research Council Committee on influenza and other respiratory virus vaccines. J Hyg (Lond) 1969 Sep;67(3):485–490. doi: 10.1017/s0022172400041905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODHOUR A. F., METZGAR D. P., STIM T. B., TYTELL A. A., HILLEMAN M. R. NEW METABOLIZABLE IMMUNOLOGIC ADJUVANT FOR HUMAN USE. I. DEVELOPMENT AND ANIMAL IMMUNE RESPONSE. Proc Soc Exp Biol Med. 1964 Jun;116:516–523. doi: 10.3181/00379727-116-29295. [DOI] [PubMed] [Google Scholar]


