Abstract
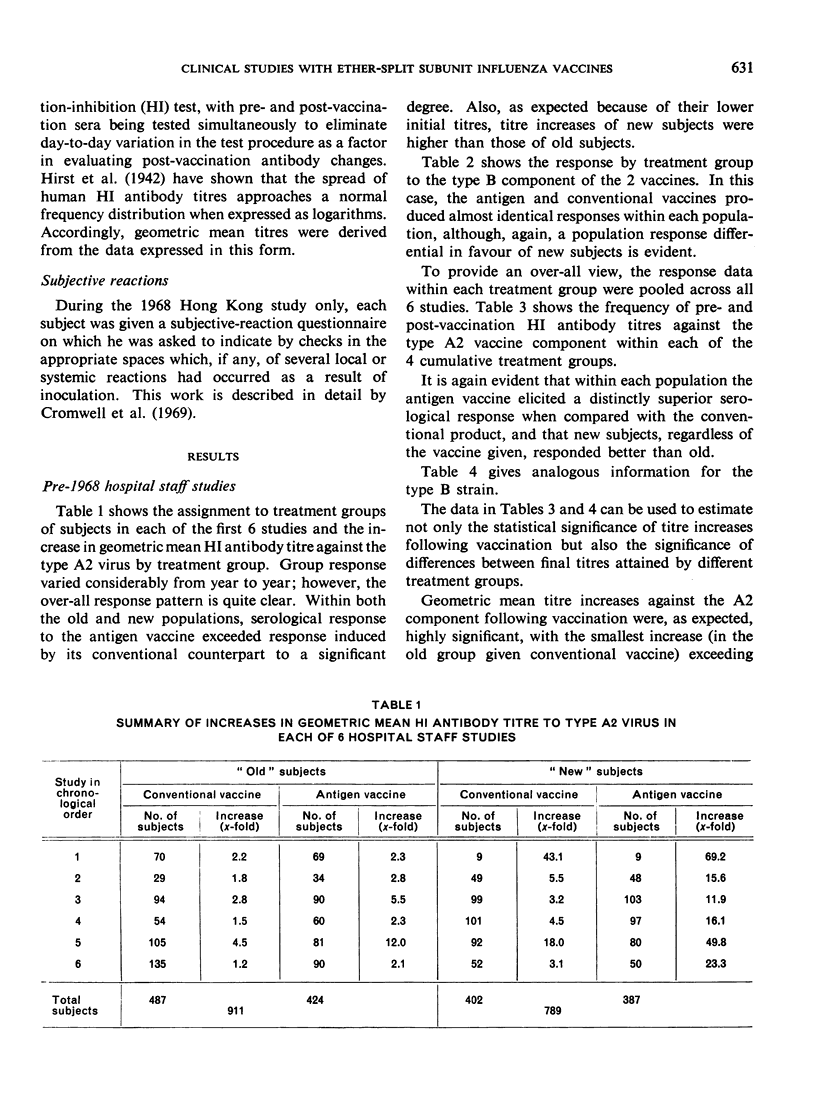
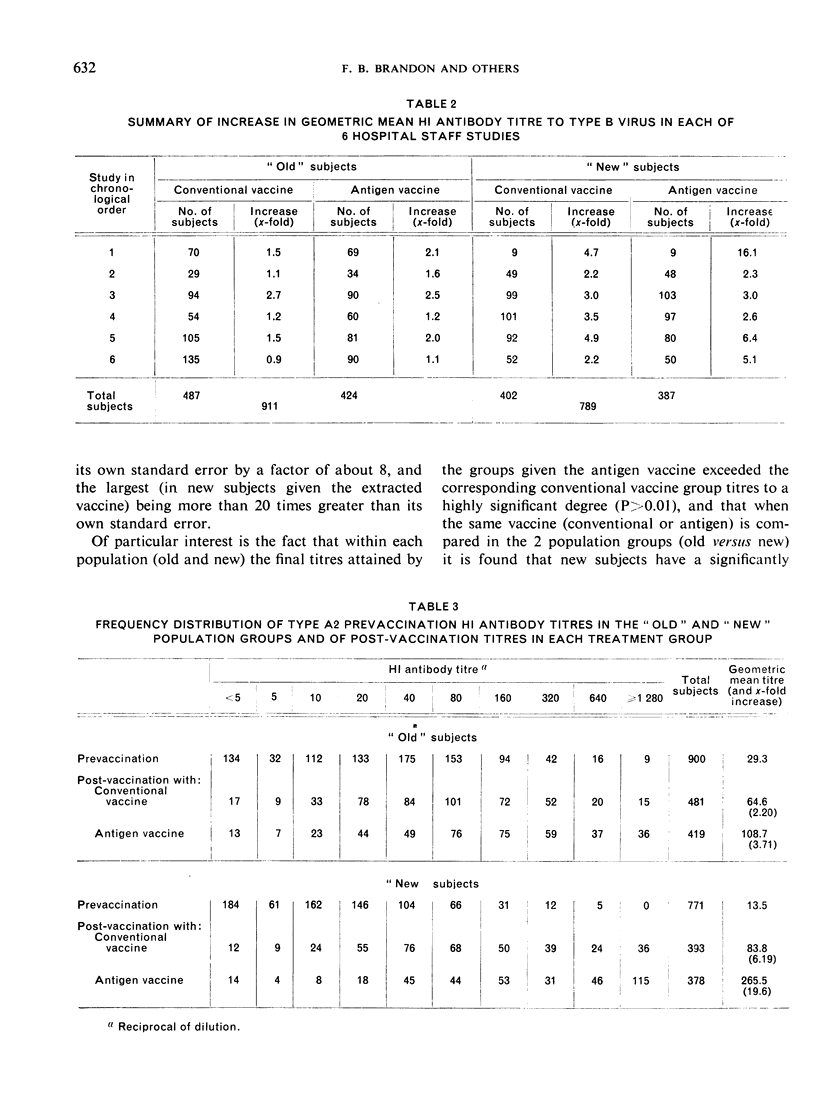
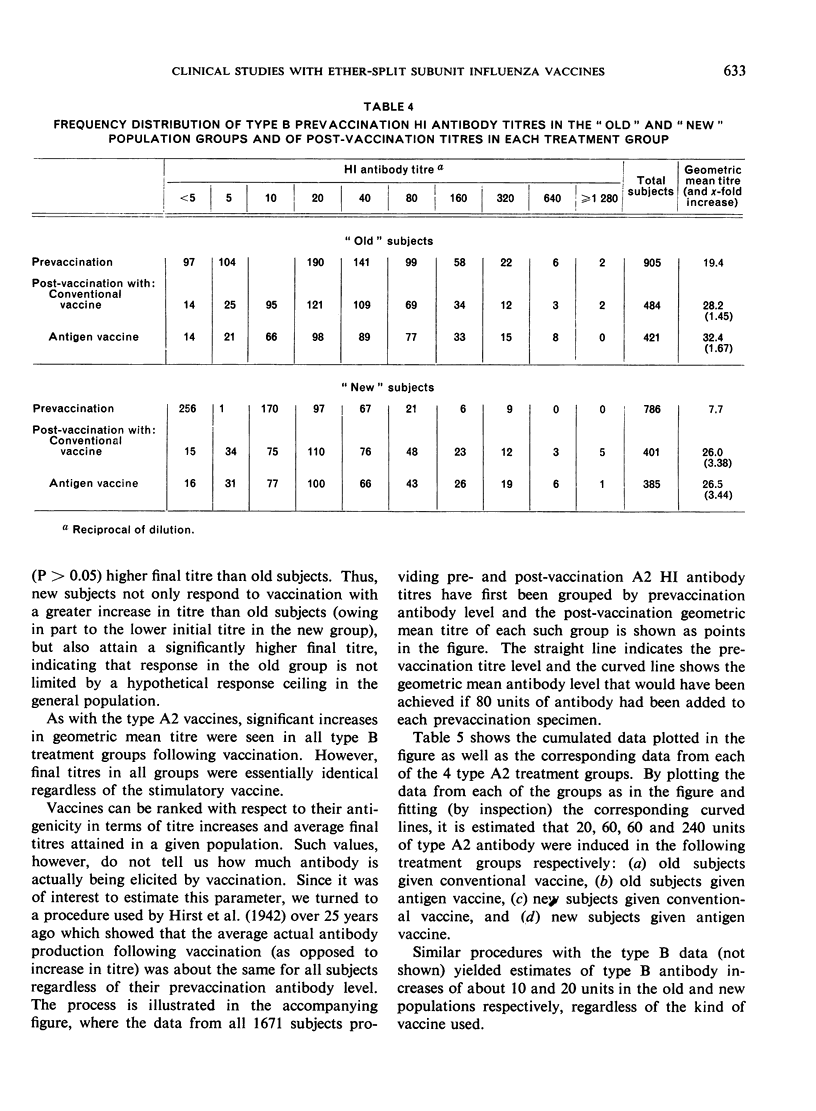
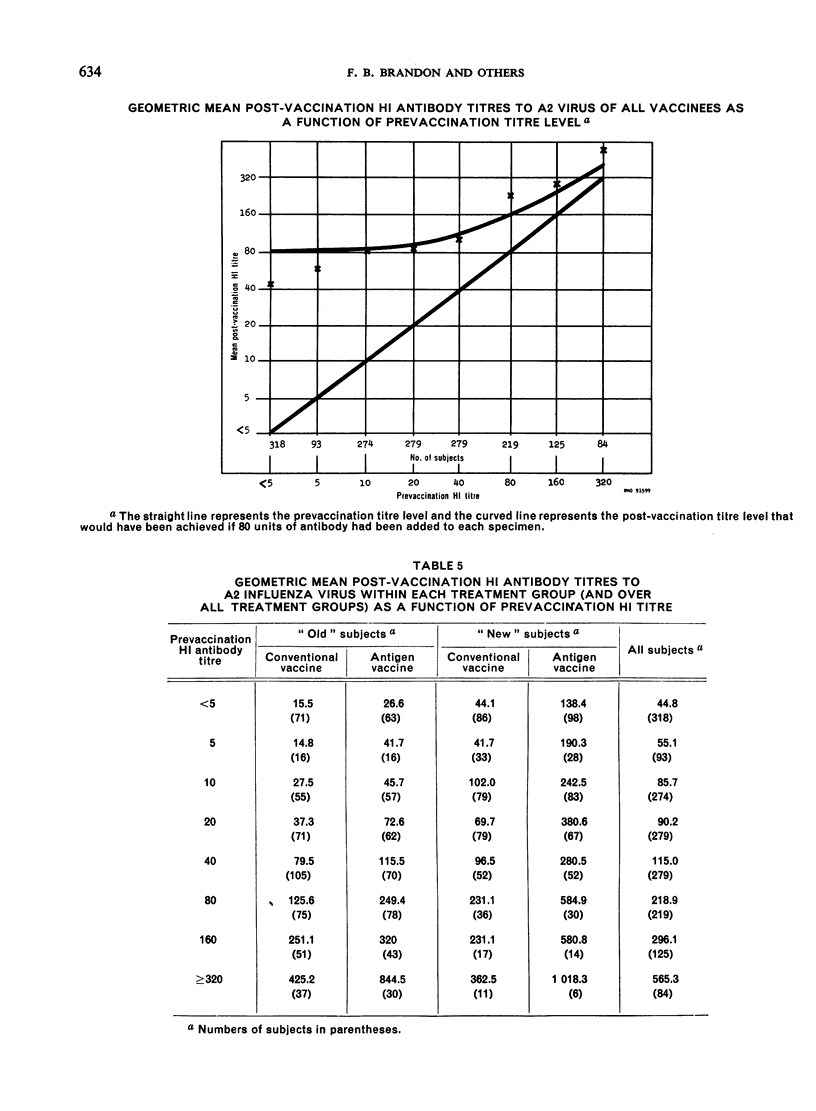
Clinical studies of ether-split influenza antigen vaccines have been in progress for almost a decade. One series of such studies, completed before the Hong Kong virus appeared, compared identically constituted conventional and antigen vaccines for serological effectiveness in 1700 vaccinees from the staff of a metropolitan hospital. A series of 6 annual trials included both ”old” subjects (vaccinated the previous year) and ”new” subjects (no vaccination the previous year). The serological response to the type A2 component of the antigen vaccines was 3-4 times better than that to intact virus in both the old and new populations. The response to either vaccine by new subjects significantly exceeded the response by the old subjects. The type B component of both vaccines induced an equivalent response in both populations. Monovalent Hong Kong vaccines, both conventional and antigen, given just prior to the Hong Kong epidemic induced an anamnestic response in a geriatric group. No influenza-like disease was seen in this high-risk group during the epidemic.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandon F. B., Barrett C. D., Jr, Hook A. E., Lease G. O. Human febrile response to influenza virus or its ether isolated hemagglutinins. Proc Soc Exp Biol Med. 1967 Jul;125(3):683–686. doi: 10.3181/00379727-125-32180. [DOI] [PubMed] [Google Scholar]
- Brandon F. B., Cox F., Lease G. O., Timm E. A., Quinn E., McLean I. W., Jr Respiratory virus vaccines. 3. Some biological properties of Sephadex-purified ether-extracted influenza virus antigens. J Immunol. 1967 Apr;98(4):800–805. [PubMed] [Google Scholar]
- Cromwell H. A., Brandon F. B., McLean I. W., Jr, Sadusk J. F., Jr Influenza immunization. A new vaccine. JAMA. 1969 Nov 24;210(8):1438–1442. [PubMed] [Google Scholar]
- DAVENPORT F. M., HENNESSY A. V., BRANDON F. M., WEBSTER R. G., BARRETT C. D., Jr, LEASE G. O. COMPARISONS OF SEROLOGIC AND FEBRILE RESPONSES IN HUMANS TO VACCINATION WITH INFLUENZA A VIRUSES OR THEIR HEMAGGLUTININS. J Lab Clin Med. 1964 Jan;63:5–13. [PubMed] [Google Scholar]
- HOYLE L. Structure of the influenza virus; the relation between biological activity and chemical structure of virus fractions. J Hyg (Lond) 1952 Jun;50(2):229–245. doi: 10.1017/s0022172400019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy A. V., Davenport F. M. Relative antigenic potency in man of polyvalent influenza virus vaccines containing isolated hemagglutinins or intact viruses. J Immunol. 1966 Aug;97(2):235–238. [PubMed] [Google Scholar]
- Hirst G. K., Rickard E. R., Whitman L., Horsfall F. L. ANTIBODY RESPONSE OF HUMAN BEINGS FOLLOWING VACCINATION WITH INFLUENZA VIRUSES. J Exp Med. 1942 May 1;75(5):495–511. doi: 10.1084/jem.75.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]



