Abstract
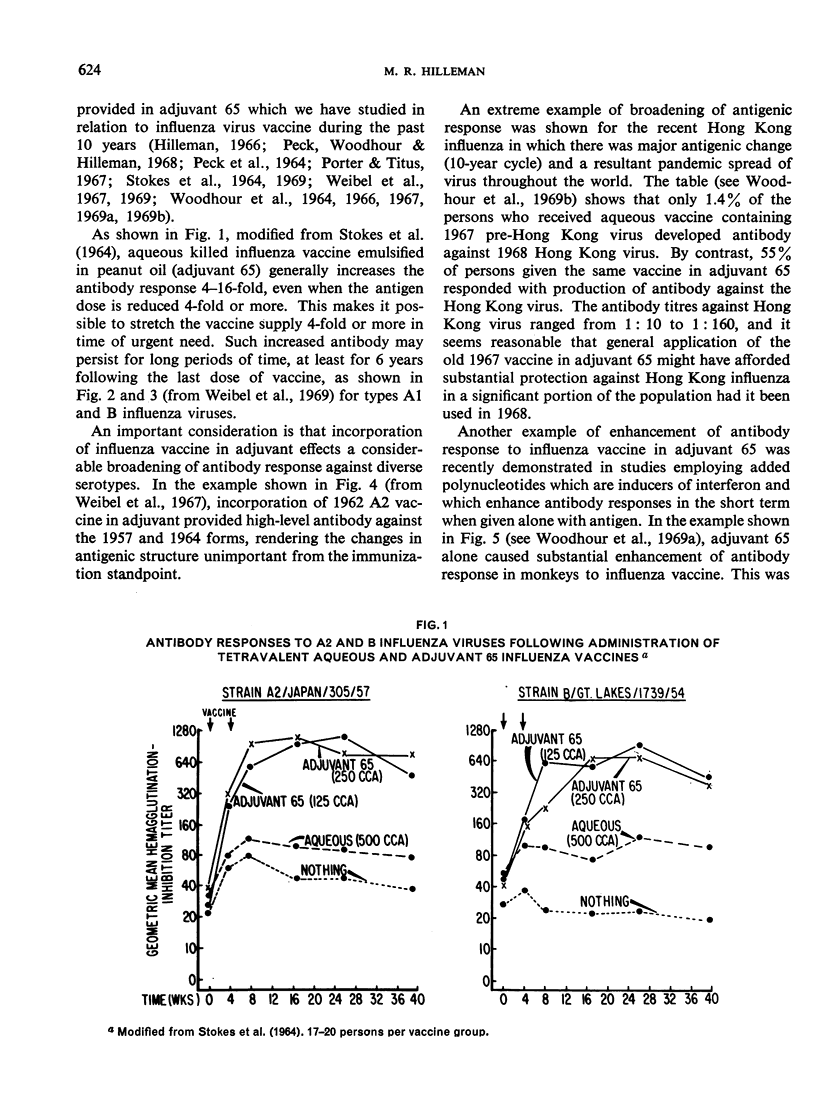
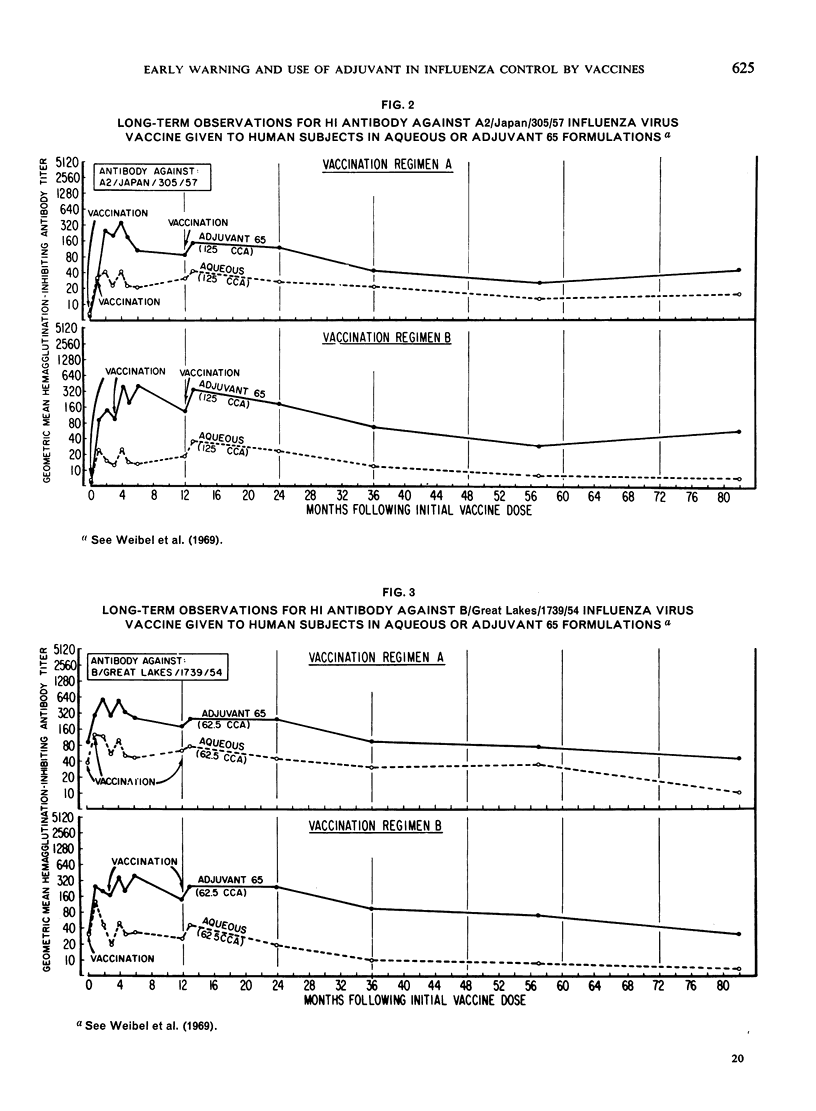
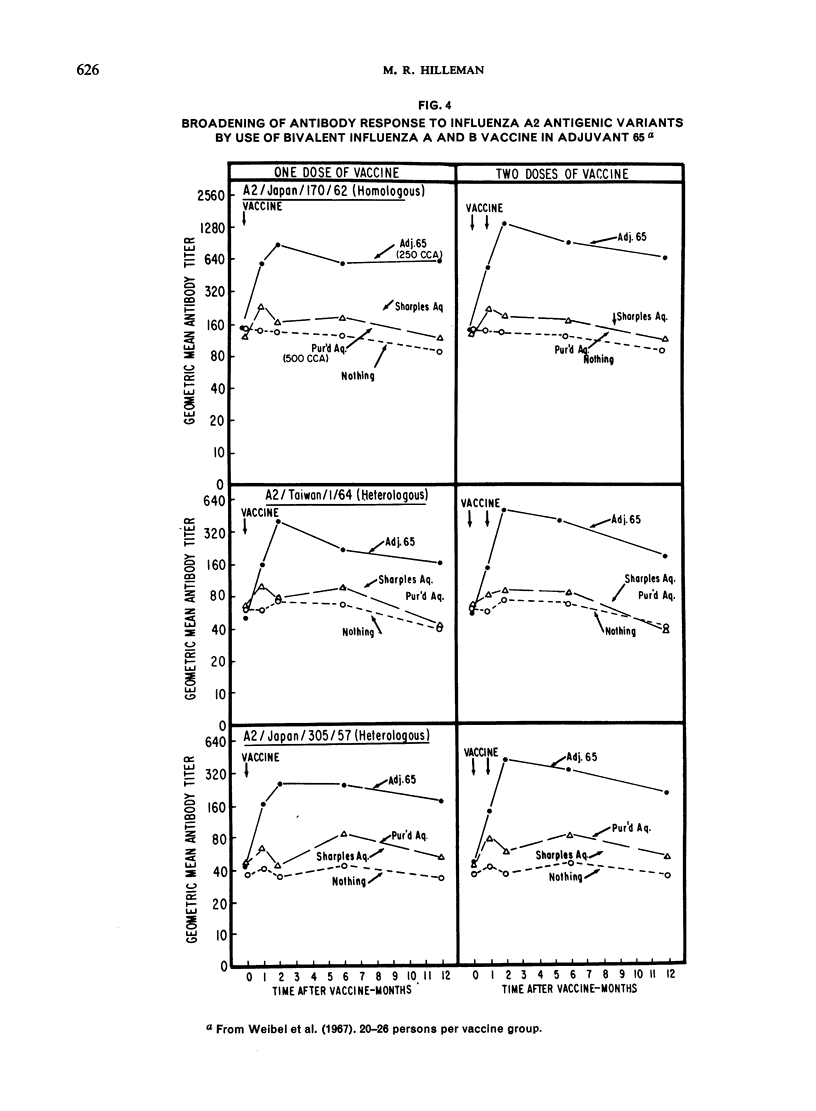
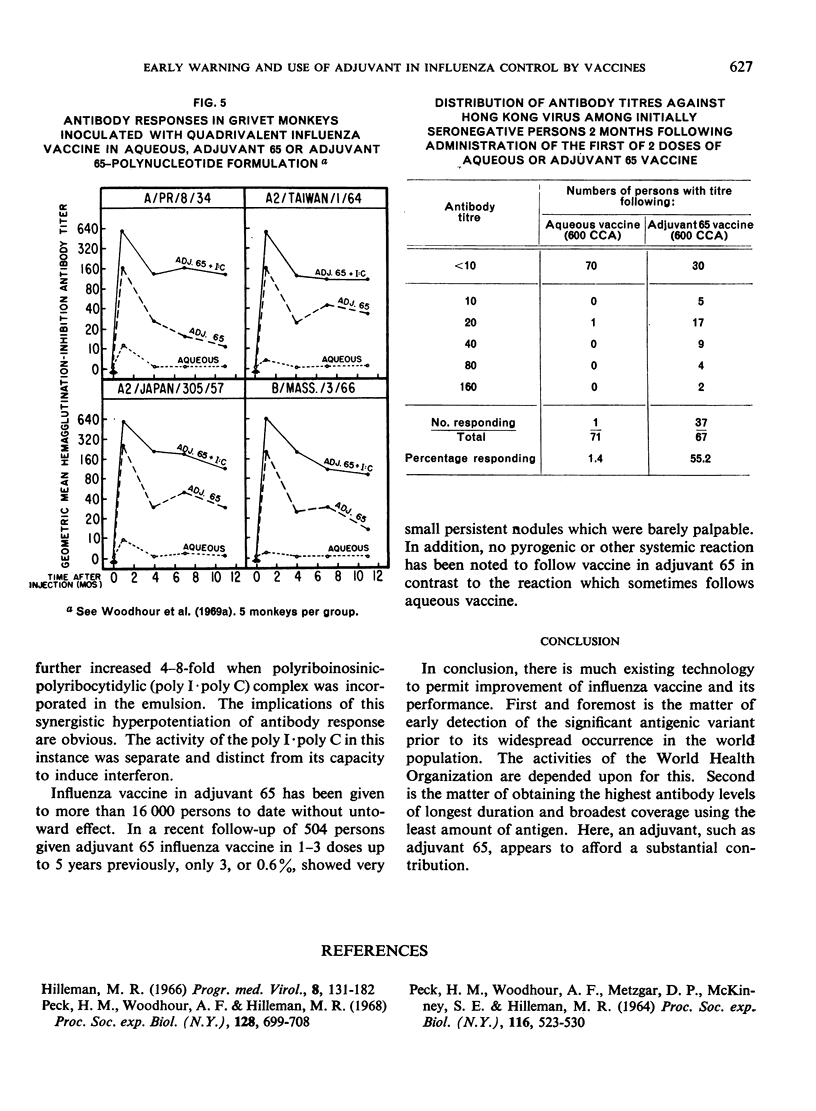
The major antigenic changes in influenza A virus that occur at 10-year intervals reduce the effectiveness of existing vaccines and pose a problem for the control of pandemics by vaccination. These difficulties may be obviated in two ways. First, by detecting the emergence of a new variant sufficiently ahead of its general spread for it to be possible to produce a corresponding vaccine in good time; the World Health Organization is widely depended upon for this early warning. Secondly, by the improvement of existing vaccines to increase and broaden their antigenicity; here, their modification by, for instance, adjuvant 65 may contribute significantly.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hilleman M. R. Critical appraisal of emulsified oil adjuvants applied to viral vaccines. Prog Med Virol. 1966;8:131–182. [PubMed] [Google Scholar]
- PECK H. M., WOODHOUR A. F., METZGAR D. P., MCKINNEY S. E., HILLEMAN M. R. NEW METABOLIZABLE IMMUNOLOGIC ADJUVANT FOR HUMAN USE. 2. SHORT-TERM ANIMAL TOXICITY TESTS. Proc Soc Exp Biol Med. 1964 Jun;116:523–530. doi: 10.3181/00379727-116-29296. [DOI] [PubMed] [Google Scholar]
- Peck H. M., Woodhour A. F., Hilleman M. R. New metabolizable immunologic adjuvant for human use. 8. Chronic toxicity and teratogenic tests. Proc Soc Exp Biol Med. 1968 Jul;128(3):699–708. doi: 10.3181/00379727-128-33104. [DOI] [PubMed] [Google Scholar]
- Porter C. C., Titus D. C. New metabolizable immunologic adjuvant for human use. 6. Disposition of radioactivity after administration of labelled vaccine to the rat. Proc Soc Exp Biol Med. 1967 Feb;124(2):500–503. doi: 10.3181/00379727-124-31774. [DOI] [PubMed] [Google Scholar]
- STOKES J., Jr, WEIBEL R. E., DRAKE M. E., WOODHOUR A. F., HILLEMAN M. R. NEW METABOLIZABLE IMMUNOLOGIC ADJUVANT FOR HUMAN USE. 3. EFFICACY AND TOXICITY STUDIES IN MAN. N Engl J Med. 1964 Sep 3;271:479–487. doi: 10.1056/NEJM196409032711001. [DOI] [PubMed] [Google Scholar]
- Stokes J., Jr, Weibel R. E., Woodhour A. F., McAleer W. J., Potkonski L. A., Hilleman M. R. New metabolizable immunologic adjuvant for human use. IX. Large-scale trials with multiple lots in different regimens. JAMA. 1969 Mar 17;207(11):2067–2072. [PubMed] [Google Scholar]
- WOODHOUR A. F., METZGAR D. P., STIM T. B., TYTELL A. A., HILLEMAN M. R. NEW METABOLIZABLE IMMUNOLOGIC ADJUVANT FOR HUMAN USE. I. DEVELOPMENT AND ANIMAL IMMUNE RESPONSE. Proc Soc Exp Biol Med. 1964 Jun;116:516–523. doi: 10.3181/00379727-116-29295. [DOI] [PubMed] [Google Scholar]
- Weibel R. E., Woodhour A. F., Stokes J., Jr, Metzgar D. P., Hilleman M. R. New metabolizable immunologic adjuvant for human use. 5. Evaluation of highly purified influenza-virus vaccine in adjuvant 65. N Engl J Med. 1967 Jan 12;276(2):78–84. doi: 10.1056/NEJM196701122760203. [DOI] [PubMed] [Google Scholar]
- Woodhour A. F., Friedman A., Tytell A. A., Hilleman M. R. Hyperpotentiation by synthetic double-stranded RNA of antibody responses to influenza virus vaccine in adjuvant 65. Proc Soc Exp Biol Med. 1969 Jul;131(3):809–817. doi: 10.3181/00379727-131-33983. [DOI] [PubMed] [Google Scholar]
- Woodhour A. F., McAleer W. J., Friedman A., Weibel R. E., Stokes J., Jr, Hilleman M. R. Antibody response in man to Hong Kong influenza following 1967 formula influenza vaccine in adjuvant 65. Proc Soc Exp Biol Med. 1969 Jun;131(2):501–506. doi: 10.3181/00379727-131-33911. [DOI] [PubMed] [Google Scholar]
- Woodhour A. F., Metzgar D. P., Lampson G. P., Machlowitz R. A., Tytell A. A., Hilleman M. R. New metabolizable immunologic adjuvant for human use. 4. Development of highly purified influenza virus vaccine in adjuvant 65. Proc Soc Exp Biol Med. 1966 Dec;123(3):778–782. doi: 10.3181/00379727-123-31601. [DOI] [PubMed] [Google Scholar]


