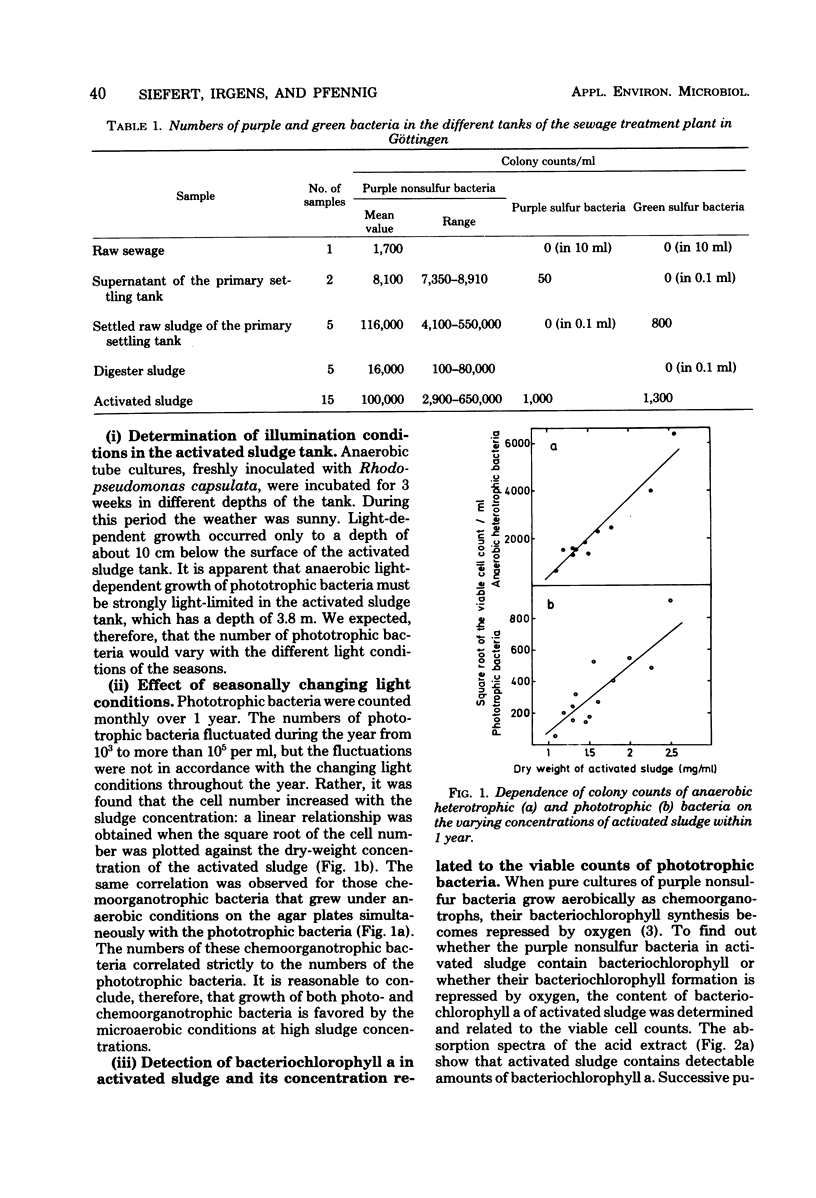
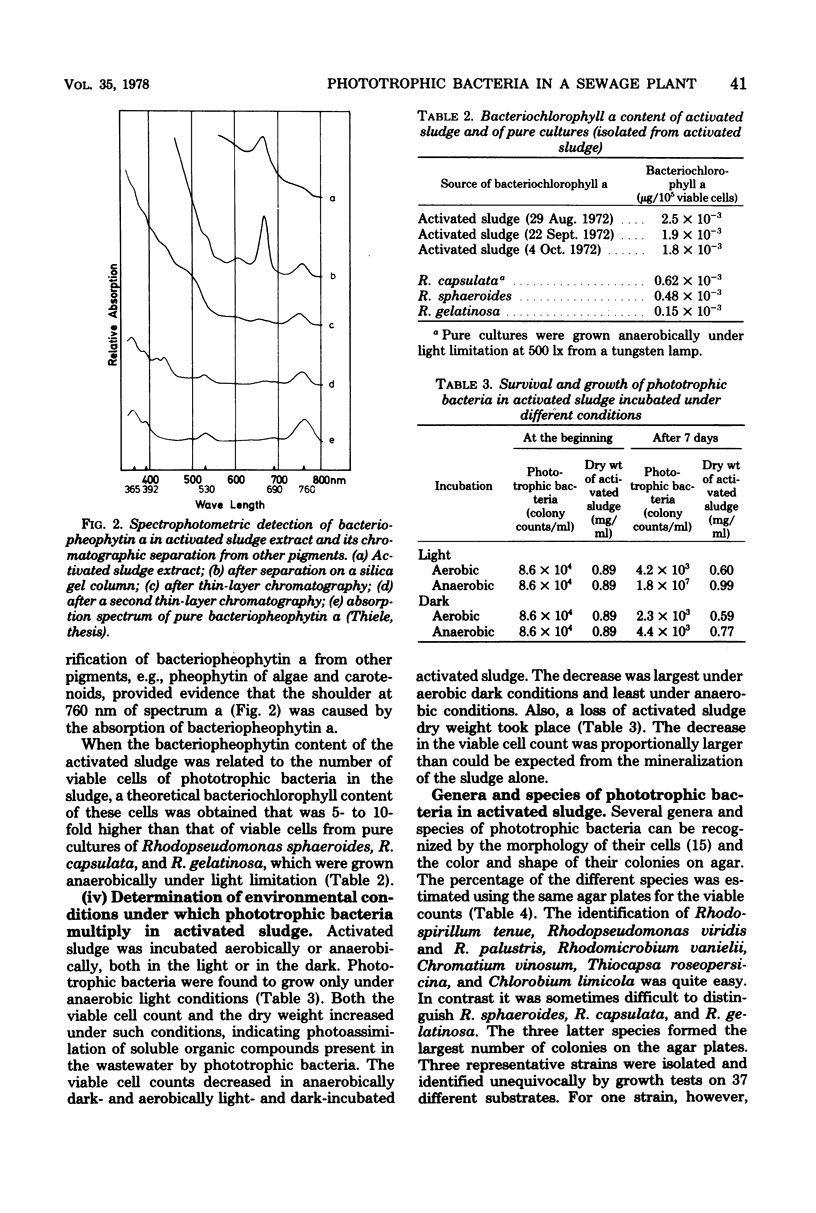
Abstract
In all purification stages of a biological sewage treatment plant, phototrophic bacteria were detected by the method of viable cell counts. The predominant species identified belonged to the genus Rhodopseudomonas of purple nonsulfur bacteria. The number of phototrophic bacteria was highest in wastewater containing sludge. In activated sludge, an average of 10(5) viable cells/ml was found; the number depended upon concentration of sludge rather than on seasonal changes in light conditions in the course of a year. Bacteriochlorophyll a was extracted from activated sludge. Relative to the viable counts of phototrophic bacteria, the content of bacteriochlorophyll a was 5- to 10-fold higher than that of three representative pure cultures. By incubation of activated and digester sludge under different environmental conditions, it was shown that phototrophic bacteria can complete with other bacteria only under anaerobic conditions in the light.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biebl H., Drews G. Das in-vivo-Spektrum als taxonomisches Merkmal bei Untersuchungen zur Verbreitung von Athiorhodaceae. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1969;123(4):425–452. [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cooper D. E., Rands M. B., Woo C. P. Sulfide reduction in fellmongery effluent by red sulfur bacteria. J Water Pollut Control Fed. 1975 Aug;47(8):2088–2100. [PubMed] [Google Scholar]
- Drews G., Lampe H. H., Ladwig R. Die Entwicklung des Photosyntheseapparates in Dunkelkulturen von Rhodopseudomonas capsulata. Arch Mikrobiol. 1969;65(1):12–28. [PubMed] [Google Scholar]
- Gürgün V., Kirchner G., Pfennig N. Vergärung von Pyruvat durch sieben Arten phototropher Purpurbakterien. Z Allg Mikrobiol. 1976;16(8):573–586. doi: 10.1002/jobm.3630160802. [DOI] [PubMed] [Google Scholar]
- Holm H. W., Vennes J. W. Occurrence of purple sulfur bacteria in a sewage treatment lagoon. Appl Microbiol. 1970 Jun;19(6):988–996. doi: 10.1128/am.19.6.988-996.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P. Contribution à l'étude de l'écologie des bactéries photosynthétiques. Ann Inst Pasteur (Paris) 1966 Dec;111(6):733–749. [PubMed] [Google Scholar]
- Pike E. B., Curds C. R. The microbial ecology of the activated sludge process. Soc Appl Bacteriol Symp Ser. 1971;1:123–147. doi: 10.1016/b978-0-12-648050-4.50012-2. [DOI] [PubMed] [Google Scholar]
- Swoager W. C., Lindstrom E. S. Isolation and counting of Athiorhodaceae with membrane filters. Appl Microbiol. 1971 Oct;22(4):683–687. doi: 10.1128/am.22.4.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L., Wolfe R. S. Anaerobic growth of purple nonsulfur bacteria under dark conditions. J Bacteriol. 1970 Oct;104(1):462–472. doi: 10.1128/jb.104.1.462-472.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


