Abstract
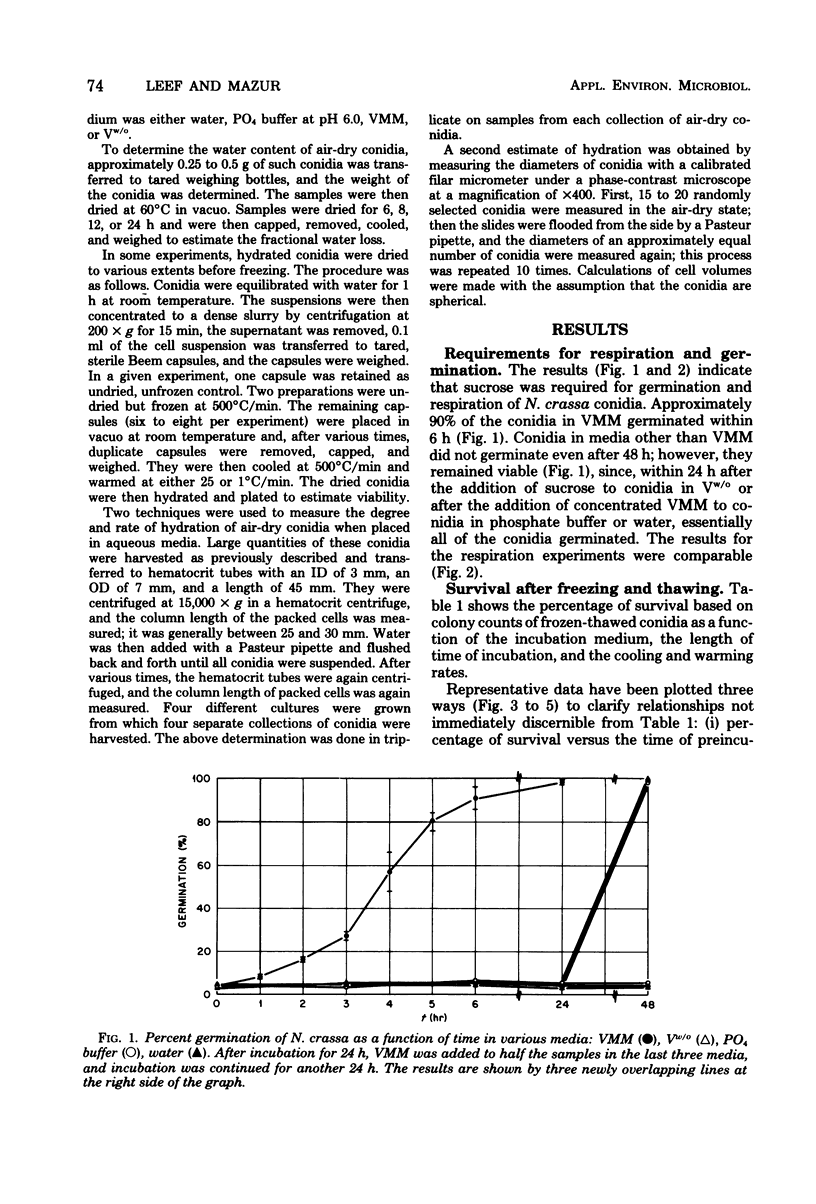
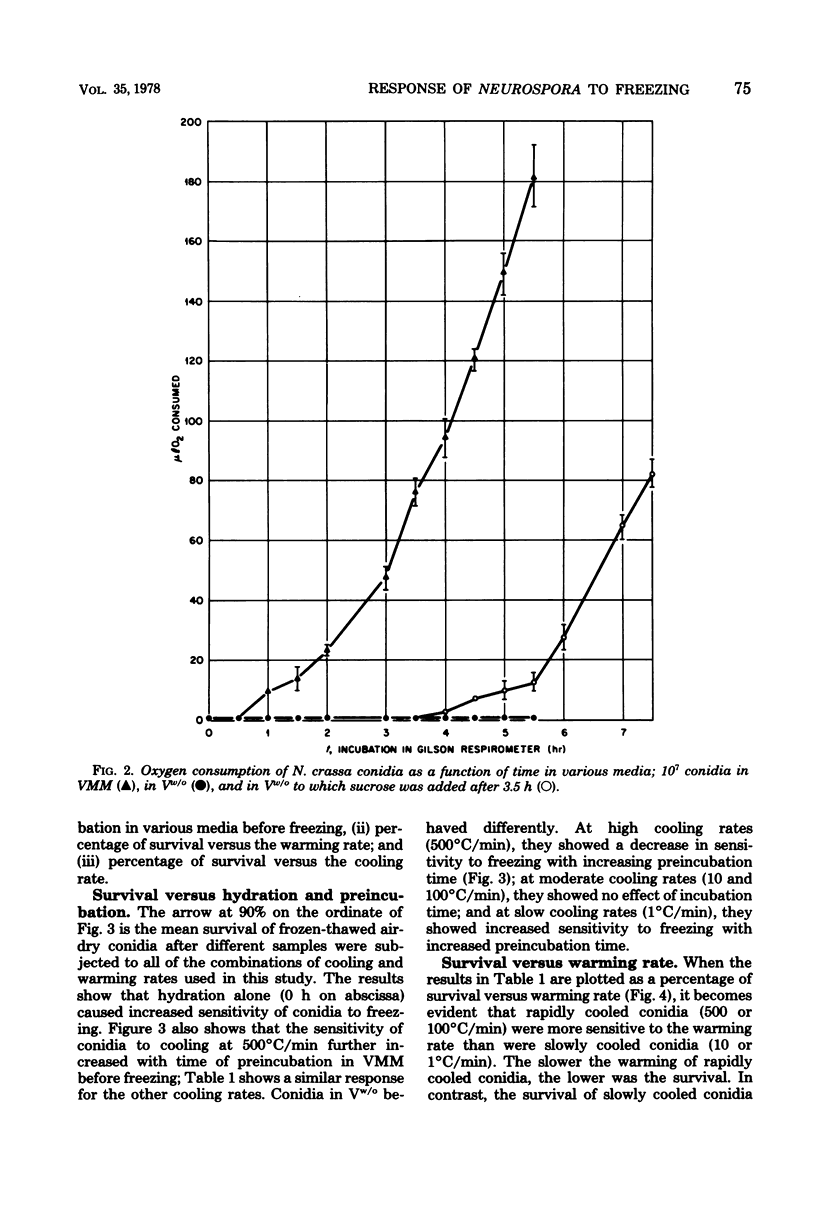
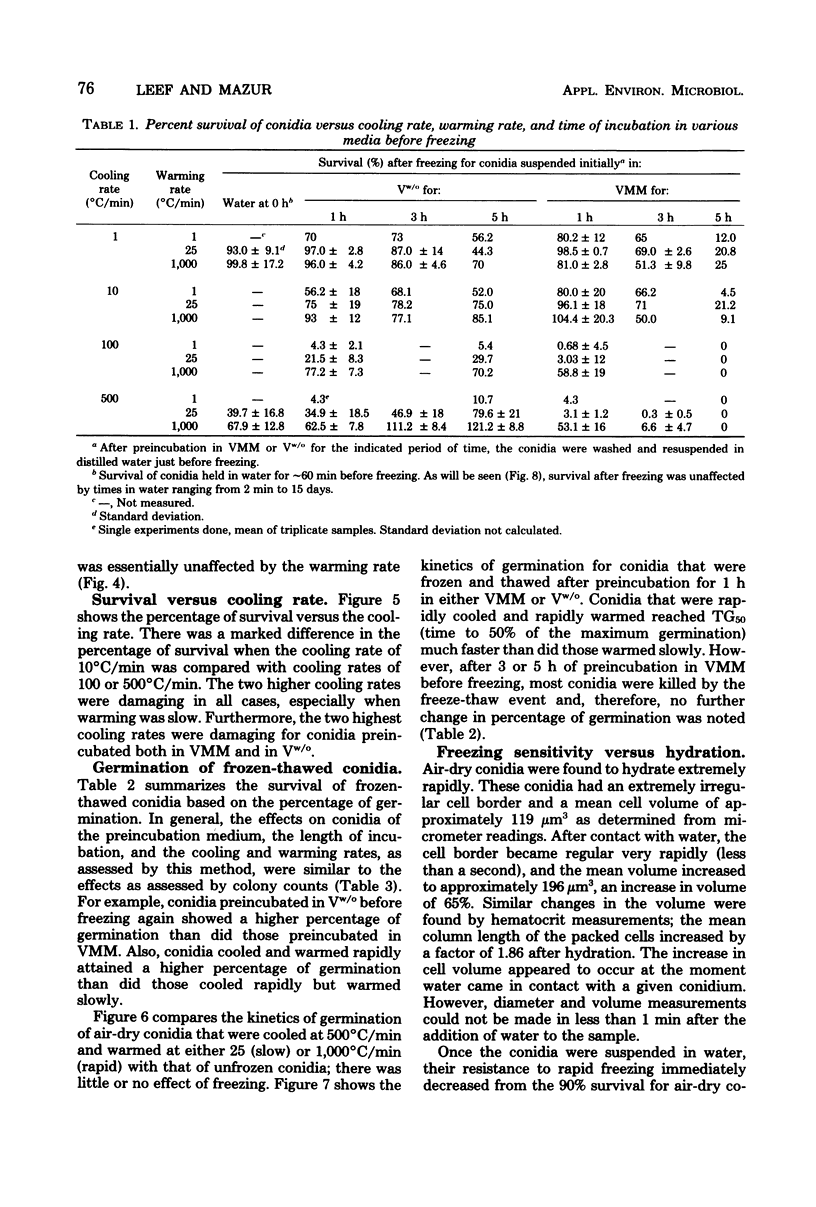
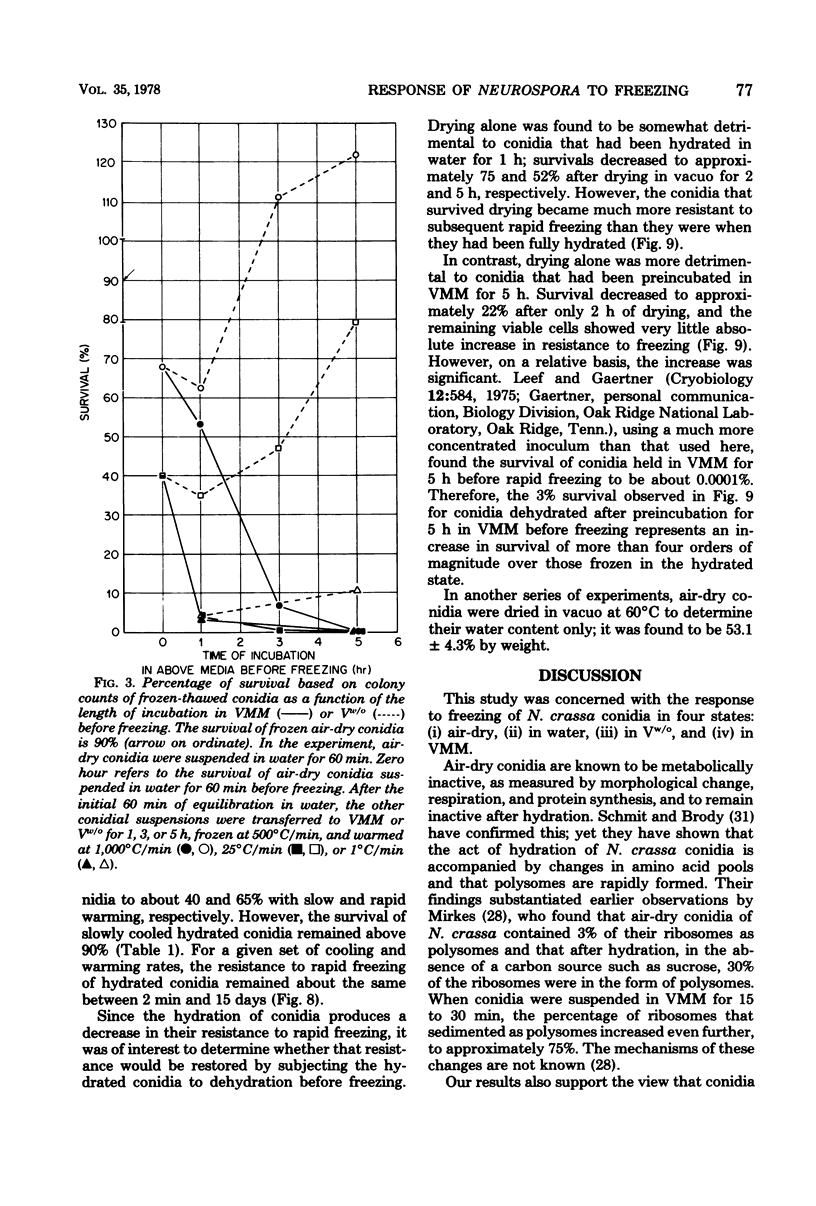
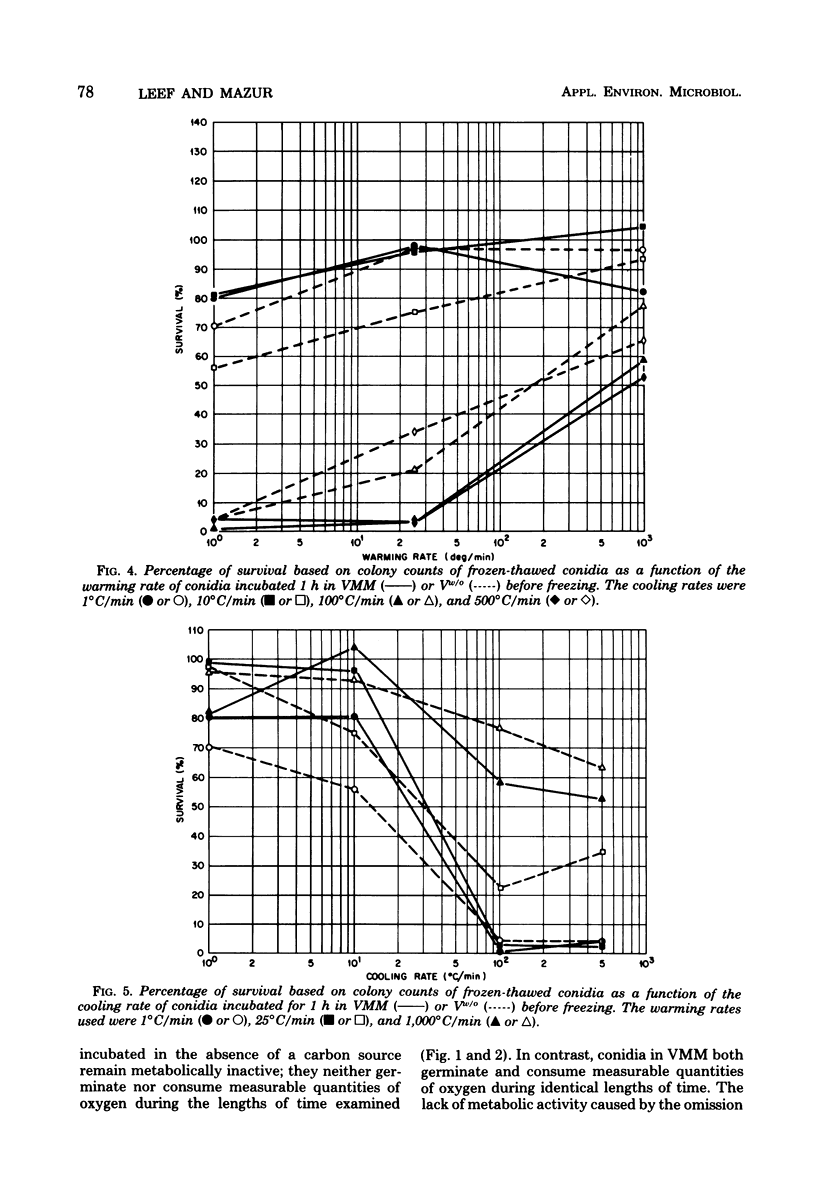
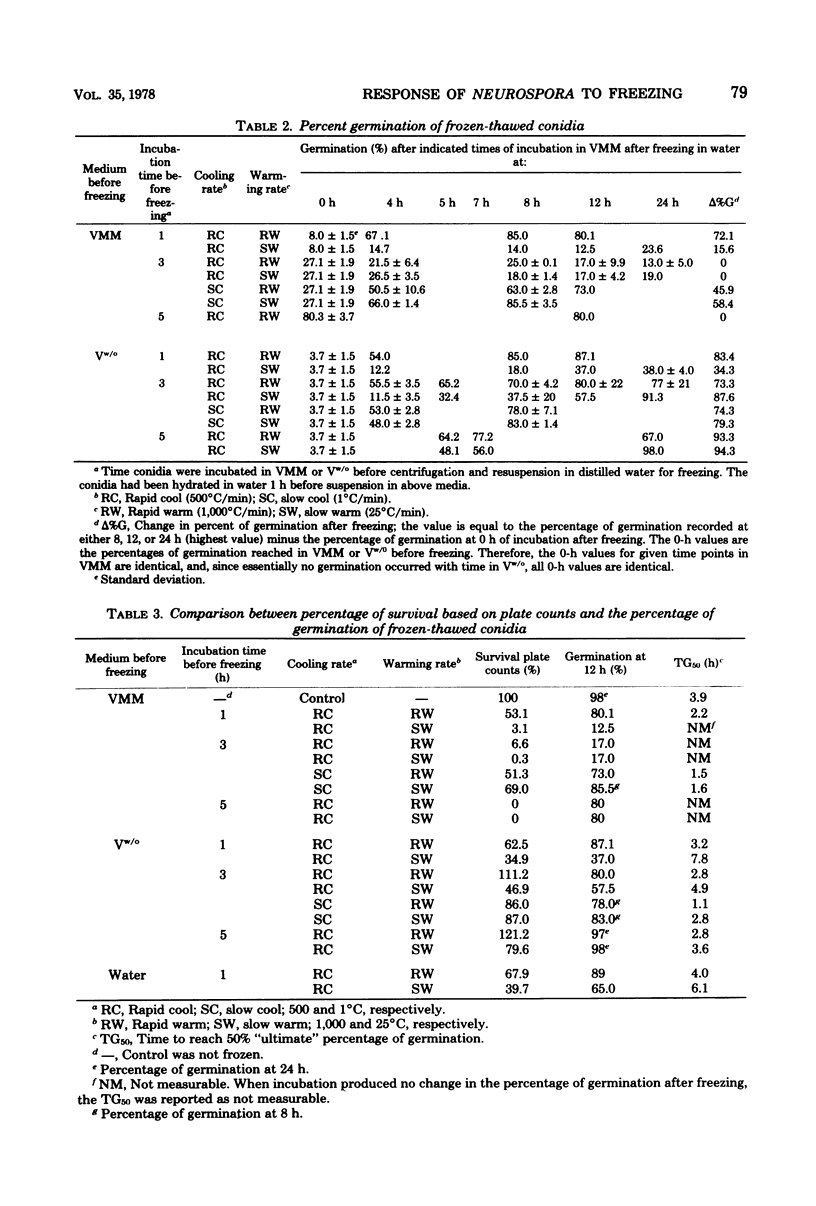
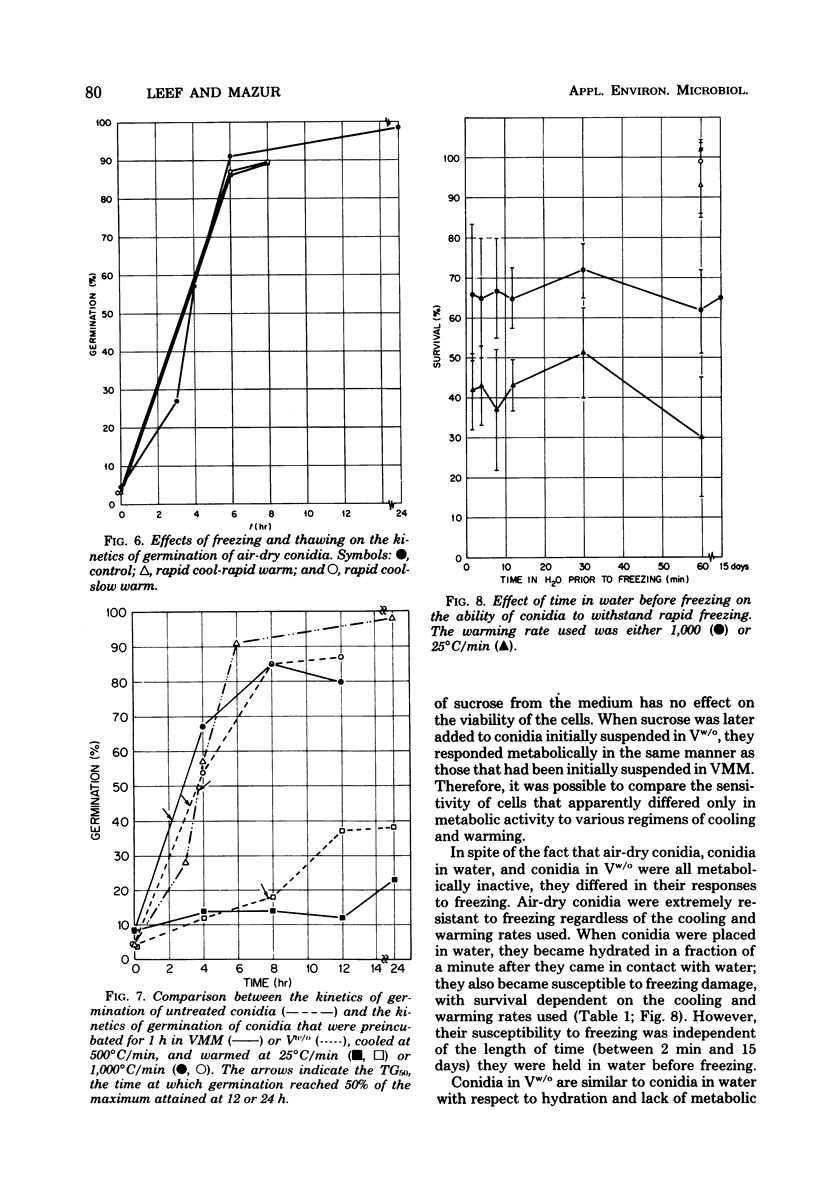
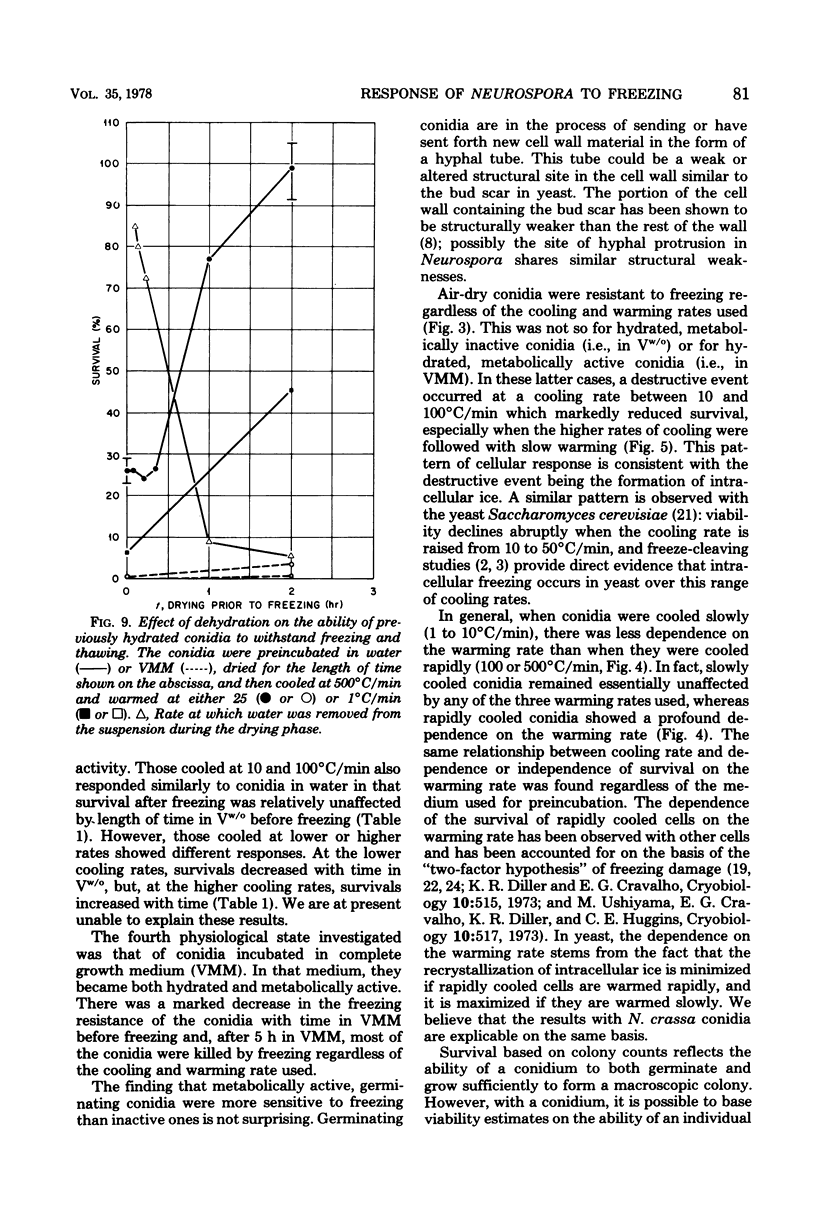
This study concerned the response to freezing of Neurospora crassa conidia in four different states: air-dry, hydrated in water, hydrated in Vogel medium lacking only sucrose, or hydrated in complete Vogel medium. All hydrated conidia were incubated in one of the above media for various times before freezing and were then washed and frozen in distilled water. Viability was estimated by three techniques, and the agreement among them was good. Hydration of air-dry conidia was found to be very rapid and, once hydrated, the conidia were much more sensitive to rapid freezing than they were before hydration. Rapidly cooled conidia survived freezing to a much higher extent when the warming rate was rapid than when it was slow; slowly cooled conidia showed little or no dependence on the warming rate. This sensitivity to rapid cooling and slow warming was attributed to the effects of intracellular ice. The sensitivity to freezing could be reversed by dehydrating the conidia in vacuo before freezing; thus, it was concluded that the presence or absence of water is the determining factor in the initial sensitivity due to freezing. In water, the sensitivity remained constant from 2 min to 15 days after hydration. Although conidia hydrated in growth medium lacking sucrose remained metabolically inactive, their sensitivity to rapid freezing decreased as a function of time in the medium before freezing. The reason for this decreased sensitivity is not understood. Conidia hydrated in complete growth medium (i.e., containing sucrose) became metabolically active and, after the initial sensitivity associated with hydration, became increasingly more sensitive to freezing as a function of their time in the medium. Drying itself was deleterious to metabolically active conidia, and those that survived dehydration did not exhibit a large absolute increase in resistance to subsequent freezing. The increase in sensitivity to freezing and to drying seems associated with the presence of metabolic activity; however, the precise cause of the sensitization remains obscure.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht R. M., Orndorff G. R., MacKenzie A. P. Survival of certain microorganisms subjected to rapid and very rapid freezing on membrane filters. Cryobiology. 1973 Aug;10(3):233–239. doi: 10.1016/0011-2240(73)90036-9. [DOI] [PubMed] [Google Scholar]
- Bank H., Mazur P. Visualization of freezing damage. J Cell Biol. 1973 Jun;57(3):729–742. doi: 10.1083/jcb.57.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank H. Visualization of freezing damage. II. Structural alterations during warming. Cryobiology. 1973 Jun;10(2):157–170. doi: 10.1016/0011-2240(73)90023-0. [DOI] [PubMed] [Google Scholar]
- Barnhart E. R., Terry C. E. Cryobiology of Neurospora crassa. II. Alteration in freeze response of Neurospora crassa conidia by additives. Cryobiology. 1971 Aug;8(4):328–332. doi: 10.1016/0011-2240(71)90126-x. [DOI] [PubMed] [Google Scholar]
- Barnhart E. R., Terry C. E. Cryobiology of neurospora crassa. I. Freeze response of Neurospora crassa conidia. Cryobiology. 1971 Aug;8(4):323–327. doi: 10.1016/0011-2240(71)90125-8. [DOI] [PubMed] [Google Scholar]
- Cunningham J. L. Proceedings: Preservation of rust fungi in liquid nitrogen. Cryobiology. 1973 Nov;10(5):361–363. doi: 10.1016/0011-2240(73)90058-8. [DOI] [PubMed] [Google Scholar]
- Darling S., Theilade J., Birch-Andersen A. Structure and chemical composition of prospheroplast envelopes of Saccharomyces cerevisiae and Hansenula anomala. J Bacteriol. 1972 Apr;110(1):336–345. doi: 10.1128/jb.110.1.336-345.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goos R. D., Davis E. E., Butterfield W. Effect of warming rates on the viability of frozen fungous spores. Mycologia. 1967 Jan-Feb;59(1):58–66. [PubMed] [Google Scholar]
- HASKINS R. H. Factors affecting survival of lyophilized fungal spores and cells. Can J Microbiol. 1957 Apr;3(3):477–485. doi: 10.1139/m57-051. [DOI] [PubMed] [Google Scholar]
- Janssen D. W., Busta F. F. Proceedings: Repair of injury in Salmonella anatum cells after freezing and thawing in milk. Cryobiology. 1973 Nov;10(5):386–392. doi: 10.1016/0011-2240(73)90063-1. [DOI] [PubMed] [Google Scholar]
- Koch G. J., Kruuv J., Bruck-Schwaiger C. W. Survival of synchronized Chinese hamster cells following freezing in liquid nitrogen. Exp Cell Res. 1970 Dec;63(2):476–477. doi: 10.1016/0014-4827(70)90245-4. [DOI] [PubMed] [Google Scholar]
- Leibo S. P., Farrant J., Mazur P., Hanna M. G., Jr, Smith L. H. Effects of freezing on marrow stem cell suspensions: interactions of cooling and warming rates in the presence of PVP, sucrose, or glycerol. Cryobiology. 1970 Jan-Feb;6(4):315–332. doi: 10.1016/s0011-2240(70)80086-4. [DOI] [PubMed] [Google Scholar]
- MAZUR P. CAUSES OF INJURY IN FROZEN AND THAWED CELLS. Fed Proc. 1965 Mar-Apr;24:S175–S182. [PubMed] [Google Scholar]
- MAZUR P. Studies on the effects of subzero temperatures on the viability of spores of Aspergillus flavus. I. The effect of rate of warming. J Gen Physiol. 1956 Jul 20;39(6):869–888. doi: 10.1085/jgp.39.6.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. Cryobiology: the freezing of biological systems. Science. 1970 May 22;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- Mazur P., Leibo S. P., Chu E. H. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res. 1972;71(2):345–355. doi: 10.1016/0014-4827(72)90303-5. [DOI] [PubMed] [Google Scholar]
- Mazur P., Schmidt J. J. Interactions of cooling velocity, temperature, and warming velocity on the survival of frozen and thawed yeast. Cryobiology. 1968 Jul-Aug;5(1):1–17. doi: 10.1016/s0011-2240(68)80138-5. [DOI] [PubMed] [Google Scholar]
- Mazur P. The role of cell membranes in the freezing of yeast and other single cells. Ann N Y Acad Sci. 1965 Oct 13;125(2):658–676. doi: 10.1111/j.1749-6632.1965.tb45420.x. [DOI] [PubMed] [Google Scholar]
- McGann L. E., Frey H. E. Effect of hypertonicity and freezing on survival of unprotected synchronized mammalian cells. Cryobiology. 1972 Apr;9(2):107–111. doi: 10.1016/0011-2240(72)90017-x. [DOI] [PubMed] [Google Scholar]
- Mirkes P. E. Polysomes, ribonucleic acid, and protein synthesis during germination of Neurospora crassa conidia. J Bacteriol. 1974 Jan;117(1):196–202. doi: 10.1128/jb.117.1.196-202.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morichi T., Irie R. Proceedings: Factors affecting repair of sublethal injury in frozen or freeze-dried bacteria. Cryobiology. 1973 Nov;10(5):393–399. doi: 10.1016/0011-2240(73)90064-3. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Speck M. L. Identification of nutritional components in trypticase responsible for recovery of Escherichia coli injured by freezing. J Bacteriol. 1966 Mar;91(3):1098–1104. doi: 10.1128/jb.91.3.1098-1104.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Biochemical genetics of Neurospora crassa conidial germination. Bacteriol Rev. 1976 Mar;40(1):1–41. doi: 10.1128/br.40.1.1-41.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman A. M., Walden D. B. Long-term cryogenic storage of Neurospora crassa spores. Cryobiology. 1971 Dec;8(6):566–569. doi: 10.1016/0011-2240(71)90008-3. [DOI] [PubMed] [Google Scholar]


