Abstract
B cell development and humoral immune responses are controlled by signaling thresholds established through the B lymphocyte antigen receptor (BCR) complex. BCR signaling thresholds are differentially regulated by the CD22 and CD19 cell surface receptors in vivo. B cells from CD22-deficient mice exhibit characteristics of chronic stimulation and are hyper-responsive to BCR crosslinking with augmented intracellular Ca2+ responses. By contrast, B cells from CD19-deficient mice are hypo-responsive to transmembrane signals. To identify signaling molecules involved in the positive and negative regulation of signaling thresholds, the signal transduction pathways activated after BCR crosslinking were examined in CD22- and CD19-deficient B cells. These comparisons revealed that tyrosine phosphorylation of Vav protein was uniquely augmented after BCR or CD19 crosslinking in CD22-deficient B cells, yet was modest and transient after BCR crosslinking in CD19-deficient B cells. Ligation of CD19 and CD22 in vivo is likely to positively and negatively regulate BCR signaling, respectively, because CD19 crosslinking was more efficient than BCR crosslinking at inducing Vav phosphorylation. However, simultaneous crosslinking of CD19 with the BCR resulted in a substantial decrease in Vav phosphorylation when CD22 was expressed. Thus, the differential regulation of Vav tyrosine phosphorylation by CD19 and CD22 may provide a molecular mechanism for adjusting BCR signaling thresholds.
Signaling thresholds established through the B lymphocyte antigen receptor (BCR) complex control B lymphocyte development, humoral immune responses, tolerance induction, and autoantibody production (1). BCR signal transduction thresholds also are regulated by other cell surface molecules, including the CD22 receptor and the CD19/CD21 complex (2–4). B cells from CD22-deficient mice exhibit characteristics of chronic stimulation and are hyper-responsive with augmented intracellular Ca2+ responses after BCR crosslinking (5–8). The ≈140-aa cytoplasmic domain of CD22 includes six tyrosines located in three tyrosine-based inhibition motifs and two tyrosine-based activation motifs that are targets for rapid phosphorylation after BCR or CD22 ligation (2). CD22 interacts with negative regulators of signal transduction such as the SH-protein tyrosine phosphatase-1 (SHP1) (9–12), which induces SHP1 enzymatic activity after BCR crosslinking (9), as well as with positive regulators of signaling such as Lyn, Syk, phosphatidylinositol 3-kinase, and phospholipase C-γ1 (PLC-γ1) (11, 13, 14). In contrast with CD22, B cells from CD19-deficient mice are hypo-responsive to transmembrane signals (15–18). The ≈240-aa cytoplasmic domain of CD19 includes nine tyrosine residues that are targets for rapid phosphorylation after BCR or CD19 ligation (3). CD19 positively regulates BCR signaling through interactions with the proteins Vav, Lyn, Fyn, Lck, phosphatidylinositol 3-kinase, and PLC-γ1. To identify signal transduction intermediates responsible for the reciprocal regulation of BCR signaling in CD22- and CD19-deficient mice, signal transduction pathways activated after BCR crosslinking in CD22- and CD19-deficient B cells were examined. These comparisons revealed that tyrosine phosphorylation of Vav was uniquely augmented after BCR crosslinking in CD22-deficient B cells, yet was diminished in CD19-deficient B cells.
MATERIALS AND METHODS
Mice.
CD22-deficient and CD19-deficient mice (129 × C57BL/6) were as described (6, 15). All experiments used 2-month-old mice housed in a specific pathogen-free barrier facility. Wild-type littermates generated from heterozygous matings were used as control mice. All procedures were approved by the Animal Care and Use Committee of Duke University.
Antibodies.
Antibodies used in this study included: anti-PLC-γ2, anti-Syk, and anti-Vav (Santa Cruz Biotechnology); anti-mitogen-activated protein kinase (MAPK), anti-SHP1 (Upstate Biotechnology, Lake Placid, NY); anti-Lyn (PharMingen); anti-SH2-containing inositol phosphatase (SHIP) (Stem Cell Technologies, Vancouver, Canada); horseradish peroxidase-conjugated donkey anti-rabbit IgG antibodies (Jackson ImmunoResearch); anti-CD22 (NIM-R6, generously provided by M. Parkhouse, Pirbright, England); and anti-CD79a (MB-1) antibodies (generously provided by L. Matsuuchi, University of British Columbia, Vancouver, Canada).
B Cell Isolation and Activation.
Spleen B cells were purified (>95% B220+) from single-cell suspensions by removing T cells with anti-Thy1.2 antibody-coated magnetic beads (Dynal). B cells were resuspended (2 × 107/ml) into RPMI 1640 medium (Life Technologies, Gaithersburg, MD) containing 5% (vol/vol) fetal calf serum (Sigma) at 37°C and stimulated with 40 or 10 μg/ml of F(ab′)2 fragments of goat anti-mouse IgM antibodies (Cappel), 60 or 10 μg/ml of mouse anti-CD19 antibodies (MB19–1, IgA), or both as described (6, 19).
Immunoprecipitation and Western Blotting.
B cells were lysed in buffer containing 1% Nonidet P-40, 150 mM NaCl, 50 mM Tris⋅HCl (pH 8.0), 1 mM sodium orthovanadate, 2 mM EDTA, 50 mM NaF, and protease inhibitors (20). Protein concentrations were determined by light absorbance at 280 nm. The lysates either were analyzed by SDS/PAGE or subjected to immunoprecipitation. The cell lysates were precleared twice by mixing with appropriate control antibodies plus protein A- or protein G-Sepharose beads (Pharmacia) for 2 hr at 4°C. For immunoprecipitation, the precleared lysates were mixed with protein A- or protein G-beads plus specific antibodies, rabbit antiserum, or rat antiserum overnight at 4°C before washing with lysis buffer. Immunoprecipitated proteins were subjected to SDS/PAGE and then electrophoretic transfer to nitrocellulose membranes. The immunoblots were incubated with horseradish peroxidase-conjugated anti-phosphotyrosine antibody (4G10; Upstate Biotechnology). These blots were developed by using an enhanced chemiluminescence kit (Amersham). To verify equivalent amounts of protein in each lane, the same blots were stripped and reprobed with antibodies against proteins of interest. In some cases, anti-MAPK antibodies were used for reprobing, because the blots were unevenly stripped of anti-phosphotyrosine antibodies.
RESULTS
Tyrosine Phosphorylation in CD22-Deficient B Cells.
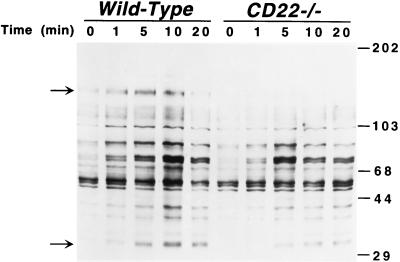
After BCR crosslinking, the spectra of tyrosine-phosphorylated proteins observed in purified splenic B cells from CD22-deficient mice were different from those observed in B cells from wild-type littermates (Fig. 1). Most notable was the loss of a ≈135-kDa phosphoprotein in CD22-deficient B cells (top arrow), which was probably CD22 because its migration was identical to that of CD22 (data not shown). Decreased phosphorylation of the ≈34-kDa (CD79a, MB-1) component of the BCR complex (Fig. 1, lower arrow) also was observed. Consistent with CD22-deficient B cells hyper-responsiveness to BCR ligation, overall tyrosine phosphorylation of proteins was maximal within approximately 5 min in CD22-deficient B cells but was maximal at approximately 10 min in wild-type B cells. The difference in kinetics of tyrosine phosphorylation was especially obvious for molecules of 85, 74, and 72 kDa (Fig. 1). These results showed that the loss of CD22 resulted in more rapid phosphorylation of subsets of cellular proteins and lower tyrosine phosphorylation of other molecules.
Figure 1.
BCR-induced protein tyrosine phosphorylation in B cells from CD22-deficient mice (CD22−/−) and wild-type littermates. Purified splenic B cells were incubated with either medium or anti-IgM antibodies (40 μg/ml) at 37°C. Cells (107) were removed from the cultures at the indicated times and solubilized. Cell lysates (40 μg/lane) were subjected to SDS/PAGE and transferred electrophoretically to a membrane for subsequent anti-phosphotyrosine immunoblotting. Molecular weight standards (×10−3) are shown on the right. Arrows indicate major bands that were different between CD22-deficient and wild-type B cells. Results are representative of those obtained with three littermate pairs of mice.
Signaling Pathways in CD22-Deficient B Cells.
Signaling molecules affected by the loss of CD22 were identified by assessing tyrosine phosphorylation of effector molecules immunoprecipitated from lysates of B cells purified from CD22-deficient or wild-type littermates. Vav was the only protein identified that was more intensity phosphorylated in CD22-deficient B cells after BCR crosslinking than in wild-type B cells (Fig. 2A). Longer autoradiography of Vav immunoprecipitates revealed that Vav was tyrosine phosphorylated at relatively low levels in resting B cells from both CD22-deficient and wild-type mice (Fig. 2A and data not shown). By contrast, tyrosine phosphorylation of PLC-γ2 was diminished at later time points (10 min) after stimulation of CD22-deficient B cells (Fig. 2B). Tyrosine phosphorylation of CD79a, CD79b, and SHIP also was decreased in CD22-deficient B cells (Fig. 2C). The Src-family protein tyrosine kinases Lyn, Fyn, and Blk were comparably tyrosine phosphorylated in CD22-deficient and wild-type B cells (Fig. 2A and data not shown), as were Syk (Fig. 2A) and SHP1 (Fig. 2D). There was no difference between CD22-deficient and wild-type B cells in activation of MAPK after BCR ligation, as determined by tyrosine phosphorylation and in vitro kinase assays (data not shown). Thus, CD22 negatively regulates BCR-induced tyrosine phosphorylation of Vav but positively regulates phosphorylation of CD79a, CD79b, SHIP, and PLC-γ2.
Figure 2.
Tyrosine phosphorylation of (A) Vav, Syk, and Lyn; (B) PLC-γ2; (C) SHIP and CD79a; and (D) SHP1 after BCR crosslinking in B cells from CD22-deficient mice and wild-type littermates. Purified splenic B cells (107/lane) were incubated with anti-IgM antibodies and processed as in Fig. 1. Proteins of interest were immunoprecipitated from cell lysates with specific antibodies either alone or in combination, as indicated. Control immunoprecipitations of lysates from unstimulated wild-type B cells with normal rabbit serum are shown (CTL). In some cases (A and C), MAPK was also simultaneously immunoprecipitated from the lysates. Immunoprecipitated proteins were fractionated by SDS/PAGE and transferred onto a membrane for subsequent anti-phosphotyrosine immunoblotting (Upper). All blots subsequently were stripped of anti-phosphotyrosine antibody and reprobed with precipitating antibodies to quantify the amount of proteins within the immunoprecipitates (Lower). Results are representative of those obtained with at least three littermate pairs of mice for each molecule shown. The same results were obtained when Vav, Syk, Lyn, SHIP, or CD79a were immunoprecipitated individually from lysates (data not shown).
Tyrosine Phosphorylation in CD19-Deficient B Cells.
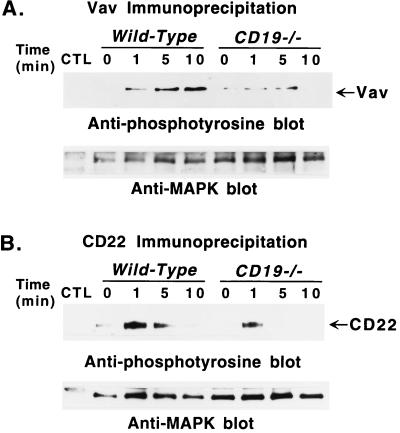
In contrast to what was observed in CD22-deficient B cells, BCR-induced Vav phosphorylation was significantly lower in CD19-deficient B cells relative to wild-type B cells 5 and 10 min after BCR crosslinking (Fig. 3A). On average, Vav was phosphorylated at similar low levels in unstimulated CD19-deficient and wild-type B cells. Tyrosine phosphorylation of CD22 also was decreased in B cells from CD19-deficient mice (Fig. 3B). As in CD22-deficient B cells, phosphorylation of CD79a, CD79b, SHIP, and PLC-γ2 was decreased in CD19-deficient B cells after BCR crosslinking (data not shown). These results show that CD19 positively regulates BCR-induced tyrosine phosphorylation of Vav as well as phosphorylation of CD79a, CD79b, SHIP, and PLC-γ2.
Figure 3.
Tyrosine phosphorylation of (A) Vav and (B) CD22 after BCR crosslinking in B cells from CD19-deficient (CD19−/−) mice and wild-type littermates. Purified splenic B cells (107/lane) were incubated with anti-IgM antibodies (40 μg/ml) and processed as in Fig. 1. Proteins of interest were immunoprecipitated with specific antibodies plus anti-MAPK antibodies, normal rabbit serum (CTL, A), or normal rat serum (CTL, B), as in Fig. 2. Immunoprecipitated proteins were fractionated by SDS/PAGE and transferred onto a membrane for subsequent anti-phosphotyrosine immunoblotting (Upper). Subsequently, all blots were stripped of anti-phosphotyrosine antibody and reprobed with anti-MAPK antibodies to quantitate the amount of proteins within the immunoprecipitates (Lower). These results are representative of those obtained with three littermate pairs of mice.
CD19 and CD22 Differentially Regulate Vav Tyrosine Phosphorylation.
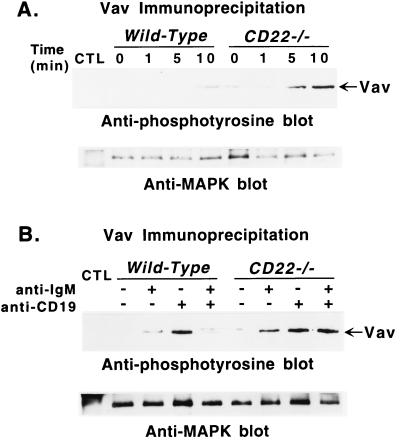
To determine if CD19 directly regulates tyrosine phosphorylation of Vav, purified B cells from CD22-deficient and wild-type littermates were stimulated with anti-CD19 antibodies. CD19 ligation alone induced more tyrosine phosphorylation of Vav in CD22-deficient B cells than in wild-type B cells (Fig. 4A). Although CD19 crosslinking was more efficient in inducing Vav phosphorylation than BCR crosslinking (Fig. 4B), CD19 crosslinking did not augment tyrosine phosphorylation of CD22 (data not shown). A surprising result was that stimulation of wild-type B cells with suboptimal concentrations of anti-IgM and anti-CD19 antibodies resulted in decreased tyrosine phosphorylation of Vav when compared with stimulation by anti-IgM or anti-CD19 antibodies alone (Fig. 4B). By contrast, coligation of CD19 and BCR did not down-regulate Vav tyrosine phosphorylation in CD22-deficient B cells (Fig. 4B). That simultaneous crosslinking of the BCR and CD19 did not induce additive phosphorylation of Vav in CD22-deficient B cells was consistent with CD19 being the most efficient inducer of Vav phosphorylation (Fig. 4B). Thus, CD19 expression positively regulates tyrosine phosphorylation of Vav after BCR crosslinking, whereas CD22 expression negatively regulates Vav phosphorylation.
Figure 4.
Tyrosine phosphorylation of Vav after (A) ligation of CD19 or (B) coligation of BCR and CD19 in B cells from CD22-deficient mice and wild-type littermates. (A) Purified splenic B cells were incubated with either medium or optimal concentrations (60 μg/ml) of anti-CD19 antibodies. Cells (107) were removed from the cultures at the indicated times and solubilized. (B) B cells also were stimulated by suboptimal concentrations of either anti-CD19 antibodies (10 μg/ml), or anti-IgM antibodies (10 μg/ml), or both, as indicated, for 5 min before solubilization. Lysates were immunoprecipitated with anti-Vav antibodies, plus anti-MAPK antibodies or normal rabbit serum (CTL) as in Fig. 2. Immunoprecipitated proteins were fractionated by SDS/PAGE and transferred onto a membrane for subsequent anti-phosphotyrosine immunoblotting (Upper). All blots were subsequently stripped of anti-phosphotyrosine antibody and reprobed with anti-MAPK antibodies to quantify the amount of proteins within the immunoprecipitates (Lower). These results are representative of those obtained with at least three littermate pairs of mice.
DISCUSSION
These studies reveal that CD22 and CD19 expression have dramatic effects on the intracellular signaling pathways activated in primary B cells after BCR ligation. CD22 loss resulted in lower tyrosine phosphorylation of subsets of molecules and more rapid phosphorylation of other cellular proteins (Fig. 1). Vav was the only protein identified that was more intensely phosphorylated in CD22-deficient B cells after BCR crosslinking. Phosphorylation of PLC-γ2, CD79a, CD79b, and SHIP was decreased in CD22-deficient B cells after BCR crosslinking, whereas Syk, Lyn, Fyn, Blk, and SHP-1 were comparably phosphorylated in CD22-deficient and wild-type B cells (Fig. 2). Decreased tyrosine phosphorylation of CD79a and CD79b correlates with the modest proliferative response observed for CD22-deficient B cells after prolonged IgM crosslinking (6, 7). Augmented intracellular Ca2+ responses observed in CD22-deficient B cells are not explained by decreased tyrosine phosphorylation of PLC-γ2. However, decreased phosphorylation of SHIP could explain this increase, because activated SHIP blocks intracellular Ca2+ responses by decreasing the availability of the major PLC-γ2 substrate, phosphatidylinositol-4,5-bisphosphate (21). The positive regulatory role for CD19 in B-cell function is supported by the finding that tyrosine phosphorylation of Vav, CD22, PLC-γ2, CD79a, CD79b, and SHIP were decreased in CD19-deficient B cells after BCR crosslinking (Fig. 3 and data not shown). Although Vav was not the only signaling molecule affected by the loss of CD19 or CD22, the finding that Vav was differentially phosphorylated in CD19-deficient and CD22-deficient B cells implicates Vav as a possible regulator of signaling thresholds in B cells.
Vav is expressed specifically in hematopoietic cells and is heavily phosphorylated on tyrosine residue(s) in response to BCR ligation or activation of other signaling pathways (22, 23). Vav is an activator of Ras and acts as a guanine-nucleotide-exchanging factor for Rac-1 through its Dbl-homologous domain (23). The guanine-nucleotide-exchanging activity of Vav is directly and positively modulated by its tyrosine phosphorylation (24). Thus, the differential phosphorylation of Vav in CD19-deficient and CD22-deficient B cells observed in this study is physiologically relevant. At a minimum, these studies demonstrate that Vav is an intermediate in the signaling pathways of both CD19 and CD22 and is likely to be important for regulating signaling thresholds via CD19 and CD22. In support of this notion, Vav-deficient mice exhibit two characteristic features: severely decreased proliferative responses after BCR ligation, and a marked loss of B-1 cells in the peritoneum (25, 26). CD19-deficient mice also exhibit severely decreased proliferative responses after BCR ligation and a marked loss of B-1 cells in the peritoneum (15, 16, 19). Consistent with Vav being more intensely phosphorylated in CD22-deficient B cells after BCR crosslinking, B cells from CD22-deficient mice generate augmented intracellular Ca2+ responses after BCR crosslinking and have increased numbers of peritoneal B-1 cells (6, 8). These findings demonstrate that Vav, CD19, and CD22 are likely to regulate similar signaling pathways.
The differential regulation of Vav tyrosine phosphorylation by CD19 and CD22 (Figs. 2A and 3A) indicates a novel model for B cell activation. Crosslinking BCR or CD19 alone activates tyrosine kinases that phosphorylate CD19 (27, 28). CD19 phosphorylation recruits Vav into a plasma membrane-bound CD19 signaling complex via SH2 interactions, which facilitates the subsequent phosphorylation of Vav by activated tyrosine kinases (29). Consistent with this process, CD19 crosslinking was more efficient than BCR crosslinking at inducing Vav phosphorylation (Fig. 4B); and Vav phosphorylation was modest and transient in CD19-deficient B cells (Fig. 3A). Vav could be subsequently dephosphorylated by SHP1 bound to tyrosine-phosphorylated CD22, because BCR- and CD19-induced tyrosine phosphorylation of Vav was augmented in CD22-deficient B cells (Figs. 2A and 4A). The previously observed association of Vav with SHP1 in hematopoietic cells supports the notion of dephosphorylation of Vav by SHP1 (30). Because CD19 crosslinking did not induce or augment tyrosine phosphorylation of CD22, BCR crosslinking is likely to induce the CD22-SHP1 association preferentially. This model explains why the simultaneous crosslinking of CD19 with BCR resulted in a substantial decrease in Vav phosphorylation when CD22 was expressed, but not in CD22-deficient B cells (Fig. 4B). Because Vav was the only signaling component identified as differentially regulated by CD19 and CD22, this model also explains some of the reciprocal characteristics of B cells from CD22- and CD19-deficient mice. Thus, the differential regulation of Vav function in vivo by CD19 and CD22 may provide a novel molecular mechanism by which BCR signaling thresholds are controlled in B lymphocytes. Altered signaling thresholds are envisioned to lead to abnormal B cell selection or clonal expansion (1). Therefore, because altered CD19 signaling thresholds correlate with autoantibody production in mice (19, 31), alterations in CD19, CD22, and Vav interactions may be implicated in autoimmunity and other defects in humoral immunity.
Acknowledgments
We thank Dr. Billie Maciunas for help with preparation of this manuscript. This work was supported by National Institutes of Health Grants AI26872, HL50985, and CA54464.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: BCR, B lymphocyte antigen receptor; MAPK, mitogen-activated protein kinase; PLC-γ, phospholipase C-γ; SHIP, SH2-containing inositol phosphatase; SHP1, SH-protein tyrosine phosphatase-1.
References
- 1.Goodnow C C. Proc Natl Acad Sci USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tedder T F, Tuscano J, Sato S, Kehrl J H. Annu Rev Immunol. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- 3.Tedder T F, Inaoki M, Sato S. Immunity. 1997;6:107–118. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 4.Cyster J G, Goodnow C C. Immunity. 1997;6:509–517. doi: 10.1016/s1074-7613(00)80339-8. [DOI] [PubMed] [Google Scholar]
- 5.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers M C. Curr Biol. 1997;7:133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Miller A S, Inaoki M, Bock C B, Jansen P J, Tang M L K, Tedder T F. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 7.Otipoby K L, Andersson K B, Draves K E, Klaus S J, Farr A G, Kerner J D, Perlmutter R M, Law C-L, Clark E A. Nature (London) 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 8.O’Keefe T L, Williams G T, Davies S L, Neuberger M S. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 9.Doody G M, Justement L B, Delibrias C C, Mathews R J, Lin J, Thomas M L, Fearon D T. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 10.Lankester A C, van Schijndel G M, van Lier R A. J Biol Chem. 1995;270:20305–20308. doi: 10.1074/jbc.270.35.20305. [DOI] [PubMed] [Google Scholar]
- 11.Law C-L, Sidorenko S P, Chandran K A, Zhao Z, Shen S-H, Fischer E H, Clark E A. J Exp Med. 1996;183:547–560. doi: 10.1084/jem.183.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell M A, Klinman N R. Eur J Immunol. 1995;25:1573–1579. doi: 10.1002/eji.1830250616. [DOI] [PubMed] [Google Scholar]
- 13.Tuscano J M, Engel P, Tedder T F, Agarwal A, Kehrl J H. Eur J Immunol. 1996;26:1246–1252. doi: 10.1002/eji.1830260610. [DOI] [PubMed] [Google Scholar]
- 14.Leprince C, Draves K E, Geahlen R L, Ledbetter J A, Clark E A. Proc Natl Acad Sci USA. 1993;90:3236–3240. doi: 10.1073/pnas.90.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel P, Zhou L-J, Ord D C, Sato S, Koller B, Tedder T F. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 16.Rickert R C, Rajewsky K, Roes J. Nature (London) 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Steeber D A, Tedder T F. Proc Natl Acad Sci USA. 1995;92:11558–11562. doi: 10.1073/pnas.92.25.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato S, Steeber D A, Jansen P J, Tedder T F. J Immunol. 1997;158:4662–4669. [PubMed] [Google Scholar]
- 19.Sato S, Ono N, Steeber D A, Pisetsky D S, Tedder T F. J Immunol. 1996;156:4371–4378. [PubMed] [Google Scholar]
- 20.Bradbury L, Kansas G S, Levy S, Evans R L, Tedder T F. J Immunol. 1992;149:2841–2850. [PubMed] [Google Scholar]
- 21.Scharenberg A M, Kinet J-P. Cell. 1996;87:961–964. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]
- 22.Bustelo X R, Barbacid M. Science. 1992;256:1196–1199. doi: 10.1126/science.256.5060.1196. [DOI] [PubMed] [Google Scholar]
- 23.Collins T L, Deckert M, Altman A. Immunol Today. 1997;18:221–225. doi: 10.1016/s0167-5699(97)01037-2. [DOI] [PubMed] [Google Scholar]
- 24.Crespo P, Schuebel K E, Ostrom A A, Gutkind J S, Bustelo X R. Nature (London) 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 25.Tarakhovsky A, Turner M, Shaal S, Mee P J, Duddy L P, Fajewsky K, Tybulewicz V. Nature (London) 1995;374:467–470. doi: 10.1038/374467a0. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Alt F W, Davidson L, Orkin S H, Swat W. Nature (London) 1995;374:470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]
- 27.Chalupny N J, Kanner S B, Schieven G L, Wee S, Gilliland L K, Aruffo A, Ledbetter J A. EMBO J. 1993;12:2691–2696. doi: 10.1002/j.1460-2075.1993.tb05930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uckun F M, Burkhardt A L, Jarvis L, Jun X, Stealey B, Dibirdik I, Myers D E, Tuel-Ahlgren L, Bolen J B. J Biol Chem. 1993;268:21772–21184. [PubMed] [Google Scholar]
- 29.Weng W K, Jarvis L, LeBien T W. J Biol Chem. 1994;269:32514–32521. [PubMed] [Google Scholar]
- 30.Kon-Kozlowski M, Pani G, Pawson T, Siminovitch K A. J Biol Chem. 1996;271:3856–3862. doi: 10.1074/jbc.271.7.3856. [DOI] [PubMed] [Google Scholar]
- 31.Inaoki, M., Sato, S., Weintraub, B. C., Goodnow, C. C. & Tedder, T. F. J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]