Abstract
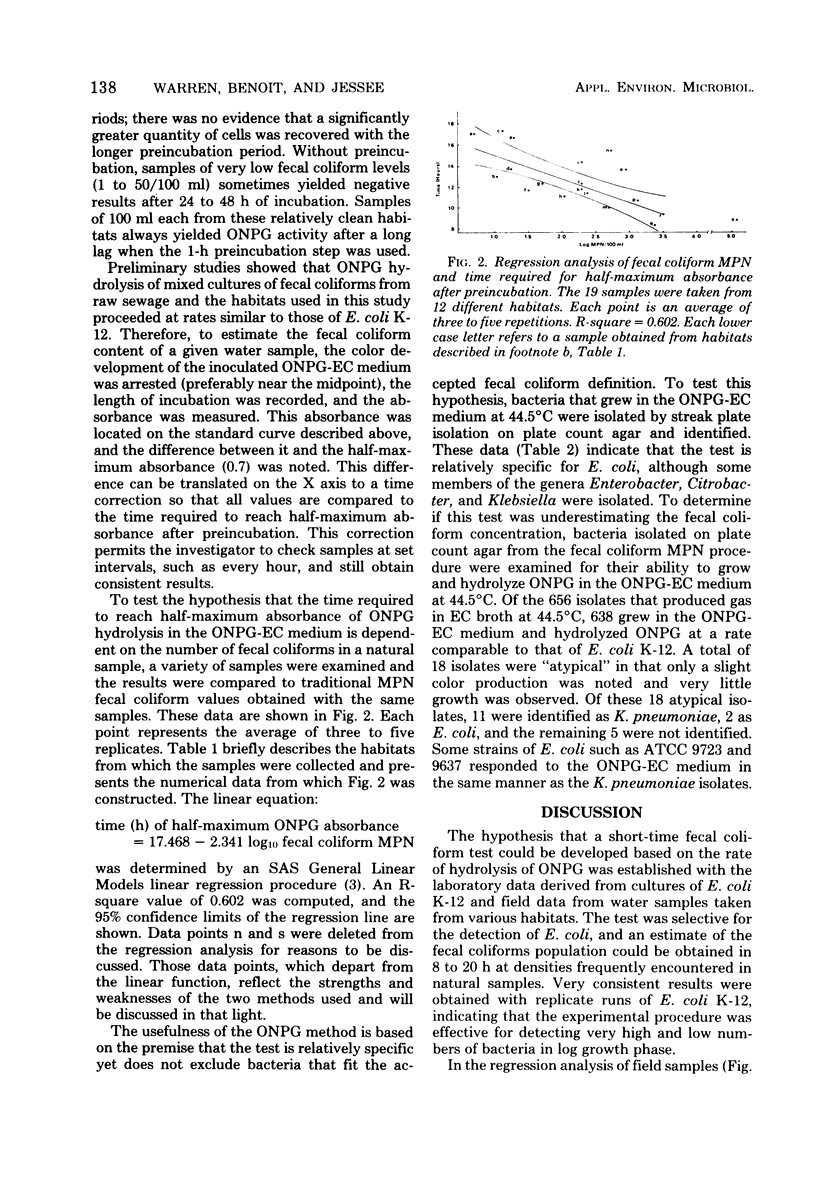
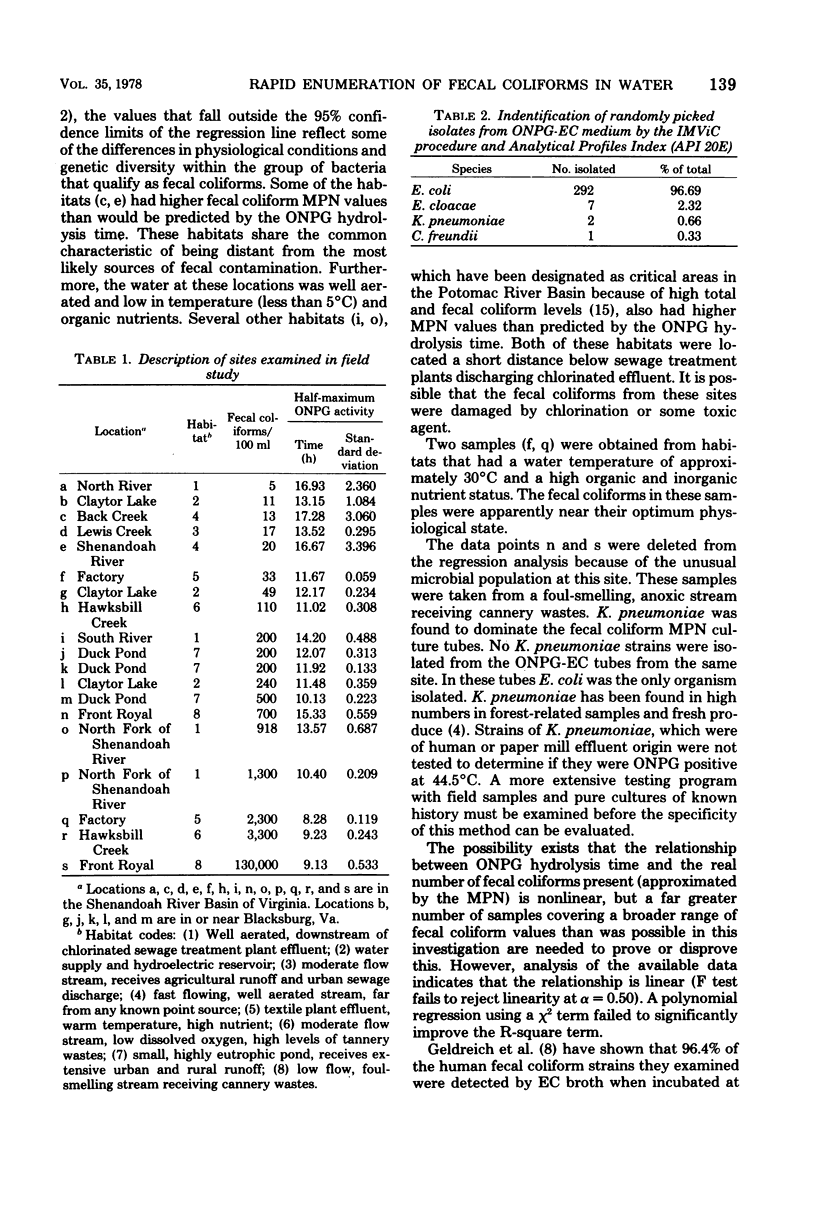
The colorimetric beta-galactosidase assay is based upon the enzymatic hydrolysis of the substrate o-nitrophenyl-beta-D-galactoside (ONPG) by fecal coliforms. This technique provides an estimate of the fecal coliform concentration within 8 to 20 h. A 100-ml portion of test sample was passed through a 0.45 micrometer membrane filter. This filter was then incubated at 37 degrees C for 1 h in EC medium followed by the addition of filter-sterilized ONPG. The incubation was continued at 44.5 degrees C until a half-maximum absorbance (at 420 nm) was reached. The time between the start of incubation and the half-maximum absorbance was proportional to the concentration of fecal coliforms present. Escherichia coli (K-12) was used to measure the kinetics of substrate hydrolysis and the response time of different cell concentrations. High cell densities produced an immediate response, whereas 1 cell/ml will produce a response in less than 20 h. In field studies in which samples were taken from a range of grossly polluted streams to relatively clean lake water, a linear correlation between ONPG hydrolysis times and fecal coliform most-probable-number values was established. A total of 302 isolates randomly selected from positive ONPG-EC media, which were derived from 11 different habitats, were identified as E. coli (96.69 percent), Enterobacter cloacae (2.32 percent), Klebsiella pneumoniae (0.66 percent), and Citrobacter freundii (0.33 percent).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachrach U., Bachrach Z. Radiometric method for the detection of coliform organisms in water. Appl Microbiol. 1974 Aug;28(2):169–171. doi: 10.1128/am.28.2.169-171.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan D. W., Razzell W. E. Klebsiella biotypes among coliforms isolated from forest environments and farm produce. Appl Microbiol. 1972 Dec;24(6):933–938. doi: 10.1128/am.24.6.933-938.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldreich E. E., Jeter H. L., Winter J. A. Technical considerations in applying the membrane filter procedure. Health Lab Sci. 1967 Apr;4(2):113–125. [PubMed] [Google Scholar]
- Hendricks C. W. Formic hydrogenlyase induction as a basis for the Eijkman fecal coliform concept. Appl Microbiol. 1970 Mar;19(3):441–445. doi: 10.1128/am.19.3.441-445.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. A., Pivnick H. Use of autocytotoxic beta-D-galactosides for selective growth of Salmonella typhimurium in the presence of coliforms. Can J Microbiol. 1970 Feb;16(2):83–89. doi: 10.1139/m70-015. [DOI] [PubMed] [Google Scholar]
- Levin M. A., Fischer J. R., Cabelli V. J. Quantitative large-volume sampling technique. Appl Microbiol. 1974 Sep;28(3):515–517. doi: 10.1128/am.28.3.515-517.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. D. Membrane filter method for recovery of fecal coliforms in chlorinated sewage effluents. Appl Environ Microbiol. 1976 Oct;32(4):547–552. doi: 10.1128/aem.32.4.547-552.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


