Abstract
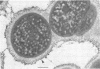
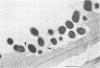
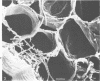
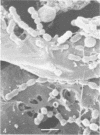
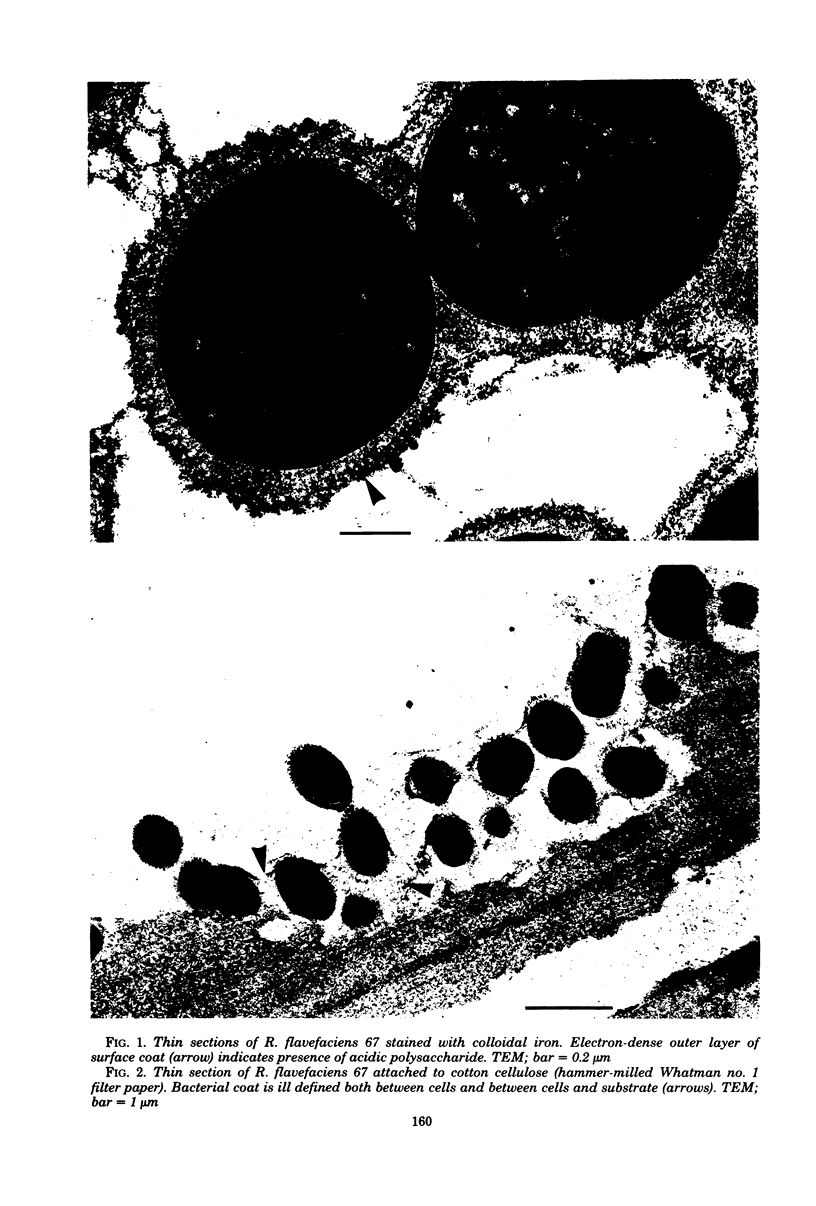
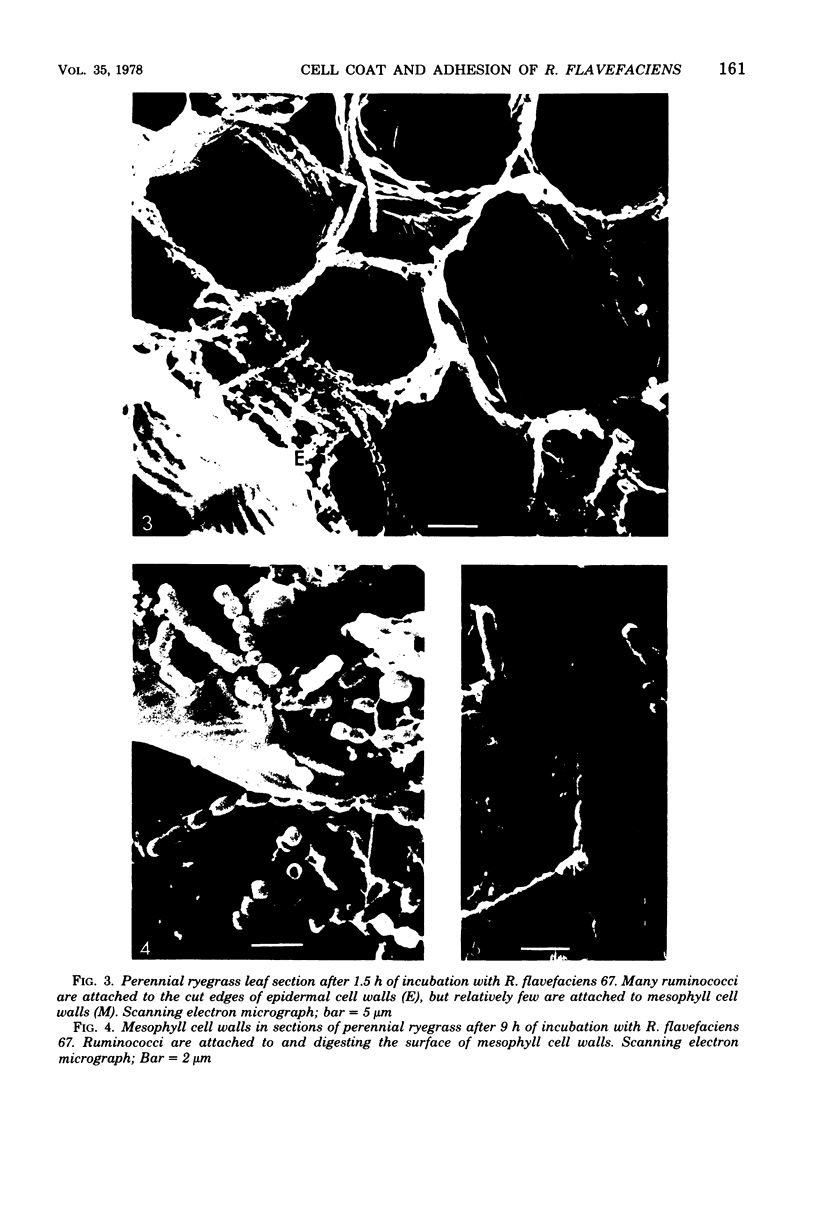
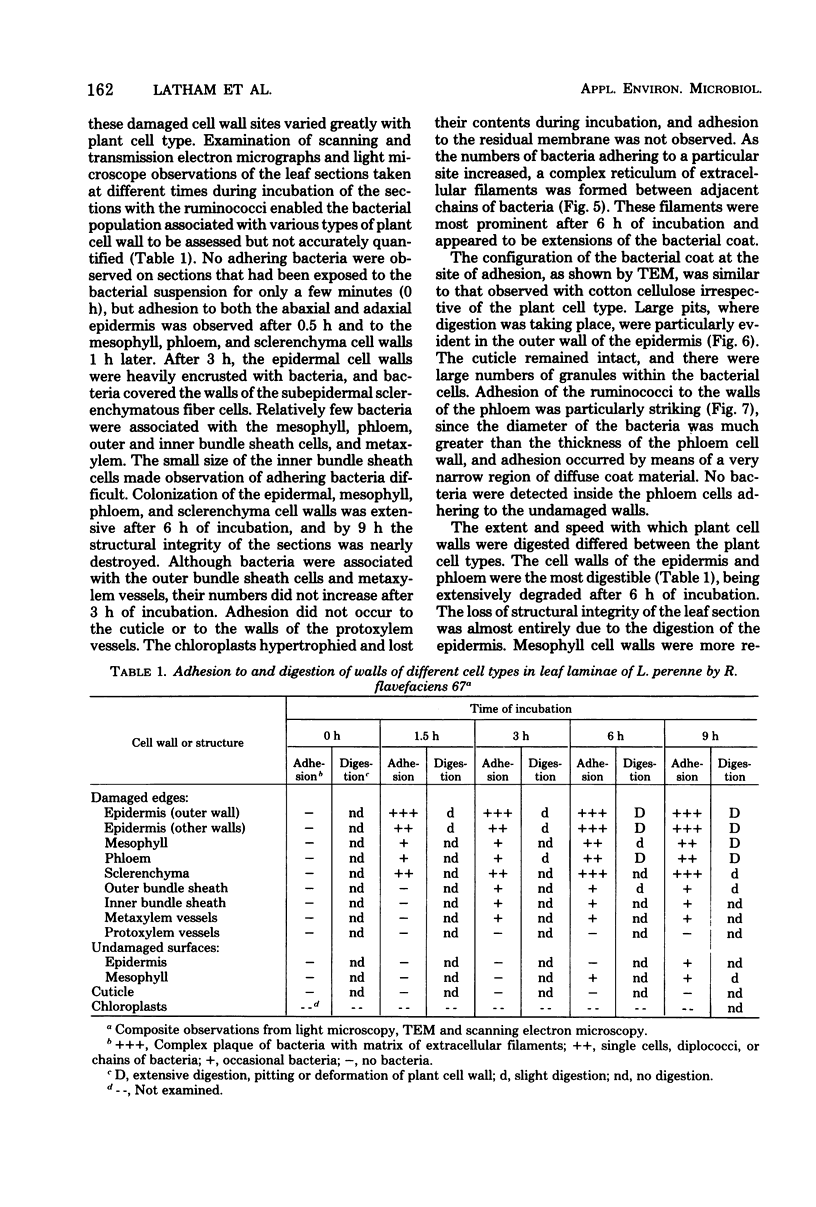
Ruminococcus flavefaciens was shown to possess a prominent glycoprotein coat, which contained rhamnose, glucose, and galactose as its principal carbohydrates. Periodate-reactive carbohydrate occurred as a surface layer of the coat. The ruminococci adhered strongly by means of this coat to cotton cellulose and to cell walls in leaf sections of Lolium perenne L. (perennial ryegrass). The coat was diffuse at the point of contact so that the bacterial cell wall was in close contact with the substrate. Adhesion was influenced by the availability of damaged plant cell walls and by the cell wall type and occurred most rapidly to cell walls of the epidermis and sclerenchyma, followed by the phloem and mesophyll. Plaques of bacteria with filamentous coat extensions developed on all these tissues. The bacteria did not readily adhere to the walls of the bundle sheath cells or metaxylem or protoxylem vessels and did not adhere to the cuticle or chloroplasts. The epidermal and phloem cell walls were more rapidly digested than the walls of other cell types.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Amos H. E. Rumen bacterial degradation of forage cell walls investigated by electron microscopy. Appl Microbiol. 1975 May;29(5):692–701. doi: 10.1128/am.29.5.692-701.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Ultrastructure of rumen bacterial attachment to forage cell walls. Appl Environ Microbiol. 1976 Apr;31(4):562–568. doi: 10.1128/aem.31.4.562-568.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg B., von Hofsten B., Pettersson G. Electronmicroscopie observations on the degradation of cellulose fibres by Cellvibrio fulvus and Sporocytophaga myxococcoides. J Appl Bacteriol. 1972 Jun;35(2):215–219. doi: 10.1111/j.1365-2672.1972.tb03692.x. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Ultrastructure of cell envelopes of bacteria of the bovine rumen. Appl Microbiol. 1975 Jun;29(6):841–849. doi: 10.1128/am.29.6.841-849.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Damgaard H. N., Cheng K. J. Cell envelope morphology of rumen bacteria. J Bacteriol. 1974 Jun;118(3):1132–1143. doi: 10.1128/jb.118.3.1132-1143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. S. Sodium borohydride as an aldehyde blocking reagent for electron microscope histochemistry. Histochemistry. 1974;42(2):141–144. doi: 10.1007/BF00533265. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- HALLIWELL G. The action of cellulolytic enzymes from Myrothecium verrucaria. Biochem J. 1961 Apr;79:185–192. doi: 10.1042/bj0790185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifkovits R. W., Ragheb H. S. Cellular fatty acid composition and identification of rumen bacteria. Appl Microbiol. 1968 Sep;16(9):1406–1413. doi: 10.1128/am.16.9.1406-1413.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latham M. J., Sharpe M. E., Sutton J. D. The microbial flora of the rumen of cows fed hay and high cereal rations and its relationship to the rumen fermentation. J Appl Bacteriol. 1971 Jun;34(2):425–434. doi: 10.1111/j.1365-2672.1971.tb02302.x. [DOI] [PubMed] [Google Scholar]
- Latham M. J., Storry J. E., Sharpe M. E. Effect of low-roughage diets on the microflora and lipid metabolism in the rumen. Appl Microbiol. 1972 Dec;24(6):871–877. doi: 10.1128/am.24.6.871-877.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. J., Spicer S. S. Letter: Concanavalin A-iron dextran technique for staining cell surface mucosubstances. J Histochem Cytochem. 1974 Mar;22(3):206–207. doi: 10.1177/22.3.206. [DOI] [PubMed] [Google Scholar]
- Muller L. L., Jacks T. J. Rapid chemical dehydration of samples for electron microscopic examinations. J Histochem Cytochem. 1975 Feb;23(2):107–110. doi: 10.1177/23.2.1117127. [DOI] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]