Abstract
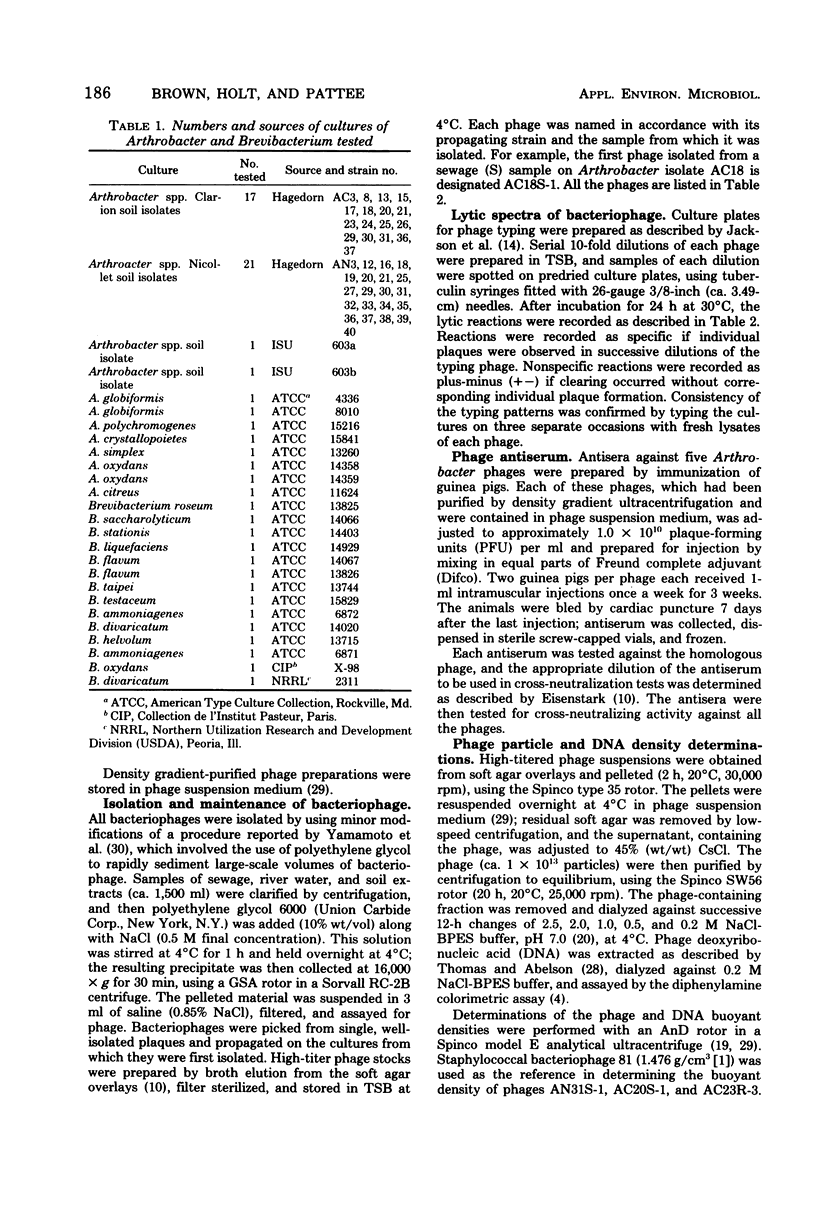
Seventeen bacteriophages, active against 19 Arthrobacter soil isolates, were isolated from concentrated samples of river water and sewage. Attempts to isolate Arthrobacter bacteriophages from filtrates of broth cultures of the soil isolates or from ultraviolet light-irradiated cultures were unsuccessful. Bacteriophages were not detected in either concentrated or unconcentrated soil extracts. Electron microscopic studies of 11 phages showed morphologies characteristic of Bradley's groups B (exhibited by 9 phages) and C (exhibited by 2 phages). Moles percent guanine plus cytosine, calculated from the deoxyribonucleic acid density of three phages, ranged from 60.2 to 65.3. The phages were characterized by their plague and virion morphology, host range, and serological specificity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill D. L., Pattee P. A. Buoyant density analysis of staphylococcal bacteriophage 80 transducing particles. J Virol. 1969 Nov;4(5):804–806. doi: 10.1128/jvi.4.5.804-806.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida L. E., Liu K. C. Arthrobacter globiformis and Its Bacteriophage in Soil. Appl Microbiol. 1974 Dec;28(6):951–959. doi: 10.1128/am.28.6.951-959.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn H. J., Bottcher E. J., Randall C. The Value of Bacteriophage in Classifying Certain Soil Bacteria. J Bacteriol. 1945 Apr;49(4):359–373. doi: 10.1128/jb.49.4.359-373.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAEMS W. T. A preliminary report on the fine structure of a bacteriophage of Arthrobacter polychromogenes Schippers-Lammertse, Muysers et Klatser-Oedekerk. Antonie Van Leeuwenhoek. 1963;29:16–21. doi: 10.1007/BF02046034. [DOI] [PubMed] [Google Scholar]
- Einck K. H., Pattee P. A., Holt J. G., Hagedorn C., Miller J. A., Berryhill D. L. Isolation and characterization of a bacteriophage of Arthrobacter globiformis. J Virol. 1973 Nov;12(5):1031–1033. doi: 10.1128/jvi.12.5.1031-1033.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn C., Holt J. G. A nutritional and taxonomic survey of Arthrobacter soil isolates. Can J Microbiol. 1975 Mar;21(3):353–361. doi: 10.1139/m75-050. [DOI] [PubMed] [Google Scholar]
- Horvath R. S., Alexander M. Cometabolism of m-chlorobenzoate by an Arthrobacter. Appl Microbiol. 1970 Aug;20(2):254–258. doi: 10.1128/am.20.2.254-258.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON G. G., DOWLING H. F., LEPPER M. H. Bacteriophage typing of staphylococci. I. Technique and patterns of lysis. J Lab Clin Med. 1954 Jul;44(1):14–28. [PubMed] [Google Scholar]
- Keddie N. C., Taylor A. W., Sykes P. A. The termination of the common bile duct. Br J Surg. 1974 Aug;61(8):623–625. doi: 10.1002/bjs.1800610808. [DOI] [PubMed] [Google Scholar]
- Loos M. A., Roberts R. N., Alexander M. Phenols as intermediates in the decomposition of phenoxyacetates by an Arthrobacter species. Can J Microbiol. 1967 Jun;13(6):679–690. doi: 10.1139/m67-090. [DOI] [PubMed] [Google Scholar]
- Owens J. D., Keddie R. M. The nitrogen nutrition of soil and herbage coryneform bacteria. J Appl Bacteriol. 1969 Sep;32(3):338–347. doi: 10.1111/j.1365-2672.1969.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Ritchie A. E., Fernelius A. L. Characterization of bovine viral diarrhea viruses. V. Morphology of characteristic particles studied by electron microscopy. Arch Gesamte Virusforsch. 1969;28(3):369–389. doi: 10.1007/BF01240951. [DOI] [PubMed] [Google Scholar]
- Sethunathan N., Pathak M. D. Development of a diazinon-degrading bacterium in paddy water after repeated applications of diazinon. Can J Microbiol. 1971 May;17(5):699–702. doi: 10.1139/m71-112. [DOI] [PubMed] [Google Scholar]
- Skyring G. W., Quadling C. Soil bacteria: comparisons of rhizosphere and nonrhizosphere populations. Can J Microbiol. 1969 May;15(5):473–488. doi: 10.1139/m69-083. [DOI] [PubMed] [Google Scholar]
- Stevenson I. L. Utilization of aromatic hydrocarbons by Arthrobacter spp. Can J Microbiol. 1967 Feb;13(2):205–211. doi: 10.1139/m67-027. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



