Abstract
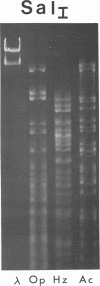
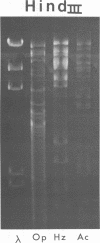
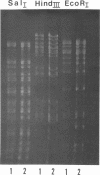
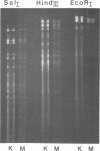
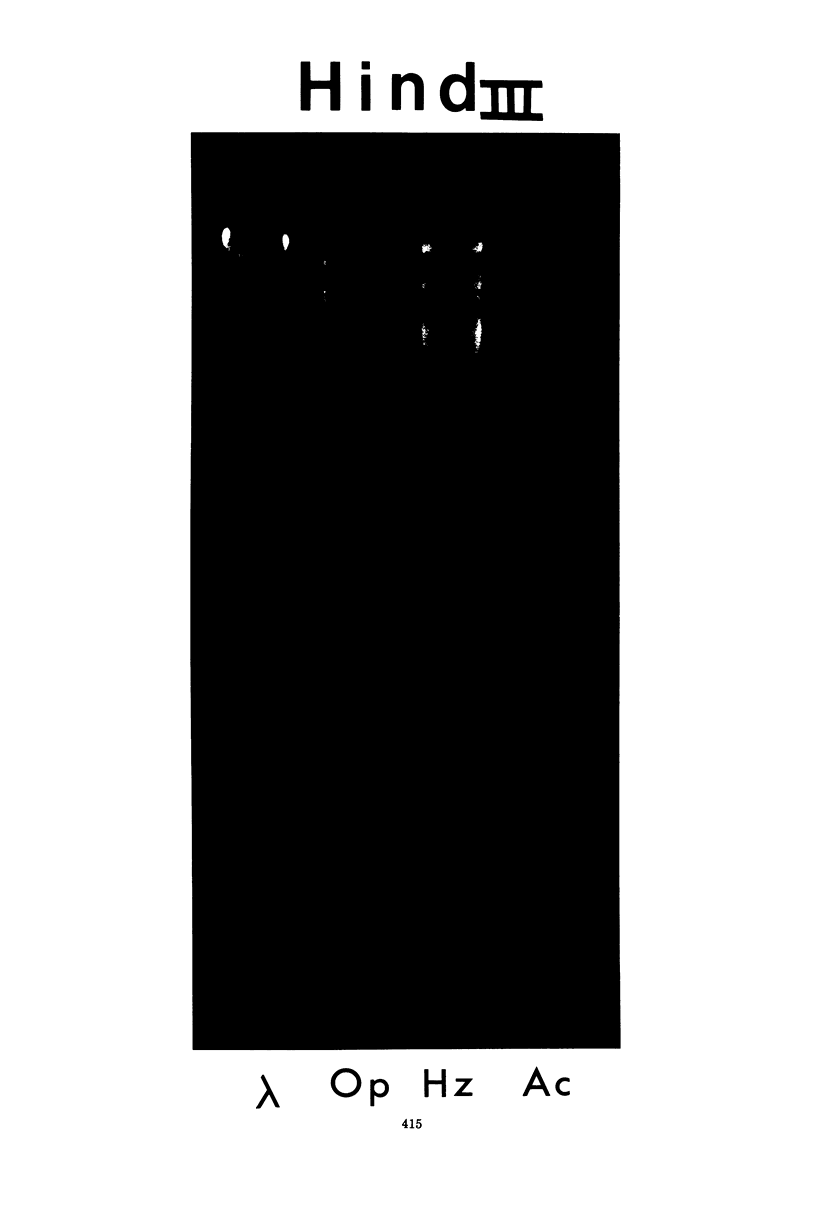
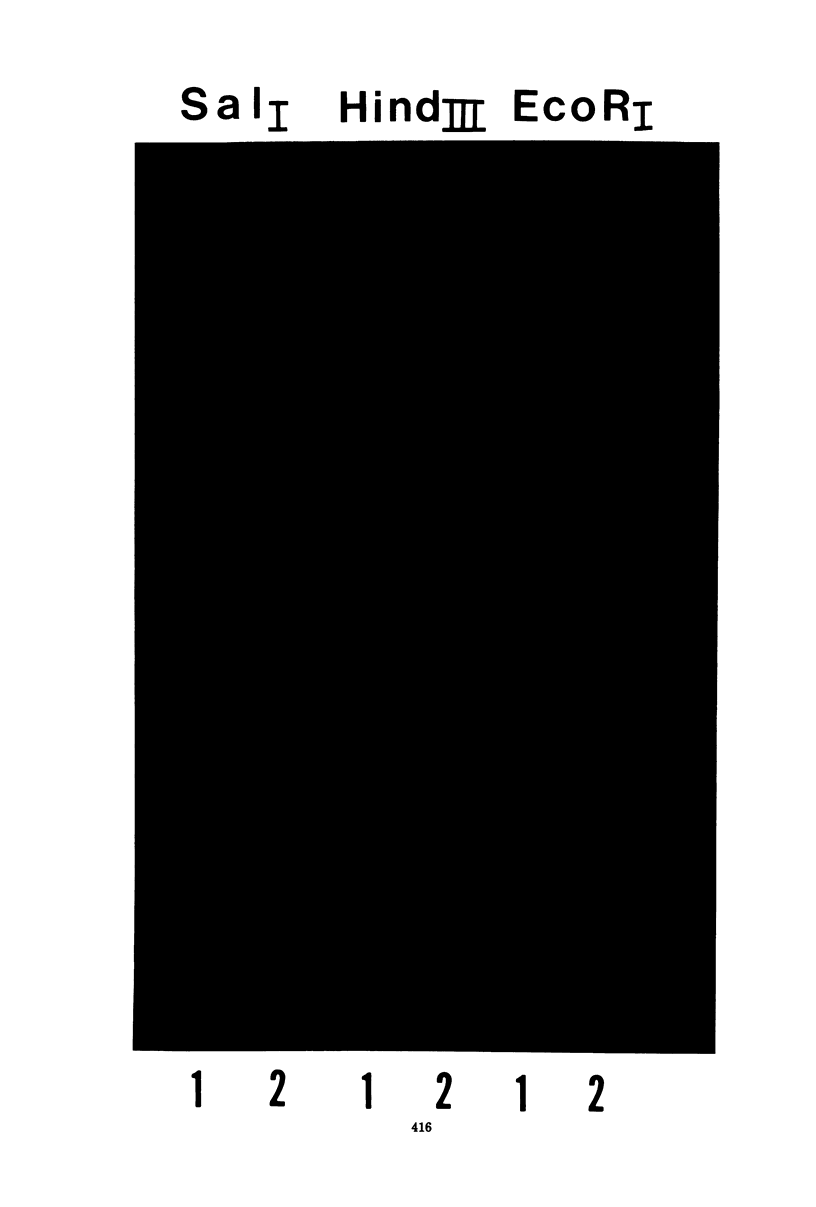
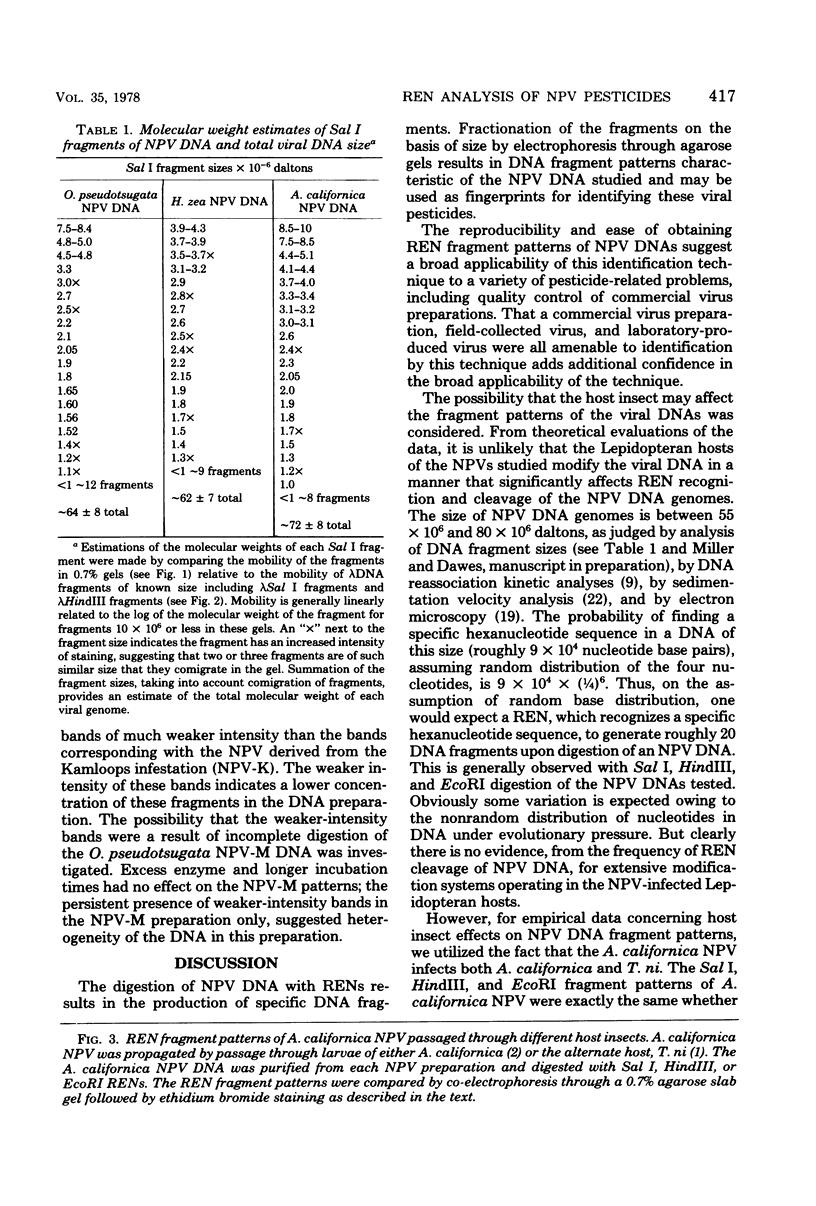
Gel electrophoresis of deoxyribonucleic acid (DNA) fragments generated by digesting the DNA genomes of nuclear polyhedrosis viruses (NPV) with restriction endonucleases provides DNA fragment patterns that may be used to identify different viruses of this group. Characteristic fragment patterns were obtained for three NPVs, which are important as biological pesticides (Autographa californica NPV, Orgyia pseudotsugata NPV, and Heliothis zea NPV). The DNA fragment patterns of the A. californica NPV genoms did not change with passage through the alternate insect host, Trichoplusia ni. Heterogeneity in one preparation of O. pseudotsugata NPV was observed. The identification procedure is direct and precise. Applications of this procedure include quality control of commercial preparations of viral pesticides and screening for genetic alterations in the viruses.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Brockman W. W., Lee T. N., Nathans D. The evolution of new species of viral DNA during serial passage of simian virus 40 at high multiplicity. Virology. 1973 Aug;54(2):384–397. doi: 10.1016/0042-6822(73)90151-7. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Jacob R. J., Honess R. W., Hayward G. S., Locker H., Roizman B. Anatomy of herpes simplex virus DNA. III. Characterization of defective DNA molecules and biological properties of virus populations containing them. J Virol. 1975 Jul;16(1):153–167. doi: 10.1128/jvi.16.1.153-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignoffo C. M. Development of a viral insecticide: concept to commercialization. Exp Parasitol. 1973 Apr;33(2):380–406. doi: 10.1016/0014-4894(73)90041-6. [DOI] [PubMed] [Google Scholar]
- Ignoffo C. M. Effects of entomopathogens on vertebrates. Ann N Y Acad Sci. 1973 Jun 22;217:141–172. doi: 10.1111/j.1749-6632.1973.tb32756.x. [DOI] [PubMed] [Google Scholar]
- Ignoffo C. M. Viruses--living insecticides. Curr Top Microbiol Immunol. 1968;42:129–167. doi: 10.1007/978-3-642-46115-6_7. [DOI] [PubMed] [Google Scholar]
- Kelly D. C. The DNA contained by nuclear polyhedrosis viruses isolated from four Spodoptera sp. (Lepidoptera, Noctuidae): genome size and homology assessed by DNA reassociation kinetics. Virology. 1977 Jan;76(1):468–471. doi: 10.1016/0042-6822(77)90325-7. [DOI] [PubMed] [Google Scholar]
- Khoury G., Fareed G. C., Berry K., Martin M. A., Lee T. N., Nathans D. Characterization of a rearrangement in viral DNA: mapping of the circular simian virus 40-like DNA containing a triplication of a specific one-third of the viral genome. J Mol Biol. 1974 Aug 5;87(2):289–301. doi: 10.1016/0022-2836(74)90150-8. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Cooke B. E., Fried M. Fate of mismatched base-pair regions in polyoma heteroduplex DNA during infection of mouse cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3073–3077. doi: 10.1073/pnas.73.9.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K., Fried M. Construction of infectious polyoma hybrid genomes in vitro. Nature. 1976 Feb 19;259(5544):598–601. doi: 10.1038/259598a0. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Fried M. Construction of the genetic map of the polyoma genome. J Virol. 1976 Jun;18(3):824–832. doi: 10.1128/jvi.18.3.824-832.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K., Sinsheimer R. L. Nature of phi chi 174 linear DNA from a DNA ligase-defective host. J Virol. 1974 Dec;14(6):1503–1514. doi: 10.1128/jvi.14.6.1503-1514.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Williams J., Sharp P. A., Grodzicker T. Physical mapping of temperature-sensitive mutations of adenoviruses. J Mol Biol. 1975 Sep 25;97(3):369–390. doi: 10.1016/s0022-2836(75)80046-5. [DOI] [PubMed] [Google Scholar]
- Scharnhorst D. W., Saving K. L., Vuturo S. B., Cooke P. H., Weaver R. F. Structural studies on the polyhedral inclusion bodies, virions, and DNA of the nuclear polyhedrosis virus of the cotton bollworm Heliothis zea. J Virol. 1977 Jan;21(1):292–300. doi: 10.1128/jvi.21.1.292-300.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Skare J., Summers W. P., Summers W. C. Structure and function of herpesvirus genomes. I. comparison of five HSV-1 and two HSV-2 strains by cleavage their DNA with eco R I restriction endonuclease. J Virol. 1975 Apr;15(4):726–732. doi: 10.1128/jvi.15.4.726-732.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D., Anderson D. L. Characterization of nuclear polyhedrosis virus DNAs. J Virol. 1973 Dec;12(6):1336–1346. doi: 10.1128/jvi.12.6.1336-1346.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne H. V. Detection of size heterogeneity in the supercoiled fraction of Polyoma virus DNA. J Mol Biol. 1968 Jul 14;35(1):215–226. doi: 10.1016/s0022-2836(68)80049-x. [DOI] [PubMed] [Google Scholar]
- Wagner M., Skare J., Summers W. C. Analysis of DNA of defective herpes simplex virus type 1 by restriction endonuclease cleavage and nucleic acid hybridization. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):683–686. doi: 10.1101/sqb.1974.039.01.082. [DOI] [PubMed] [Google Scholar]
- Yoshiike K. Studies on DNA from low-density particles of SV40. I. Heterogeneous defective virions produced by successive undiluted passages. Virology. 1968 Mar;34(3):391–401. doi: 10.1016/0042-6822(68)90059-7. [DOI] [PubMed] [Google Scholar]