Abstract
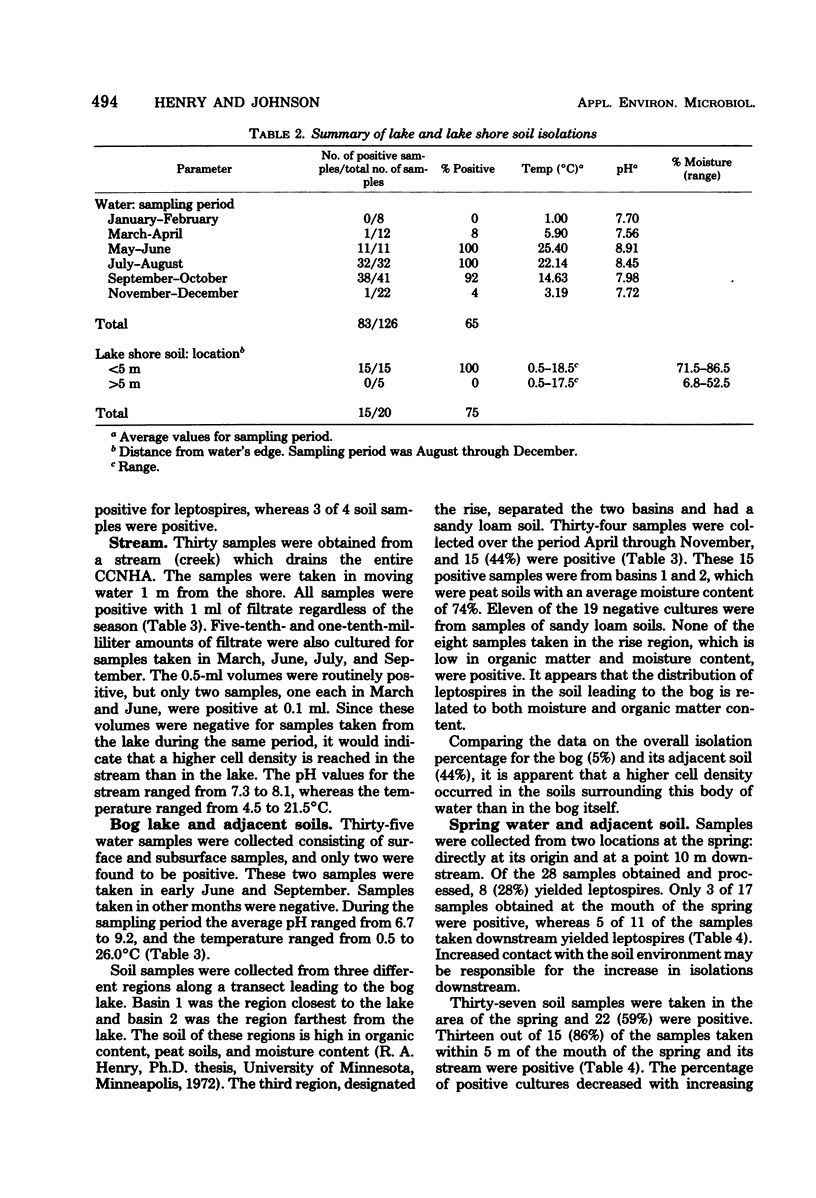
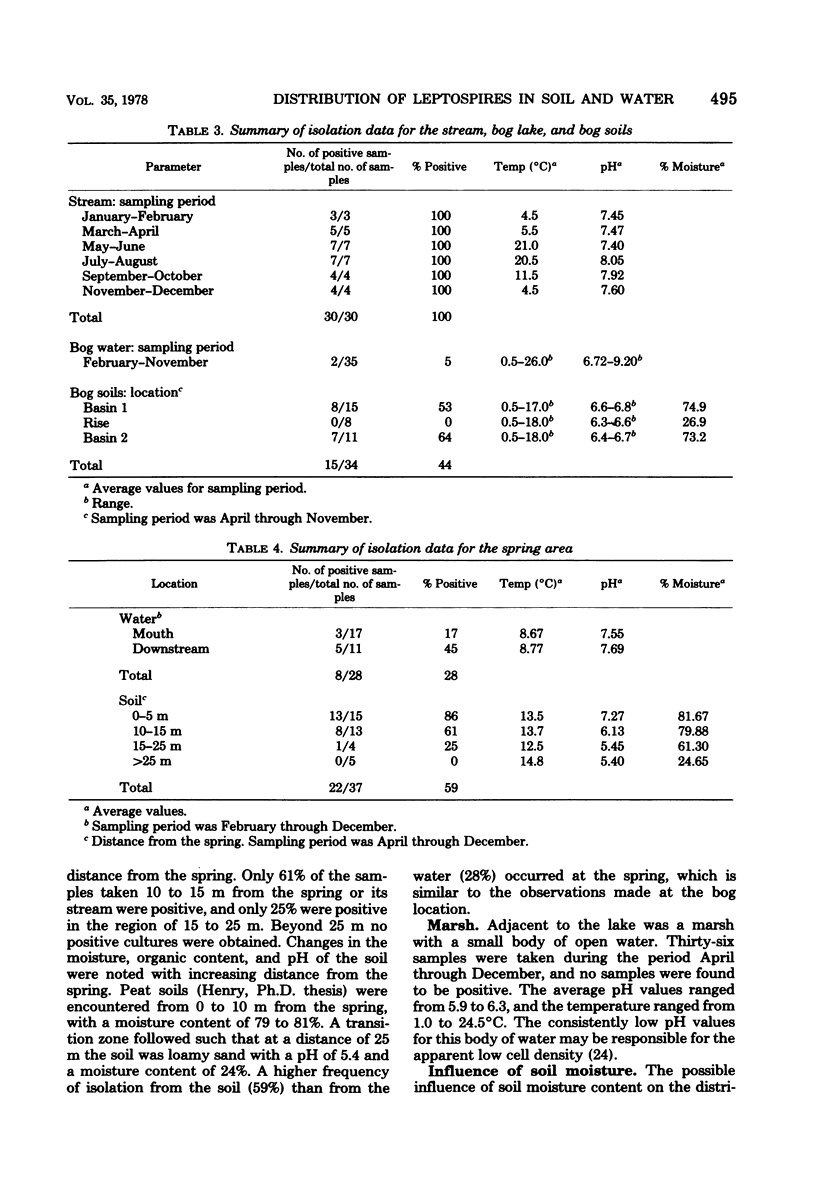
The distribution of the aerobic spirochetes Leptospira in surface waters, soil, and aquatic animals was investigated. Isolates from water and soil exhibited physiological characteristics common to members of the "biflexa complex," none were capable of infecting experimental animals, and leptospires could not be isolated from the eight genera of aquatic animals examined. The isolation frequencies from surface waters were: stream, 100%; lake, 65%; spring, 28%; bog lake, 5%; and marsh, 0%. With the exception of the stream, more isolations were obtained from the soil adjacent to the water than from the water. Leptospires were most frequently associated with soils of high moisture and organic matter content.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander A. D., Evans L. B., Baker M. F., Baker H. J., Ellison D., Marriapan M. Pathogenic leptospiras isolated from Malaysian surface waters. Appl Microbiol. 1975 Jan;29(1):30–33. doi: 10.1128/am.29.1.30-33.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. F., Baker H. J. Pathogenic Leptospira in Malaysian surface waters. I. A method of survey for Leptospira in natural waters and soils. Am J Trop Med Hyg. 1970 May;19(3):485–492. doi: 10.4269/ajtmh.1970.19.485. [DOI] [PubMed] [Google Scholar]
- Charon N. W., Johnson R. C., Muschel L. H. Antileptospiral activity in lower-vertebrate sera. Infect Immun. 1975 Dec;12(6):1386–1391. doi: 10.1128/iai.12.6.1386-1391.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesch S. L., McCulloch W. F., Braun J. L., Crawford R. P., Jr Environmental studies on the survival of leptospires in a farm creek following a human leptospirosis outbreak in Iowa. Wildl Dis. 1969 Jul;5(3):166–173. doi: 10.7589/0090-3558-5.3.166. [DOI] [PubMed] [Google Scholar]
- Diesch S. L., McCulloch W. F., Braun J. L., Ellinghausen H. C., Jr Leptospires isolated from frog kidneys. Nature. 1966 Feb 26;209(5026):939–940. doi: 10.1038/209939a0. [DOI] [PubMed] [Google Scholar]
- Diesch S. L., McCulloch W. F. Isolation of pathogenic leptospires from waters used for recreation. Public Health Rep. 1966 Apr;81(4):299–304. [PMC free article] [PubMed] [Google Scholar]
- GRAY P. H., WALLACE R. H. Correlation between bacterial numbers and organic matter in a field soil. Can J Microbiol. 1957 Aug;3(5):711–714. doi: 10.1139/m57-078. [DOI] [PubMed] [Google Scholar]
- Hagedorn C., Holt J. G. Ecology of soil arthrobacters in clarion-webster toposequences of iowa. Appl Microbiol. 1975 Feb;29(2):211–218. doi: 10.1128/am.29.2.211-218.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrici A. T. Studies of Freshwater Bacteria: IV. Seasonal Fluctuations of Lake Bacteria in Relation to Plankton Production. J Bacteriol. 1938 Feb;35(2):129–139. doi: 10.1128/jb.35.2.129-139.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. A., Johnson R. C., Bohlool B. B., Schmidt E. L. Detection of Leptospira in soil and water by immunofluorescence staining. Appl Microbiol. 1971 May;21(5):953–956. doi: 10.1128/am.21.5.953-956.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. 5-FLUOROURACIL AS A SELECTIVE AGENT FOR GROWTH OF LEPTOSPIRAE. J Bacteriol. 1964 Feb;87:422–426. doi: 10.1128/jb.87.2.422-426.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. DIFFERENTIATION OF PATHOGENIC AND SAPROPHYTIC LEPTOSPIRES WITH 8-AZAGUANINE. J Bacteriol. 1964 Dec;88:1618–1623. doi: 10.1128/jb.88.6.1618-1623.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967 Jul;94(1):27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G., Walby J. K. Characterization of leptospires according to fatty acid requirements. J Gen Microbiol. 1969 Mar;55(3):399–407. doi: 10.1099/00221287-55-3-399. [DOI] [PubMed] [Google Scholar]
- Karaseva E. V., Chernukha Y. G., Piskunova L. A. Results of studying the time of survival of pathogenic leptospira under natural conditions. J Hyg Epidemiol Microbiol Immunol. 1973 Mar;17(3):339–345. [PubMed] [Google Scholar]
- Kingscote B. F. Correlation of bedrock type with the geography of leptospirosis. Can J Comp Med. 1970 Jan;34(1):31–37. [PMC free article] [PubMed] [Google Scholar]
- Kingscote B. F. Leptospires in finger nail clams. J Wildl Dis. 1971 Jul;7(3):178–185. doi: 10.7589/0090-3558-7.3.178. [DOI] [PubMed] [Google Scholar]
- OKAZAKI W., RINGEN L. M. Some effects of various environmental conditions on the survival of Leptospira pomona. Am J Vet Res. 1957 Jan;18(66):219–223. [PubMed] [Google Scholar]
- SMITH D. J., SELF H. R. Observations on the survival of Leptospira australis A in soil and water. J Hyg (Lond) 1955 Dec;53(4):436–444. doi: 10.1017/s0022172400000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torten M., Birnbaum S., Klingberg M. A., Shenberg E. Epidemiologic investigation of an outbreak of leptospirosis in the Upper Galilee, Israel. Am J Epidemiol. 1970 Jan;91(1):52–58. doi: 10.1093/oxfordjournals.aje.a121112. [DOI] [PubMed] [Google Scholar]
- Turner L. H. Leptospirosis. 3. Maintenance, isolation and demonstration of leptospires. Trans R Soc Trop Med Hyg. 1970;64(4):623–646. doi: 10.1016/0035-9203(70)90087-8. [DOI] [PubMed] [Google Scholar]
- Turner L. H. Leptospirosis. I. Trans R Soc Trop Med Hyg. 1967;61(6):842–855. doi: 10.1016/0035-9203(67)90045-4. [DOI] [PubMed] [Google Scholar]
- Ward D. M., Brock T. D. Environmental factors influencing the rate of hydrocarbon oxidation in temperate lakes. Appl Environ Microbiol. 1976 May;31(5):764–772. doi: 10.1128/aem.31.5.764-772.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


