Abstract
We show here that elevated levels of gonadotropins (luteinizing hormone and follicle stimulating hormone), as found in menopause or after ovariectomy, promote growth of human ovarian carcinoma by induction of tumor angiogenesis. Human epithelial ovarian cancer tumors progressed faster in ovariectomized mice. This induced growth could be attributed to the elevated levels of gonadotropins associated with loss of ovarian function because direct administration of gonadotropins also was effective in promoting tumor progression in vivo. On the other hand, gonadotropins had no direct effect on the proliferation of human ovarian cancer cells in vitro. Using MRI, we demonstrated that ovariectomy significantly (P < 0.02) induces neovascularization of human ovarian carcinoma spheroids implanted in nude mice. Moreover, conditioned medium of gonadotropin-treated human ovarian carcinoma cells showed increased mitogenic activity to bovine endothelial cells, and this activity could be blocked by neutralizing antibodies against luteinizing hormone and against vascular endothelial growth factor. Accordingly, gonadotropin stimulation resulted in a dose-dependent-induced expression of vascular endothelial growth factor in monolayer culture as well as in the outer proliferating cells of human ovarian cancer spheroids. These results demonstrate the significance of the elevated levels of gonadotropins, as found in menopause and in all ovarian cancer patients, on the progression of ovarian cancer and could explain the protective effect of estrogen replacement therapy. Based on these results, we suggest that hormonal therapy aimed at lowering the circulating levels of gonadotropins may possibly prolong remission in ovarian cancer by extending tumor dormancy.
Human epithelial ovarian carcinoma presents a major challenge to oncological research. This cancer frequently is diagnosed late and shows poor prognosis and low survival despite the apparent success of chemotherapy in achieving remission with no evidence of disease. The goal of this work was to evaluate the role of gonadotropins [luteinizing hormone (LH) and follicular-stimulating hormone (FSH)], which are elevated during menopause or after ovariectomy, in promoting ovarian cancer progression. The involvement of gonadotropins in human epithelial ovarian carcinoma is backed by a number of epidemiological studies showing, on one hand, an increased occurrence of the disease with exposure to high levels of these hormones during menopause or during fertility treatments and, on the other hand, reduced risk associated with multiple pregnancies, oral contraceptives, and estrogen replacement therapy, all of which reduce the exposure to gonadotropins (1–3). However, no biological mechanism linking progression of ovarian cancer and gonadotropins has been demonstrated so far (1). An indirect mechanism of action of gonadotropins was suggested in a study of a gonadotropin-releasing hormone agonist that caused a latent transient inhibition of growth of human ovarian carcinoma in nude mice (4). In the study reported here, we show that loss of ovarian function, as occurs in menopause or after ovariectomy, promotes growth of human epithelial ovarian tumors in vivo by accelerating angiogenesis. We further demonstrate that this enhanced angiogenesis could be caused by elevated secretion of vascular endothelial growth factor (VEGF), that is in turn induced by the elevated levels of the gonadotropin hormones LH and FSH.
Neovascularization occurs rarely in normal physiology (5), the ovary being one of a few exceptions. Control of angiogenesis in the human ovary is mediated by VEGF (6), expressed mainly in the large preovulatory follicles (7) but also in the outer cortex (8), namely in the epithelial cells that are the progenitors of the most common cancer of the ovary. Expression of VEGF was reported also in neoplastic human ovaries (7, 9), and the critical role of VEGF in ovarian cancer was demonstrated recently and independently by two approaches. Dominant negative inhibition of flk-1 VEGF receptor as well as administration of a neutralizing antiserum to VEGF inhibited ovarian carcinoma-associated ascites formation and tumor growth in mice (10, 11).
VEGF received considerable attention recently because of its induction by hypoxia as well as by hypoglycemia (12, 13). The formation of extreme nutrient deficiencies, conducive for VEGF induction, is characteristic of solid tumors. Reasoning that the overall angiogenic output of human epithelial ovarian carcinoma tumors includes contributions of stress-induced stimuli in addition to the gonadotropin-induced stimuli, we analyzed the expression of VEGF in multicellular spheroids. Spheroids simulate the microenvironmental heterogeneity in small avascular tumors (14) and, in conjunction with in situ analysis of gene expression (13, 15, 16), enabled us to determine the dynamic interplay between stress and hormonal induction of angiogenic growth factors.
We conclude that the elevated levels of gonadotropins as occurs in menopause or after ovariectomy can facilitate the implantation of existing dormant ovarian microtumors by promoting angiogenesis and could thus provide an accelerated pathway for the eruption of the disease and favorable conditions for its recurrence.
METHODS
Cell Culture.
OC109 and OC238 human epithelial ovarian carcinoma cells (17) were cultured in DMEM supplemented with 5% fetal calf serum (Biological Industries, Beit Haemek, Israel) and antibiotics: 50 units/ml penicillin, 50 μg/ml streptomycin, and 125 μg/ml fungizone (Biolab, Jerusalem, Israel). MLS human epithelial ovarian carcinoma cells were obtained from R. M. Sutherland (Varian Biofocus, Palo Alto, CA) and were cultured in αMEM supplemented with 10% fetal calf serum and antibiotics (18). Spheroids were cultured as reported (18, 19) for 6 weeks at a spinning rate of 80 rpm and a change of medium every 48 h. A mixture of 95% air and 5% CO2 was blown over the medium every 48 h for 5 min. Bovine capillary endothelial (BCE) cells and A21 bovine aortic endothelial cells were obtained from I. Vlodavsky (Hadassah Medical Center, Jerusalem) and were cultured in low glucose DMEM (1 g/l) supplemented with 10% calf serum (GIBCO), a serum-free supplement [Biogro 1 (Beth Haemek, Israel)], and antibiotics [100 units/ml penicillin and 0.1 mg/ml streptomycin (Beth Haemek, Israel)].
Animal Protocols.
Ovariectomy. Female, CD1 nude mice (2 months old, 30-g body weight) were anesthetized i.p. with ketamine HCl (75 μg/g) and xylazine HCl (3 μg/g). Both ovaries were removed through 4-mm incisions, and the mice were allowed to recover fully for 2–3 weeks. Sham surgeries, i.e., anesthesia and incision (the ovaries left intact) were performed on 5 of 16 mice in the control group.
Gonadotropin administration.
The circulating levels of gonadotropins were elevated in normal cycling female CD-1 nude mice by s.c. administration (9 units every 48 h) of a 1:1 LH and FSH preparation used in fertility programs (Pergonal, Teva, Israel).
Tumor growth in the peritoneum.
Tumors were initiated by i.p injection of three MLS or OC109 spheroids (200 μm in diameter).
Spheroid implantation s.c..
For analysis of angiogenesis in vivo, MLS spheroids were encapsulated in agarose beads (19) to improve MRI visibility and reduce the possibility of an immune response. Mice were anesthetized, and one bead containing a single spheroid was implanted s.c. in each mouse as reported (20). For each experiment, a careful manual selection was performed to choose identical spheroid as well as bead size for implantation in all mice.
MRI and NMR Studies.
Gradient echo images were acquired on a horizontal 4.7-T Bruker–Biospec (Germany) spectrometer using an actively radio frequency-decoupled 2-cm surface coil with slice thickness of 0.6 mm, time of repetition 100 ms, time to echo 10.5 ms, and 256 × 256 pixels, in plane resolution of 110 μm and scan time of 7 min (16 averages) per mouse (20). Vascularization reduced the mean intensity in a region of 1 mm surrounding the spheroid-containing bead relative to a distant tissue (angiogenic contrast, 20). The apparent density of the blood vessels determined by MRI (apparent vessel density = −ln Sa/So, where Sa and So refer to the intensity measured at the rim of the implanted tumor and at a control point ≈10 mm from the tumor, respectively) correlated well with the density of blood vessels determined from skin specimens of the same mice. MRI analysis enabled us to follow each mouse independently and to determine the peak of the angiogenic activity in a noninvasive manner. 31P NMR spectroscopy was performed on a Bruker 500AM spectrometer on perfused ovarian cancer spheroids embedded in agarose beads as reported (19).
In Vitro Hormonal Stimulation.
Ovarian cancer cells (MLS, OC238) were plated at a density of 50000 cells/flask on a 25-cm2 tissue culture flask for preparation of conditioned medium as well as for analysis of VEGF expression.
Human LH and human FSH (hLH and hFSH) were provided by the United States National Hormone and Pituitary Program. Hormones were dissolved in PBS and stored at −20°C at a concentration of 100–400 μg/ml.
Thymidine Incorporation.
BCE and A21 cells were seeded in 24-well plates and incubated with medium conditioned by the ovarian carcinoma cells for 24 h. Cellular DNA was labeled with 3H-methyl-thymidine 5 μCi/ml (Rotem Industries, Beer Sheva, Israel) for an additional 6 h. The medium was removed, and cells were rinsed sequentially with methanol, chilled 5% trichloroacetic acid, and double distilled water and finally lysed with NaOH (0.5 M). Radioactive label was counted in an LKB Pharmacia (Uppsala, Sweden) β counter using scintillation fluid (ULTIMA GOLD, Packard). Neutralizing antibodies for hLH were provided by Fortune Kohen (Weizmann Institute). Neutralizing antibodies for VEGF were obtained from R & D Systems.
Reverse Transcriptase-PCR Analysis.
RNA was isolated from MLS and OC238 cells using TRI (RNA/DNA/protein isolation) reagent (ref. 21; Molecular Research Center, Cincinnati, OH). Complementary DNA was synthesized from 300 ng of total RNA using oligo(dT) as a primer and avian myeloblastosis virus reverse transcriptase. PCR amplification was carried out in the presence of [32P]-dCTP tracer (2 μCi in a 100-μl reaction volume), 1 mM of each dNTP, 2.5 mM MgCl2, and 2.5 units of Taq polymerase. Twenty five amplification cycles were used, each consisting of 1 min at 94°C, 2 min at 65°C, and 3 min at 72°C. cDNAs were coamplified with oligonucleotides GGAGAGATGAGCTTCCTACAG and TCACCGCCTTGGCTTGTCACA (corresponding to aa 92–98 and to the six carboxy terminal amino acids of VEGF) and L19 (serving as an internal standard, CTGAAGGTCAAAGGGAATGTG and GGACAGAGTCTTGATGATCTC, corresponding to forward and reverse sequences). Amplified fragments were resolved in a 6% nondenaturing polyacrylamide gel, visualized by autoradiography, and analyzed by a phosphoimager.
In Situ Hybridization.
Spheroids were fixed in 4% paraformaldehyde. Frozen specimens were sectioned (10 μm thick), processed, and hybridized in situ as described (13, 15). Digitized maps of the in situ hybridization sections were analyzed using nih image software. A binary presentation was derived and used in determination of the stain density (five spheroids, eight regions of interest per spheroid for each treatment).
RESULTS
Gonadotropins promoted growth of human epithelial ovarian carcinoma tumors in female CD-1 nude mice. This effect was observed using two different hormonal interventions: ovariectomy, leading to reduced estrogen production and thus elevated release of LH and FSH (due to loss of the normal negative feedback loop), as occurs in menopause, and direct administration of a gonadotropin preparation used in fertility programs (human LH/FSH, 1:1; Pergonal, Teva, Israel). We tested two different human epithelial ovarian carcinoma cell lines implanted s.c. or i.p. Tumors that developed from small MLS spheroids (200 μm) implanted intraperitoneum were significantly larger in ovariectomized mice relative to the control group. Mean tumor wet weight after 40 days was 0.67 ± 0.50 g for the ovariectomized group and 0.11 ± 0.11 g for the control group (n = 8 per group; P = 0.01 for unpaired t test). Similarly, administration of Pergonal (9 units every 48 h) enhanced growth of OC109 spheroids implanted intraperitoneum (n = 4) or in the left ovary (n = 7; 3 spheroids per ovary) in hypergonadotropic mice relative to mice treated with phosphate buffer. Tumor wet weight at 30 days was 0.21 ± 0.05 g for control and 0.37 ± 0.08 g for mice treated with LH + FSH (P = 0.05 for two-tailed unpaired t test). Similar enhancement of tumor growth was measured for s.c. implanted spheroids.
In contrast with their pronounced effect in vivo, gonadotropins did not affect the proliferation and metabolism of human epithelial ovarian carcinoma cells in vitro. MLS human epithelial ovarian carcinoma cells cultured in monolayer were exposed to hLH (1 μg/ml), hFSH (1 μg/ml), or Pergonal (1 μg/ml). No significant effect was observed on cell proliferation, protein content, or thymidine incorporation (data not shown). In addition, 31P NMR spectra showed no change in the content of NTP after stimulation with hLH, hFSH, or Pergonal.
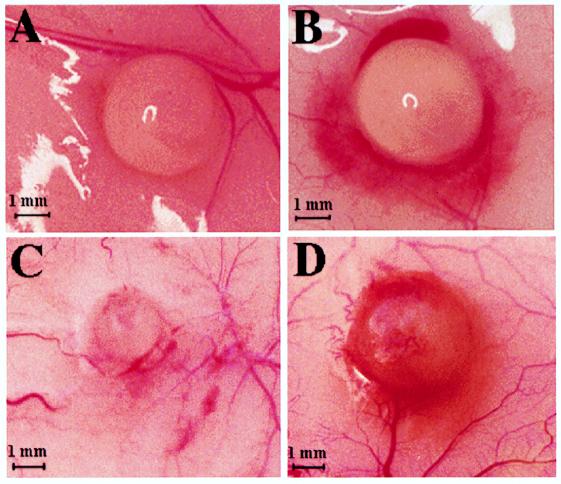
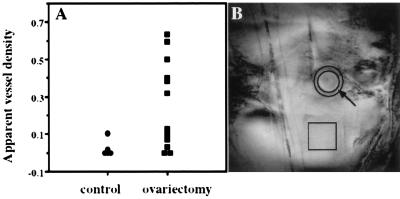
To reconcile between the different outcomes of gonadotropin administration in vivo and in vitro with respect to human epithelial ovarian carcinoma growth, we postulated that gonadotropins induce tumor growth in vivo by promoting tumor neovascularization. We therefore examined, by using MRI, tumor neovascularization at the early days after implantation of MLS human epithelial ovarian carcinoma spheroids. MLS spheroids of ≈800 μm in diameter were encapsulated in a 1- to 3-mm diameter agarose bead and implanted s.c. in ovariectomized mice and in age-matched controls (n = 20 and n = 16, respectively). Apparent vessel density was determined from gradient echo MR images as reported for C6 glioma spheroids (20). Massive angiogenesis was observed after 11 days in ovariectomized mice, and these mice developed tumors that burst the bead borders (Fig. 1D). Tumor development in nonovariectomized mice was minimal, and no sign of angiogenesis was evident (Fig. 1 A and C). In the control group, no difference was found between mice that were not subjected to surgery and mice subjected to sham surgery. The mean apparent vessel density was determined by MRI on day 11 for some of the animals. Five controls (out of 16) and 12 ovariectomized mice (out of 20) were picked randomly for MRI, and the measured vessel density was ≈7-fold larger in the ovariectomized mice (P < 0.02) (Fig. 2). Visual examination of the skin specimens dissected at the end of the experiment from all mice confirmed the MRI result, showing a more frequent occurrence of vascular tumors in the ovariectomized mice. Eleven days after spheroid implantation, 70% (14 of 20) of the tumors in the ovariectomy group were vascular whereas only 12.5% (2 of 16) in the control group were vascular.
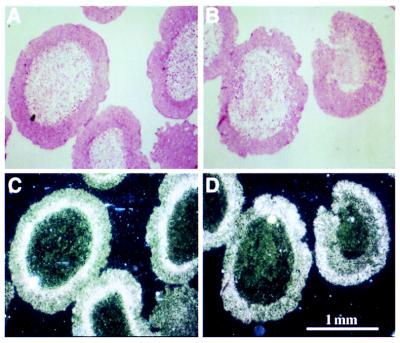
Figure 1.
Primary neovascularization of implanted human epithelial ovarian carcinoma spheroids. Skin specimens were obtained 11 days after implantation of an 800-μm MLS spheroid embedded in a 1- to 3-mm agarose bead. (A) Control mouse subjected to a sham operation. (C) Control mouse, no sham operation. (B and D) Ovariectomized mouse. Early neovascularization is characterized by very thin capillaries and redness surrounding the tumor. (A-D) Representative examples of two independent experiments in which the bead size was chosen to be 3 mm in A and B and 1 mm in C and D. Spheroid diameter was 800 μm in both experiments.
Figure 2.
Apparent vessel density of implanted ovarian carcinoma spheroids. (A) Apparent vessel density was determined from gradient echo MRI as reported (20). Note the enhanced angiogenic activity of spheroids in ovariectomized mice relative to control mice and mice that underwent sham surgery. (B) MRI of an implanted spheroid in an ovariectomized mouse. The circular region (arrow) contains the developing microvasculature surrounding the tumor. The mean intensity in this region was scaled to the mean intensity in a distant tissue (square) to yield the apparent vessel density (20).
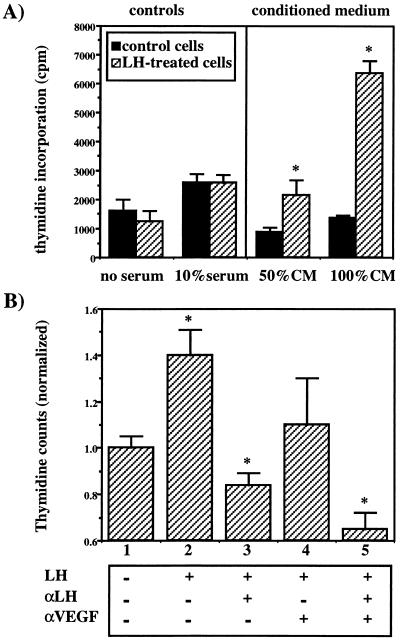
Augmented production and secretion of an endothelial cell mitogen in hormonally stimulated human epithelial ovarian carcinoma cells also was shown in vitro. BCE and A21 bovine endothelial cells were incubated for 16 h with conditioned medium derived from hLH-treated (1 μg/ml, 48 h) or untreated MLS cells. DNA synthesis in the endothelial cells increased significantly and in a dose-dependent manner after treatment with hLH-conditioned medium relative to control conditioned medium (Fig. 3A). Treatment of the ovarian cancer cells with specific neutralizing antibody against LH inhibited the secretion of endothelial cell mitogenic activity induced by hLH whereas the mitogenic activity itself was inhibited by neutralizing antibodies against VEGF (Fig. 3B). These results confirmed the role of LH as an inducer of angiogenesis in ovarian cancer and point to VEGF as at least one of the angiogenic promoters stimulated by LH.
Figure 3.
The effect of medium conditioned by LH-stimulated ovarian cancer cells on endothelial cell proliferation in vitro. (A) Subconfluent MLS cells were cultured in 25-cm2 flasks for 48 h in the medium of the endothelial cells. Half of the flasks were supplemented with hLH (1 μg/ml). The conditioned medium (CM) was collected, filtered at 0.2 μm, and applied immediately on BCE and A21 endothelial cells for a 30-h incubation. Direct stimulating effect of LH on the endothelial cells was ruled out by incubating the cells with or without LH as shown in the left side of A. Cell proliferation was monitored by 3H-thymidine incorporation. Note the dose-dependent enhancement of endothelial cell proliferation in hLH-conditioned medium (P < 0.05). (B) MLS cells were incubated in the presence or absence of hLH and neutralizing antibodies for LH (αLH) for 48 h. The conditioned medium was applied as in A on the endothelial cells in the presence or absence of neutralizing antibodies for VEGF (αVEGF). Cell proliferation was monitored by 3H-thymidine incorporation. A statistically significant difference (one-tailed t test; ∗, P < 0.05) was observed between: columns 1 and 2 (P = 0.006); columns 2 and 3 (P = 0.002); columns 2 and 5 (P = 0.001); columns 3 and 5 (P = 0.024); and columns 4 and 5 (P = 0.023). For columns 2 and 4, P = 0.08.
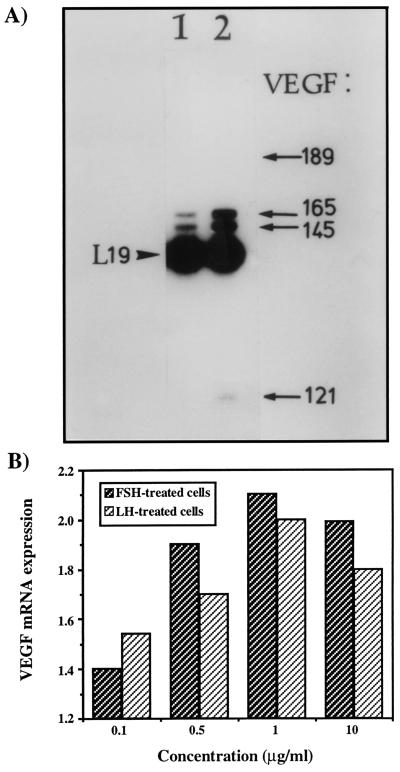
To examine the possibility that hormonally induced angiogenesis in ovarian microtumors can be accounted for, at least in part, by hLH-induced VEGF expression, RNA was extracted from MLS and OC238 cells exposed to hLH or hFSH, and steady-state levels of VEGF mRNA were compared with those of noninduced cells by reverse transcriptase-PCR analysis. Both hLH and hFSH stimulated VEGF mRNA expression in a dose-dependent manner. Maximal induction was measured for a dose of 1 μg/ml in which the expression of VEGF was elevated ≈3-fold (Fig. 4). Interesting to note, among the VEGF splicing variants induced by hLH, we detected in OC238 cells abundant expression of VEGF145, a rare species in most cell types, found previously in the reproductive system (22).
Figure 4.
Expression of VEGF in OC238 human epithelial ovarian carcinoma after hLH stimulation. (A) RNA was extracted from control (lane 1) and hLH-treated cells (1 μg/ml, 48 h) (lane 2). VEGF mRNA levels were compared by reverse transcriptase-PCR analysis. Amplified VEGF cDNA fragments (indicated by arrows) correspond to mRNA species encoding VEGF189, VEGF165, VEGF145, and VEGF121. The arrowhead points at the 194 bp-long fragment of coamplified ribosomal L19 cDNA. (B) Both LH and FSH stimulated the expression of VEGF165 in human ovarian cancer cells in a dose-dependent manner. Maximal induction was obtained for a dose of 1 μg/ml.
The relative contributions of hormonal and stress induction of VEGF were analyzed in spheroids derived from MLS cells by in situ hybridization (Fig. 5; see Fig. 6A). Stress-induced VEGF was evident as high levels of expression in cells surrounding the necrotic center and was independent of the hormonal status. The latter result reproduced for MLS spheroids previous observations from tumors (23) as well as C6 glioma spheroids (13, 15, 16). In addition, semi-quantitative analysis of VEGF expression showed a 2-fold induction in the outer cell layers of hLH- and hFSH-treated spheroids.
Figure 5.
Hormonal and stress regulation of VEGF expression in MLS spheroids. MLS spheroids were treated with 1 μg/ml of hFSH or hLH for 48 h and then stained with a VEGF-specific probe. (A and C) Control. (B and D) hLH-treated MLS spheroids stained with a specific VEGF mRNA antisense. (A and B) bright field images. (C and D) Dark field images. Note the hypoxia-induced expression of VEGF in the inner layers of the viable rim in the control as well as in the hLH-treated spheroids. On the other hand, the outer proliferating cells expressed low levels of VEGF in the control spheroids and showed significantly induced expression in the gonadotropin-stimulated spheroids.
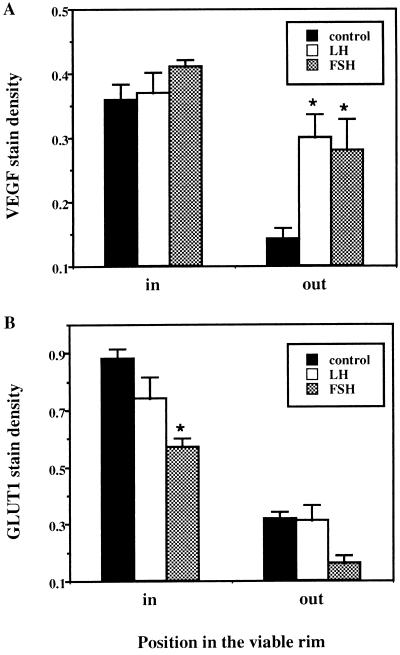
Figure 6.
Relative expression of VEGF and GLUT1 in MLS spheroids. The relative expression of (A) VEGF and (B) GLUT1 was derived from the inner and outer layers of the viable rim of the spheroids by quantitating the stain density of the in situ hybridization images (∗, P < 0.01). Note the similar pattern of stress-induced expression of VEGF and GLUT1 in the inner hypoxic regions. On the other hand, the outer proliferating cell layers show significant gonadotropin-induced expression of VEGF but not GLUT1.
GLUT-1, a glucose transporter showing stress-induced expression patterns similar to that of VEGF in spheroids (15), was expressed in the same cell layers as VEGF in control spheroids but was not induced by either LH or FSH in the proliferating layers (Fig. 6B). In addition, gonadotropins had no effect on cellular energetics measured by NMR spectroscopy on perfused spheroids. Thus, we conclude that LH and FSH induce VEGF expression directly and not via hormonally induced metabolic stress. Our data suggest that gonadotropin-induced expression would be the prevalent mode of VEGF production in small microtumors, and larger tumors would show increased contribution of VEGF induced by hypoxic stress.
DISCUSSION
Hypergonadotropic hormonal milieu is common to almost all ovarian cancer patients, who are usually in menopause and frequently undergo ovariectomy. Thus, it is important to note that human ovarian tumors implanted in nude mice progressed faster when exposed to increased gonadotropin stimulation obtained either by direct hormonal administration or by removal of the ovaries. In accord with this finding, it was previously shown that development of human ovarian cancer in mice can be inhibited by treatment with gonadotropin-releasing hormone antagonists (24). However, the mechanism by which gonadotropins promote ovarian cancer progression was not known. The inability of gonadotropin stimulation to induce growth of human epithelial ovarian carcinoma cells in vitro argues against a direct effect of gonadotropins on tumor cell growth. Our MRI studies demonstrate that ovariectomy can stimulate progression of human epithelial ovarian carcinoma by stimulation of tumor angiogenesis. We furthermore show that the induced angiogenesis of ovarian cancer after ovariectomy can be mediated by gonadotropin-induced expression of VEGF. The induction of tumor angiogenesis by ovariectomy is not general for all angiogenic processes but rather seems to represent a unique feature of ovarian cancer. For example, implants with known angiogenic stimuli were reported to show reduced angiogenesis in ovariectomized mice (25).
The experimental data presented here support the following mechanism for the role of gonadotropins in progression of human epithelial ovarian carcinoma: When the levels of gonadotropins are low, tumors will progress slowly or will remain dormant as small avascular microtumors. When the levels of gonadotropins rise, as occurs during menopause or after ovariectomy, an accelerated pathway for vascularization arises. Gonadotropin-induced expression of VEGF can contribute significantly to the angiogenic potential of the tumors and could enable implantation of very small microtumors. These findings provide a possible mechanistic explanation for the epidemiological observation of increased risk of ovarian cancer associated with elevated levels of systemic gonadotropins.
The clinical implications of our results for hormonal intervention in human epithelial ovarian carcinoma are apparent. It is clear that the elevated levels of gonadotropins as found in all ovarian cancer patients create conditions that are favorable for tumor implantation and progression and could aggravate prognosis. However, at the time of diagnosis, the lesions are larger than 1 mm and contain an established vascular system and usually a significant hypoxic cell fraction. In this case, stress-induced expression of VEGF may predominate, and, thus, tumors are not likely to respond to hormonal intervention. This mode of hormonal “resistance” can explain the failure of previous treatments that were limited to patients with apparent tumors (1). An appropriate time window for hormonal intervention may be found during the period of clinically “complete remission.” It is during that period that dormancy of micrometastatic lesions (5) may be extended by avoiding a hormonal milieu that promotes angiogenesis.
Acknowledgments
We thank Professor Nava Dekel for stimulating discussion. Human ovarian carcinoma MLS cells were provided by Dr. R. M. Sutherland (Varian Biofocus, Palo Alto, CA). BCE and A21 endothelial cells were provided by Professor Israel Vlodavsky. Human LH and FSH were obtained through The United States National Hormone and Pituitary Program. Pergonal was provided by Teva. Neutralizing antibodies for LH were kindly provided by Dr. Fortune Kohen. This work was supported by grants from the Israel Cancer Association (to M.H. and M.N.); from the Israel Cancer Research Fund, the Frenkel Foundation, and the Minerva Foundation (to M.N.); and from the Mireille and James Levy foundation (United States) (to E.K); M.N. is an incumbent of the Dr. Phil Gold Career Development Chair in cancer research.
ABBREVIATIONS
- LH
luteinizing hormone
- FSH
follicular-stimulating hormone
- VEGF
vascular endothelial growth factor
- BCE
bovine capillary endothelial
References
- 1.Rao B R, Slotman B J. Endocrine Rev. 1991;12:14–26. doi: 10.1210/edrv-12-1-14. [DOI] [PubMed] [Google Scholar]
- 2.Shoham Z. Fertil Steril. 1994;62:433–448. doi: 10.1016/s0015-0282(16)56928-3. [DOI] [PubMed] [Google Scholar]
- 3.Rossing M A, Daling J R, Weiss N S, Moore D E, Self S G. N Engl J Med. 1994;331:771–776. doi: 10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- 4.Peterson C M, Jolles C J, Carrell D T, Straight R C, Jones K P, Poulson A M, Hataska H H. Gynecol Oncol. 1994;52:26–30. doi: 10.1006/gyno.1994.1006. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak H F, Brown L F, Detmar M, Dvorak A M. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 7.Olson T A, Mohanraj D, Carson L F, Ramakrishnan S. Cancer Res. 1994;54:276–280. [PubMed] [Google Scholar]
- 8.Dissen G A, Lara H E, Fahrenbach W H, Costa M E, Ojeda S R. Endocrinology. 1994;134:1146–1154. doi: 10.1210/endo.134.3.8119153. [DOI] [PubMed] [Google Scholar]
- 9.Boocock C A, Charnock-Jones D S, Sharkey A M, McLaren J, Barker P J, Wrigth K A, Twentyman P R, Smith S K. J Natl Cancer Inst. 1995;87:506–516. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- 10.Millauer B, Longhi M P, Plate K H, Shawver L K, Risau W, Ullrich A, Strawn L M. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- 11.Olson T A, Mohanraj D, Ramakrishnan S. Int J Oncol. 1996;8:505–511. doi: 10.3892/ijo.8.3.505. [DOI] [PubMed] [Google Scholar]
- 12.Shweiki D, Itin A, Soffer D, Keshet E. Nature (London) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 13.Shweiki D, Neeman M, Itin A, Keshet E. Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutherland R M. Science. 1988;240:177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 15.Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Mol Cell Biol. 1995;15:5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waleh N S, Brody M D, Knapp M A, Mendonca H L, Lord E M, Koch C J, Laderoute K R, Sutherland R M. Cancer Res. 1995;55:6222–6226. [PubMed] [Google Scholar]
- 17.Maymon R, Maymon B B, Holzinger M, Tartakovsky B, Leibovici J. Gynecol Oncol. 1994;55:265–270. doi: 10.1006/gyno.1994.1288. [DOI] [PubMed] [Google Scholar]
- 18.Rofstad E K, Sutherland R M. Br J Cancer. 1989;59:28–35. doi: 10.1038/bjc.1989.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiffenbauer Y S, Meir G, Cohn M, Neeman M. Am J Physiol. 1996;270:C160–C169. doi: 10.1152/ajpcell.1996.270.1.C160. [DOI] [PubMed] [Google Scholar]
- 20.Abramovitch R, Meir G, Neeman M. Cancer Res. 1995;55:1956–1962. [PubMed] [Google Scholar]
- 21.Chomczynski P A. BioTechniques. 1993;15:532–537. [PubMed] [Google Scholar]
- 22.Anthony F W, Wheeler T, Elcock C L, Pickett M, Thomas E J. Placenta. 1994;15:557–561. doi: 10.1016/s0143-4004(05)80424-2. [DOI] [PubMed] [Google Scholar]
- 23.Plate K H, Breier G, Weich H A, Risau W. Nature (London) 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 24.Yano T, Pinski J, Halmos G, Szepeshazi K, Groot K, Schally A V. Proc Natl Acad Sci USA. 1994;91:7090–7094. doi: 10.1073/pnas.91.15.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morales D E, McGowan K A, Grant D S, Maheshwari S, Bhartiya D, Cid M C, Kleinman H K, Schnaper H W. Circulation. 1995;91:755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]