Abstract
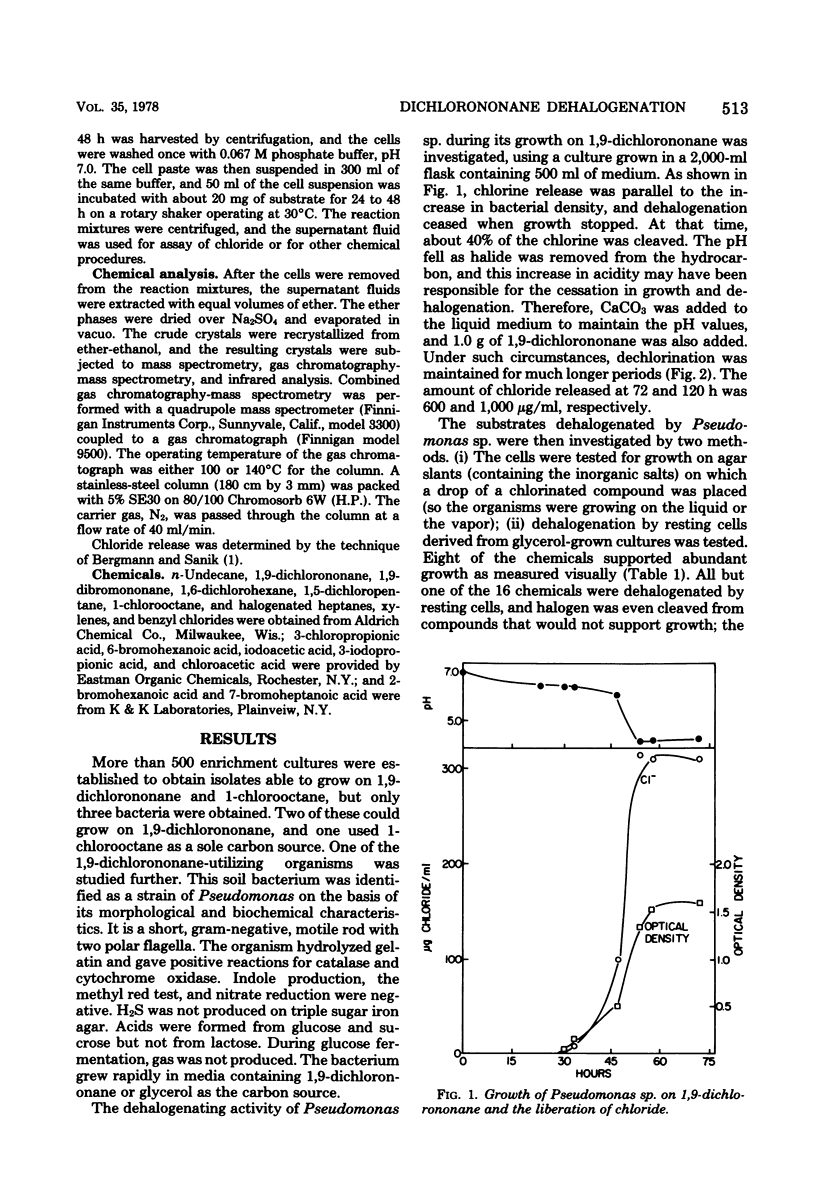
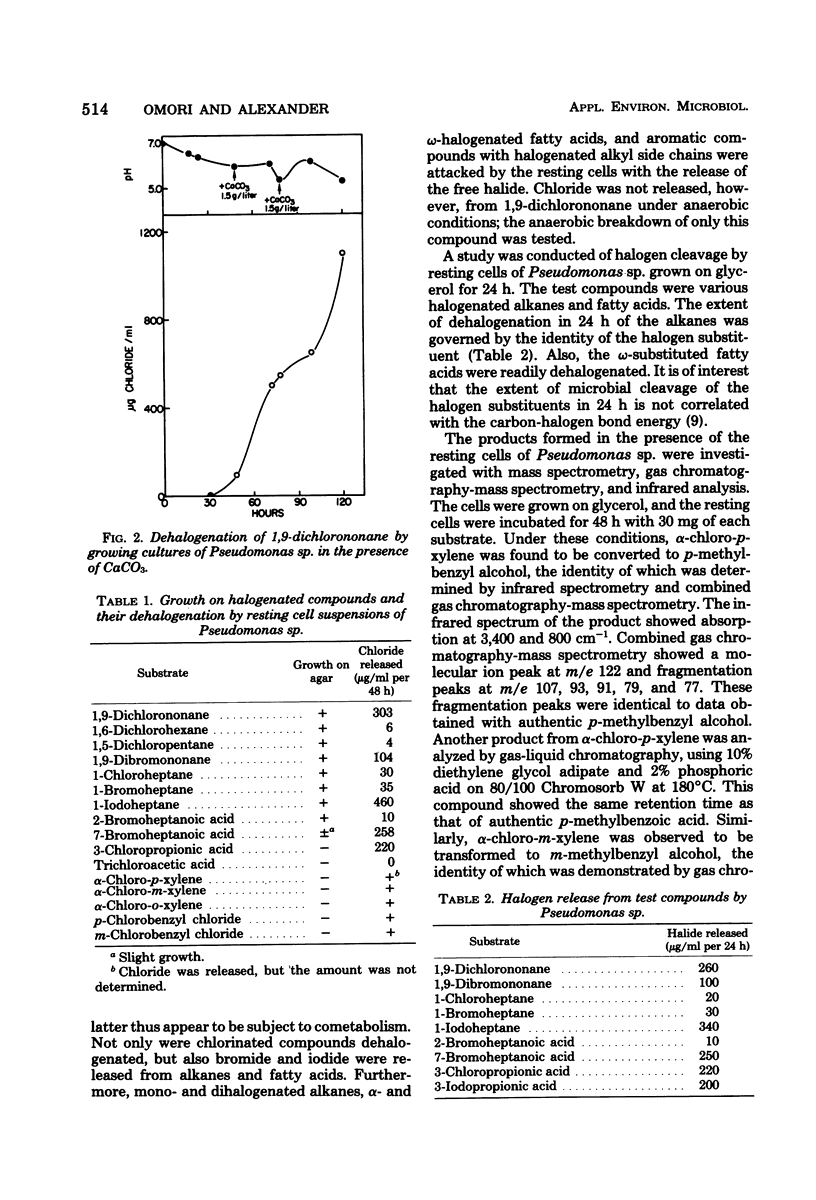
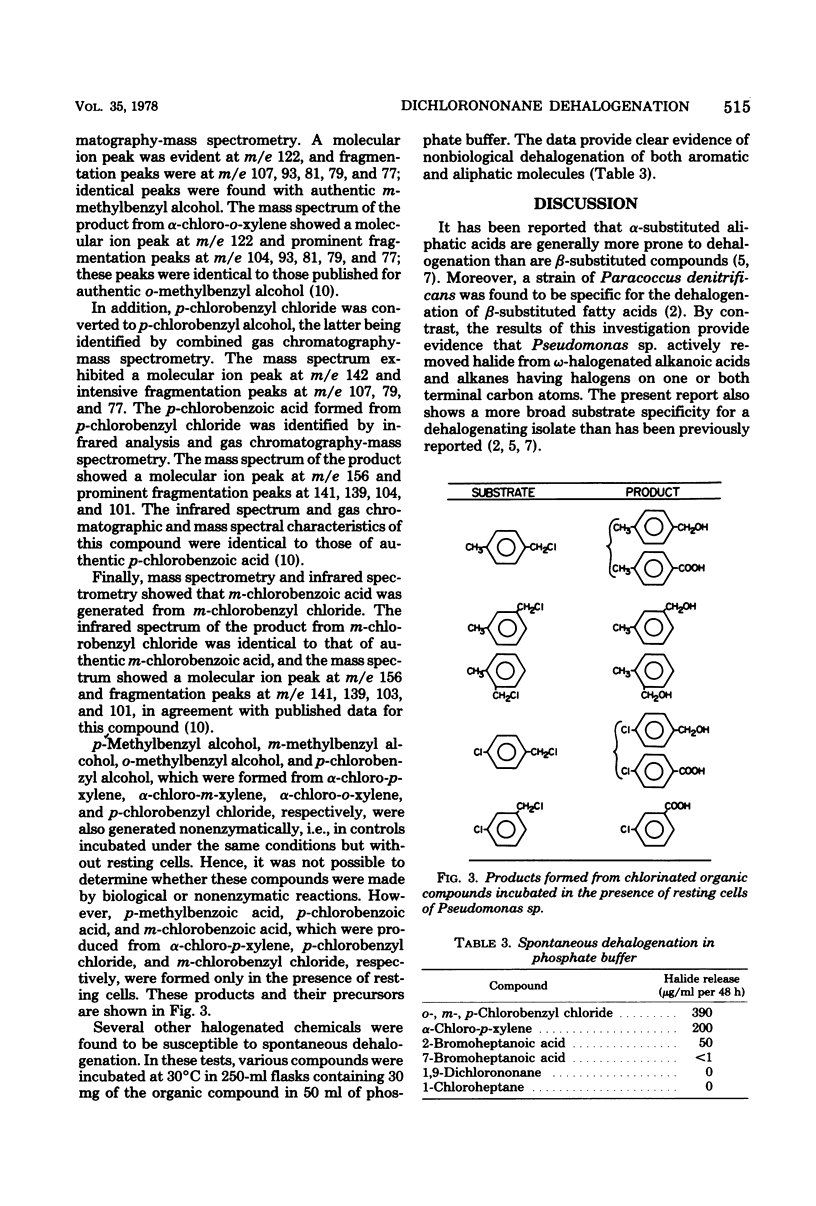
Only 3 of more than 500 soil enrichments contained organisms able to use 1,9-dichlorononane as a sole carbon source. One isolate, a strain of Pseudomonas, grew on the compound and released much of the halogen as chloride. Resting cells dehalogenated 1,9-dichlorononane aerobically but not anaerobically. Pseudomonas sp. grew on and resting cells dehalogenated 1,6-dichlorohexane, 1,5-dichloroheptane, 2-bromoheptanoate, and 1-chloro-, 1-bromo-, and 1-iodoheptane, but the bacterium cometabolized but did not grow on 3-chloropropionate. p-Methylbenzyl alcohol, chloride, and p-methylbenzoate were formed when resting cells were incubated with alpha-chloro-p-xylene; the first two products were also formed in the absence of the bacteria. Similarly, o- and m-methylbenzyl alcohols were generated from the corresponding chlorinated xylenes in the presence or absence of Pseudomonas sp. The formation of m- and p-chlorobenzoic acid from m- and p-chlorobenzyl chloride proceeded only in the presence of the cells, but p-chlorobenzyl alcohol was generated from p-chlorobenzyl chloride even in the absence of the bacterium. These results are discussed in terms of possible mechanisms of dehalogenation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASTRO C. E., BARTNICKI E. W. BIOLOGICAL CLEAVAGE OF CARBON-HALOGEN BONDS. METABOLISM OF 3-BROMOPROPANOL BY PSEUDOMONAS SP. Biochim Biophys Acta. 1965 May 4;100:384–392. doi: 10.1016/0304-4165(65)90007-3. [DOI] [PubMed] [Google Scholar]
- Goldman P., Milne G. W., Keister D. B. Carbon-halogen bond cleavage. 3. Studies on bacterial halidohrolases. J Biol Chem. 1968 Jan 25;243(2):428–434. [PubMed] [Google Scholar]
- HIRSCH P., ALEXANDER M. Microbial decomposition of halogenated propionic and acetic acids. Can J Microbiol. 1960 Jun;6:241–249. doi: 10.1139/m60-028. [DOI] [PubMed] [Google Scholar]
- Kearney P. C., Kaufman D. D., Beall M. L. Enzymatic dehalogenation of 2,2-dichloropropionate. Biochem Biophys Res Commun. 1964;14:29–33. doi: 10.1016/0006-291x(63)90205-5. [DOI] [PubMed] [Google Scholar]
- Milne G. W., Goldman P., Holtzman J. L. The metabolism of 2-fluorobenzoic acid. II. Studies with 18-O2. J Biol Chem. 1968 Oct 25;243(20):5374–5376. [PubMed] [Google Scholar]
- STEENSON T. I., WALKER N. The pathway of breakdown of 2:4-dichloro- and 4-chloro-2-methyl-phenoxyacetic acid by bacteria. J Gen Microbiol. 1957 Feb;16(1):146–155. doi: 10.1099/00221287-16-1-146. [DOI] [PubMed] [Google Scholar]


