Abstract
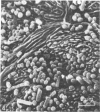
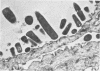
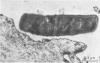
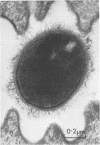
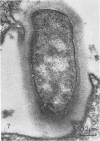
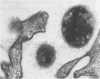
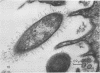
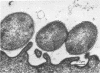
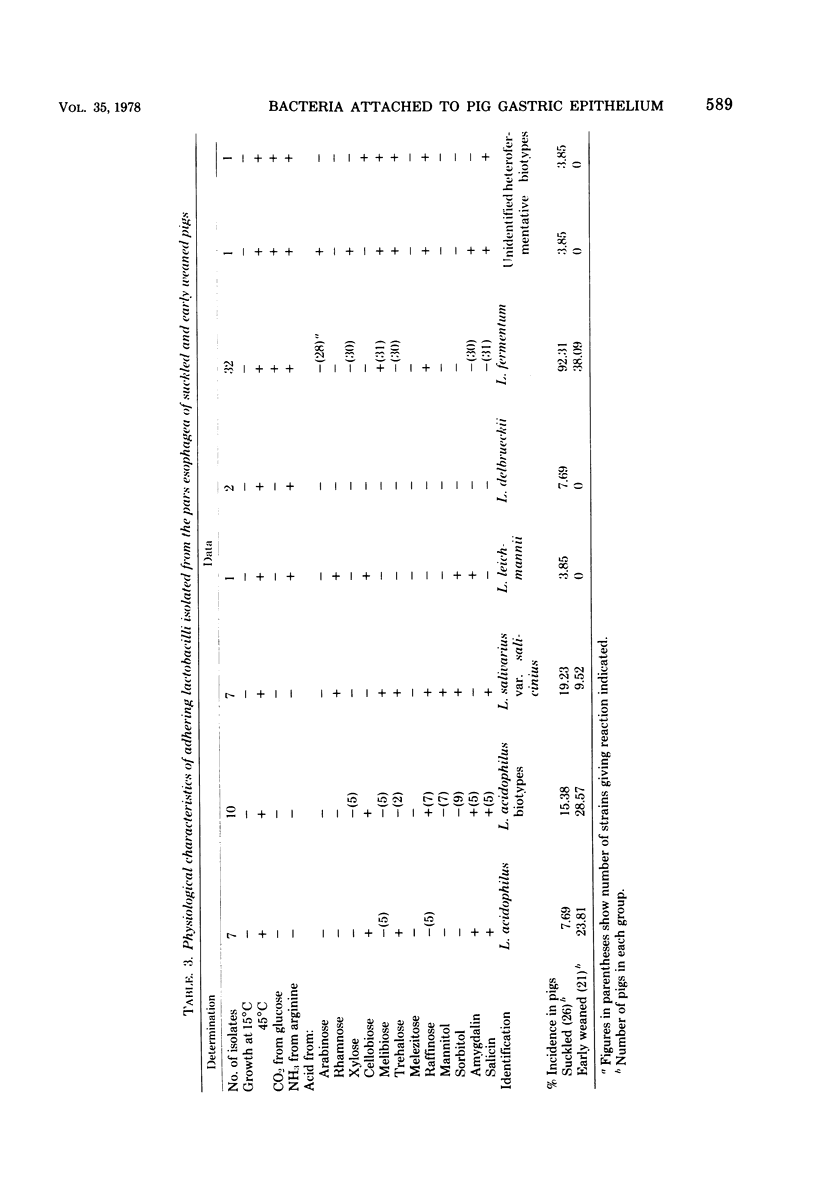
Light and electron microscopy showed lactobacilli and, to a lesser degree, streptococci to be closely associated with the squamous area of the pig stomach known as the pars esophagea. Several different types of extracellular layers were seen on bacteria attached to the epithelial surface. The total number of bacteria per square centimeter did not change with age up to 10 days, and there was no effect of weaning at 2 days. Lactobacillus fermentum, L. salivarius, and Streptococcus salivarius were isolated more frequently from sucking pigs than from those that were early weaned, whereas the reverse was true of L. acidophilus and S. bovis. All isolates recovered from washed macerated pars esophagea adhered to pig esophageal epithelial cells when tested in vitro.
Full text
PDF
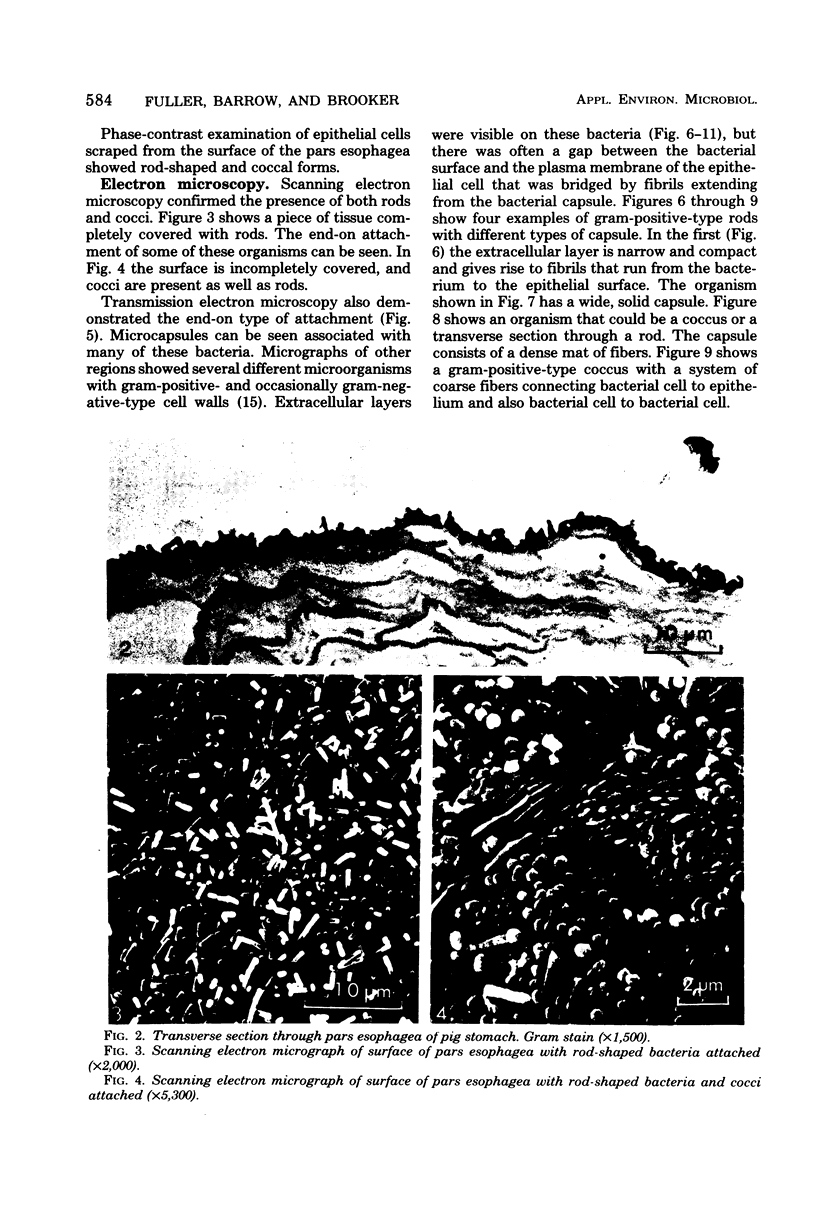
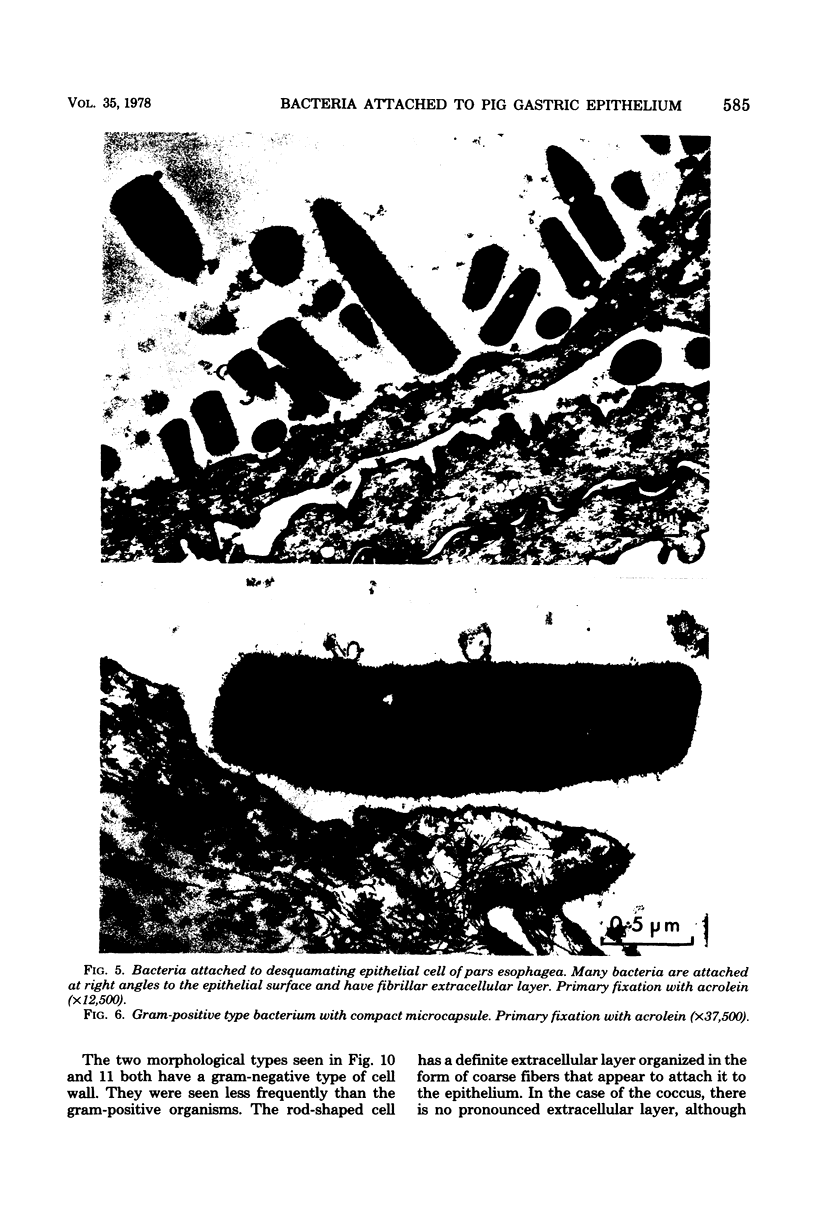

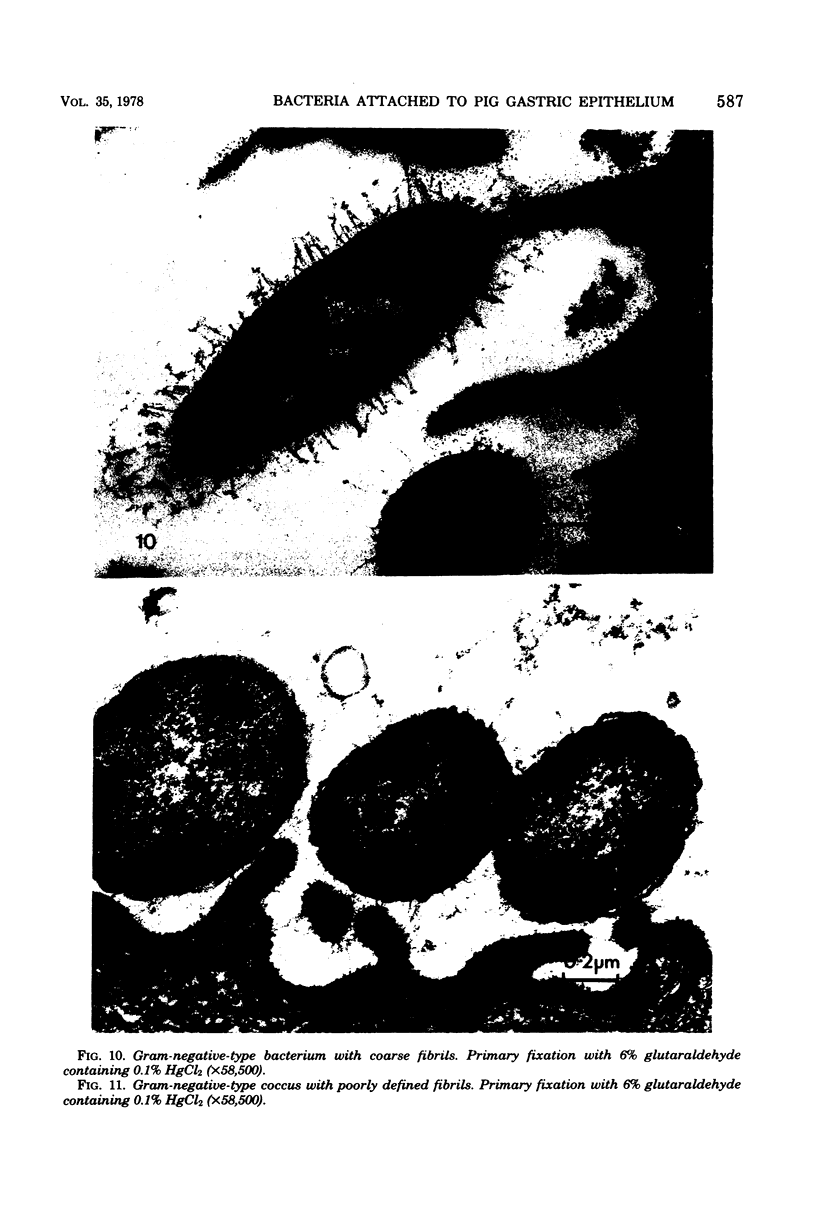



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E. Ultrastructure of rumen bacterial attachment to forage cell walls. Appl Environ Microbiol. 1976 Apr;31(4):562–568. doi: 10.1128/aem.31.4.562-568.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWNLEE A., MOSS W. The influence of diet on lactobacilli in the stomach of the rat. J Pathol Bacteriol. 1961 Oct;82:513–516. doi: 10.1002/path.1700820227. [DOI] [PubMed] [Google Scholar]
- Barrow P. A., Fuller R., Newport M. J. Changes in the microflora and physiology of the anterior intestinal tract of pigs weaned at 2 days, with special reference to the pathogenesis of diarrhea. Infect Immun. 1977 Dec;18(3):586–595. doi: 10.1128/iai.18.3.586-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. M., Gray W. A., Lara-Garcia W. Localization of bacteria on the rat tongue with scanning and transmission electron microscopy. J Dent Res. 1975 Jul-Aug;54(4):777–782. doi: 10.1177/00220345750540041401. [DOI] [PubMed] [Google Scholar]
- Brooker B. E., Fuller R. Adhesion of Lactobacilli to the chicken crop epithelium. J Ultrastruct Res. 1975 Jul;52(1):21–31. doi: 10.1016/s0022-5320(75)80019-0. [DOI] [PubMed] [Google Scholar]
- Collan Y., Sainio P. Relation of bacteria to exfoliated oral cells. An electron microscopic study. Acta Cytol. 1970 Nov-Dec;14(9):570–573. [PubMed] [Google Scholar]
- DUBOS R., SCHAEDLER R. W., COSTELLO R., HOET P. INDIGENOUS, NORMAL, AND AUTOCHTHONOUS FLORA OF THE GASTROINTESTINAL TRACT. J Exp Med. 1965 Jul 1;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. Some morphological and physiological characteristics of gram negative anaerobic bacteria isolated from the alimentary tract of the pig. J Appl Bacteriol. 1966 Aug;29(2):375–379. doi: 10.1111/j.1365-2672.1966.tb03486.x. [DOI] [PubMed] [Google Scholar]
- Fuller R. The importance of Lactobacilli in maintaining normal microbial balance in the crop. Br Poult Sci. 1977 Jan;18(1):85–94. doi: 10.1080/00071667708416332. [DOI] [PubMed] [Google Scholar]
- Fuller R., Turvey A. [Bacteria associated with the intestinal wall of the fowl (Gallus domesticus)]. J Appl Bacteriol. 1971 Sep;34(3):617–622. doi: 10.1111/j.1365-2672.1971.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Jayne-Williams D. J. The application of miniaturized methods for the characterization of various organisms isolated from the animal gut. J Appl Bacteriol. 1976 Apr;40(2):189–200. doi: 10.1111/j.1365-2672.1976.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Ability of Veillonella and Neisseria species to attach to oral surfaces and their proportions present indigenously. Infect Immun. 1971 Sep;4(3):264–268. doi: 10.1128/iai.4.3.264-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa M. Acidaminococcus gen. n., Acidaminococcus fermentans sp. n., anaerobic gram-negative diplococci using amino acids as the sole energy source for growth. J Bacteriol. 1969 May;98(2):756–766. doi: 10.1128/jb.98.2.756-766.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Smith J. M. The microflora of the pig stomach and its possible relationship to ulceration of the pars oesophagea. J Comp Pathol. 1970 Jul;80(3):359–367. doi: 10.1016/0021-9975(70)90066-6. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Ecology of human oral lactobacilli. Infect Immun. 1972 Nov;6(5):723–729. doi: 10.1128/iai.6.5.723-729.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]