Abstract
Laron syndrome [growth hormone (GH) insensitivity syndrome] is a hereditary dwarfism resulting from defects in the GH receptor (GHR) gene. GHR deficiency has not been reported in mammals other than humans. Many aspects of GHR dysfunction remain unknown because of ethical and practical limitations in studying humans. To create a mammalian model for this disease, we generated mice bearing a disrupted GHR/binding protein (GHR/BP) gene through a homologous gene targeting approach. Homozygous GHR/BP knockout mice showed severe postnatal growth retardation, proportionate dwarfism, absence of the GHR and GH binding protein, greatly decreased serum insulin-like growth factor I and elevated serum GH concentrations. These characteristics represent the phenotype typical of individuals with Laron syndrome. Animals heterozygous for the GHR/BP defect show only minimal growth impairment but have an intermediate biochemical phenotype, with decreased GHR and GH binding protein expression and slightly diminished insulin-like growth factor I levels. These findings indicate that the GHR/BP-deficient mouse (Laron mouse) is a suitable model for human Laron syndrome that will prove useful for the elucidation of many aspects of GHR/BP function that cannot be obtained in humans.
Laron syndrome, a recessively inherited disease with growth hormone (GH) resistance, was first described in 1966 (1). To date, more than 220 cases have been reported worldwide. Affected patients are characterized by very short stature, facial dysmorphism, truncal obesity, delayed puberty, recurrent hypoglycemia, low serum insulin-like growth factor I (IGF-I), elevated serum GH, absent, low, or dysfunctional serum GH binding protein (GHBP), and resistance to GH (2). The primary defect causing this disease is a mutated GH receptor (GHR). Absence of GH binding to GHRs in the liver has been demonstrated in two patients (3). A spectrum of ≈30 inactivating mutations affecting the expression or functions of the GHR and GHBP has been reported (2, 4–8). They include deletions, nonsense, frameshift, missense, and splice site mutations, mostly within the extracellular domain of the GHR, resulting in truncations, abnormal processing, or inability to bind ligand. Other mutations disable GHR dimerization or truncate the intracellular portion, with resulting inability to transduce a signal (9, 10). The phenotype of Laron syndrome is primarily defined on clinical and blood biochemical grounds because of ethical and practical limitations in defining tissue GHR status and its biology in humans. Several other issues related to GH resistance, such as long term IGF-I responsivity, longevity, immune function, oncogenesis, etc., are only partially known because of the slow growth phase and long life-span of humans. A suitable animal model would greatly facilitate a full investigation of the consequences of GHR dysfunction. However, to date, only one animal with a mutated GHR, the sex-linked dwarf chicken (11), has been discovered, and no mammalian model exists for the human disorder. The dwarf chicken is not an ideal paradigm for a human disease because of the significant taxonomic differences between birds and mammals. To create a mammalian model for Laron syndrome, we disrupted the mouse GHR/BP gene by homologous recombination and studied the growth and endocrine status of the resultant GHR/BP-deficient mice (Laron mice).
The GHR and GHBP are encoded by a single GHR/BP gene in mammalian species (4, 12, 13). The GHR is a single chain glycoprotein whereas the GHBP is a secreted short form that corresponds to the extracellular domain of the GHR (12). GHBP is generated either by proteolysis from the GHR (humans, rabbits, and pigs) (12, 14, 15) or through alternative splicing of a common GHR/BP pre-mRNA (rodents) (13, 16–18). The GHR and GHBP are expressed in virtually all tissues (19–21). The existence of other types of GHRs has been postulated (22) but thus far none has been identified.
MATERIALS AND METHODS
Gene Disruption Strategy.
We aimed to disrupt the mouse (m)GHR/BP gene by homologous recombination. The GHR/BP is encoded in both humans and mouse by 10 exons (4, 13), among which exon 4 encodes a portion of the GH binding domain (23). To produce the GHR/BP null mutation, a 500-bp DraIII fragment containing the 3′ portion of exon 4 and the adjacent 5′ region of intron 4/5 was replaced by a neo cassette (Fig. 1A). The rationale for disrupting the gene at exon 4 is 2-fold: (i) mutations in exon 4 have been reported in patients with Laron syndrome (4–7), and (ii) if alternative pre-mRNA splicing should unexpectedly occur between exons 3 and 5, the resulting frameshift would result in a premature stop codon in exon 5 (4, 13).
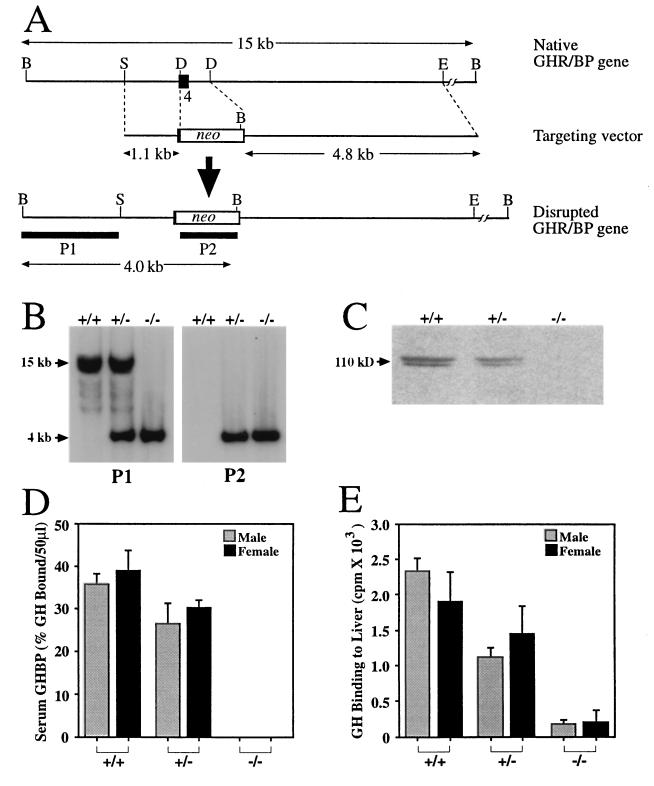
Figure 1.
Targeted disruption of the mGHR/BP gene. (A) Strategy for gene disruption. To construct the targeting vector, a DraIII–DraIII fragment containing a major portion of GHR exon 4 and ≈500 bp of intron 4/5 was replaced by the neomycin resistance (neo) gene. A herpes simplex virus thymidine kinase gene cassette (not shown) was placed at the 3′ end of the targeting vector for negative selection. The lengths (kilobases) of the left and right arms of the targeting vector and the important restriction fragments are indicated in the figure. B, BamHI; D, DraIII; E, EcoRI; and S, SacI. P1 and P2 denote hybridization probes. (B) Mouse genotyping with Southern blots using genomic DNA isolated from GHR/BP+/+, GHR/BP+/−, and GHR/BP−/− mice, digested with BamHI, and hybridized with 32P-labeled probes P1 or P2. The size of the native BamHI band was 15 kb, and that of the recombinant fragment was 4 kb. (C) Western blot analysis of liver GHR in a 4–12% gradient SDS/PAGE gel. The doublet appearance of the GHR is likely caused by variations in glycosylation. (D) GHBP activity in sera collected at 30 and 60 days of age (n = 6–7 mice in each group; data from 30- and 60-day-old animals pooled because they were not significantly different). No GHBP was detected in GHR/BP−/− mice (<30 pM). (E) Specific binding of [125I]-bGH to liver membrane preparations from 60-day-old mice (n = 9 animals in each group).
Construction of a GHR/BP Targeting Vector.
A 15-kb EcoRI fragment containing exon 4 of the mGHR/BP gene was isolated from a C57/Black mouse genomic library (13). To construct a GHR/BP targeting vector, a 500-bp DraIII fragment of the 6.4-kb SacI–EcoRI fragment was replaced by a 1.1-kb neomycin resistance gene (neo) cassette through blunt-end ligation (Fig. 1A). This replacement deleted the major portion of exon 4 and ≈500 bp of intron 4/5 of the GHR/BP gene. The neo cassette was flanked by mGHR/BP gene homologous sequences of 1.1 kb at the 5′ end and 4.8 kb at the 3′ terminus. A herpes simplex virus thymidine kinase gene (tk) cassette, which served as a negative selection marker (24), was placed at the 3′ end of the downstream mGHR/BP homologous sequence. Both the neo and tk cassettes were derived from plasmid pSSC9 (25).
Production of GHR/BP-Disrupted Mice.
Embryonic stem (ES) cell transfection and selection were performed as described with minor modifications (26, 27). In brief, 107 E14 ES cells derived from 129/Ola mice (28) were transfected with 30–40 μg of linearized mGHR/BP targeting vector by electroporation, followed by positive-negative selection with G418 and ganciclovir (24). Colonies of ES cells with homologous recombination were identified by Southern blot analysis after BamHI digestion of genomic DNA. The 1.7-kb BamHI–SacI fragment (P1) 5′ to the vector fragment and the neo cassette (P2) were labeled with 32P by random priming and were used as hybridization probes (Fig. 1A). Heterozygous GHR/BP-disrupted (GHR/BP+/−) ES cells were injected into BALB/c blastocysts. The injected blastocysts were transplanted into uteri of pseudopregnant BALB/c mice. Chimeric males were crossed to BALB/c females to produce GHR/BP+/− mice. The germ line transmission of GHR/BP+/− ES cells was monitored by detecting agouti-colored mice among the F1 offspring, and GHR/BP disruption was confirmed by Southern blotting. Homozygous GHR/BP-disrupted (GHR/BP−/−) mice were produced by GHR/BP+/− inbreeding and confirmed by Southern blot analysis.
Western Blot Analysis of GHR Expression.
Liver from GHR/BP+/+, GHR/BP+/−, and GHR/BP−/− mice was homogenized in lysis buffer as described (29). Total lysates (50 μg of protein) were fractionated on a 4–12% SDS/PAGE gel, transferred to Hybond membranes, and membranes probed with an antiserum directed against the mouse GHR intracellular domain (a gift from P. Frick and H. M. Goodman). The enhanced chemiluminescence (Amersham) system was used to visualize GHR protein.
GHBP Measurements.
GHBP in mouse serum was measured by a standardized GH binding assay as described (30, 31). In brief, 50 μl of serum was incubated with 105 cpm [125I]human (h)GH for 45 min at 37°C. (hGH binds with high affinity to the mouse GHBP and GHR.) Bound GH was separated from free GH by gel filtration and quantitated by peak integration. Nonspecific binding was determined in the presence of 2 μg/ml unlabeled hGH. Correction for the saturation by endogenous GH was made according to a saturation standard curve as described (30, 31).
GHR Determinations.
GH binding to liver microsomal membranes was determined as described (29). Membrane aliquots (200 μg of protein) were incubated with [125I]bovine (b)GH (50,000 cpm) at 4°C for 48 h. bGH, like hGH, binds to the mouse GHR. Here, bGH rather than hGH was used because the latter also binds to prolactin receptors that are present in liver membranes. (This is not a concern with serum, in which there is no detectable prolactin receptor/BP.) Nonspecific binding was determined in the presence of 200 nM of unlabeled bGH.
Hormone Measurements.
Serum mouse (m)GH was measured by a polyclonal radioimmunoassay using reagents (mGH as tracer and standard, anti-rat GH antiserum) kindly provided by A. F. Parlow and the National Hormone and Pituitary Program. Serum IGF-I levels were determined after acid–ethanol extraction using a human IGF-I RIA kit as described (29). [Because mouse IGF-I differs from human IGF-I in 4 of 70 residues (32), the human assay underestimates rodent IGF-I by a factor of 2–3 (33).]
Statistical Analysis.
Data were analyzed by contingency table, ANOVA, ANOVA for repeated measures, or Kruskal–Wallis ANOVA as appropriate, followed by the Newman–Keuls test. Data are given as mean ± SD. A P level of <0.05 was considered statistically significant.
RESULTS
Targeted Disruption of the GHR/BP Gene.
Out of 512 clones dual-resistant to both G418 and ganciclovir, two contained the desired mutation in one allele of the GHR/BP gene. No additional integrations of the targeting vector were detected when analyzed by Southern blotting by using a neo hybridization probe (data not shown). Six chimeric animals were produced from both clones when microinjected into BALB/c blastocysts, and two chimeric males transmitted the disrupted allele to offspring, yielding GHR/BP+/− heterozygotes. Inbreeding of the F1 generation of GHR/BP+/− mice produced homozygous GHR/BP−/− mice.
The complete disruption of the GHR/BP gene was confirmed by Southern blotting (Fig. 1B). In homozygous animals, only the mutant band was detected. In addition, we have been unable to detect GHR/BP mRNA in livers of GHR/BP−/− animals even by reverse transcription-PCR (data not shown). Genotyping of 243 offspring from GHR/BP+/− inbreeding revealed a frequency of 26% GHR/BP+/+ (34 male, 28 female), 51% GHR/BP+/− (54 male, 70 female), and 23% GHR/BP−/− (33 male, 24 female), consistent with a Mendelian pattern of inheritance. The sex distribution was statistically not different among the three genotypes (χ2 = 4.06, P > 0.1).
Western blot analyses demonstrated no detectable GHR protein in the liver of GHR/BP−/− mice and decreased GHR levels in GHR/BP+/− animals (Fig. 1C). No GHBP was detected in sera of GHR/BP−/− mice (<30 pM) (Fig. 1D). Heterozygous GHR/BP+/− littermates had mildly decreased GHBP levels—a finding that did not reach statistical significance in this study. These findings are consistent with those in Laron syndrome individuals bearing mutations in the extracellular domain of the GHR gene (34, 35). As shown in Fig. 1E, the ability of liver from GHR/BP−/− mice to bind bGH was greatly decreased compared with GHR/BP+/+ littermates although a small amount of residual binding was detectable. The residual binding is minimal but unexplained. Because bGH does not bind to prolactin receptors and because excess prolactin (1 μg/ml) did not diminish binding, the finding may indicate the presence of other type(s) of GH receptors. Alternatively, it may represent nonspecific background that is difficult to differentiate from zero binding. Heterozygous GHR/BP+/− mice had moderately decreased GH binding to liver, which differed statistically from both GHR/BP+/+ and GHR/BP−/− animals.
Viability and Fertility of GHR/BP Knockout Mice.
The average litter size from GHR/BP+/− inbreeding was 6.57 pups (Table 1). This is a typical litter size for GHR/BP+/+ crosses at the same breeding age. The average age of first pregnancy for GHR/BP−/− females was delayed, implying delayed sexual maturation, as also has been reported in Laron syndrome patients (2). Inbreeding of GHR/BP−/− mice gave an average of 2.71 pups per litter, which is significantly smaller than those derived from GHR/BP+/+ or GHR/BP+/− breeding. In addition, the perinatal or immediate postnatal mortality of newborns of GHR/BP−/− breeding was significantly higher than that of newborns of GHR/BP+/− or GHR/BP+/+ breeding. The reason for smaller litter size and higher mortality of offspring from GHR/BP−/− breeding is not certain but may relate to placentation, “obstetrical problems” due to maternal–fetal size mismatch, and/or insufficient lactation to nourish the pups. Inspection of the stomach contents of neonates suggested decreased milk consumption in offspring from GHR/BP−/− parents compared with those of GHR/BP+/− parents.
Table 1.
Viability and fertility of GHR/BP knockout mice
| Cross | Litter size mean (range) | Perinatal mortality, % | Female sexual maturation, weeks |
|---|---|---|---|
| +/+ × +/+ | 6.90 pups (3–13) | 5 | 6 |
| +/− × +/− | 6.57 pups (3–13) | 6 | 6 |
| −/− × −/− | 2.71 pups (1–6) | 26 | 10 |
Retarded Growth of GHR/BP Knockout Mice.
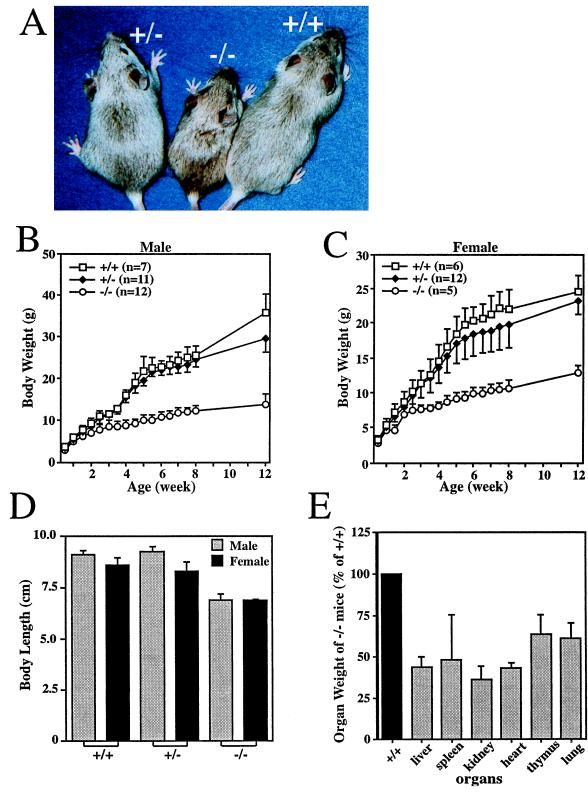
At birth, no significant body size or weight differences were observed among GHR/BP+/+, GHR/BP+/−, and GHR/BP−/− littermates. At 3 weeks after birth, the GHR/BP−/− mice were significantly smaller than either GHR/BP+/+ or GHR/BP+/− mice. With age, the difference in body size or weight increased progressively (Fig. 2A–C). Analysis of the growth curves showed a highly significant difference (P < 10−6) for both sexes between GHR/BP−/− and either GHR/BP+/− or GHR/BP+/+ animals. The growth curve up to 12 weeks for heterozygous males was indistinguishable from that of GHR/BP+/+ males (P ≈ 0.35) whereas for females the growth of heterozygotes was mildly but significantly retarded compared with GHR/BP+/+ females (P < 0.05). At 8 weeks of age, body length (nose–anus) in both sexes was significantly shorter in GHR/BP−/− than in GHR/BP+/− or GHR/BP+/+ mice (P < 0.01) whereas heterozygous and GHR/BP+/+ mice had similar body lengths. The length of 8-week-old GHR/BP−/− mice was significantly decreased when compared with GHR/BP+/− and GHR/BP+/+ littermates (Fig. 2D). Female GHR/BP+/− mice were shorter than female GHR/BP+/+ mice (P < 0.05), but the length difference between male GHR/BP+/− and GHR/BP+/+ mice was not statistically significant. The weights of major organs of GHR/BP−/− mice also were significantly decreased (P < 0.01) compared with GHR/BP+/+ or GHR/BP+/− littermates (Fig. 2E). Except for their smaller size, the organs exhibited no gross anatomic abnormalities.
Figure 2.
Retarded somatic growth of GHR/BP-deficient mice. (A) Photograph of female GHR/BP+/+, GHR/BP+/−, and GHR/BP−/− mice at 4 weeks of age. (B and C) Growth curves of male and female mice. (D) Body length at 60 days of age, determined from the tip of the nose to the anus (n = 3 in each group). (E) Organ weights of 60-day-old GHR/BP−/− mice, expressed as a percentage of the corresponding mean organ weight in GHR/BP+/+ mice (n = 3 for both groups).
Abnormal GH–IGF-I Axis of GHR/BP Knockout Mice.
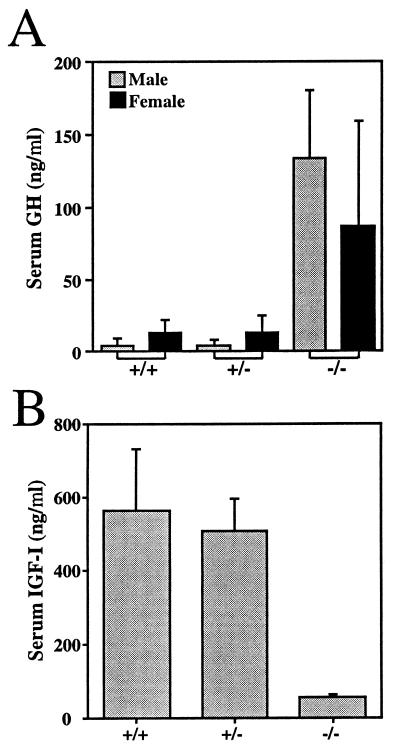
Serum GH levels for GHR/BP−/− mice were elevated greatly for both sexes compared with GHR/BP+/+ and GHR/BP+/− mice (P < 0.05) (Fig. 3A). In contrast, the GH levels of GHR/BP+/+ and GHR/BP+/− mice were comparable. The differences in GH levels between male and female mice were not statistically significant for any group. Serum IGF-I levels in GHR/BP−/− mice were decreased by ≈90% (P < 0.001) (Fig. 3B). IGF-I levels in GHR/BP+/+ and GHR/BP+/− mice were not significantly different. These results are similar to those seen in human Laron syndrome and can be attributed to defective IGF-I generation in response to GH and lack of negative feedback of IGF-I on GH secretion.
Figure 3.
Serum GH (A) and IGF-I (B) concentrations of GHR/BP+/+, GHR/BP+/−, and GHR/BP−/− mice. Blood was collected from 3–4 mice of each genotype on day 30 and day 60 after birth. Results from both time points were pooled.
Other Potential Abnormalities.
No other abnormalities were evident in the homozygous or heterozygous knockout mice. They appear healthy and vigorous up to the date of this writing. In particular, their behavior was indistinguishable from that of their wild-type littermates. Lactation in GHR/BP+/− mice appeared adequate to feed their young.
DISCUSSION
The GHR/BP knockout mouse (Laron mouse) we generated appeared to be a valid and suitable mammalian model for human Laron syndrome. Its physical and biochemical phenotype greatly resembled the human disease. Salient features shared between the two species are the absence of the GHR and GHBP, the severe postnatal growth failure, delayed puberty, and low IGF-I and elevated GH levels. The degree of growth retardation is similar to that in the human syndrome. It can thus be anticipated that this model will be useful in providing new answers to a number of unresolved questions in this field. Examples are: the long term growth response and final height/size achievement with IGF-I therapy; the effect of GHR/BP deficiency on longevity, immune function, gonadal function, fertility, and fecundity; as well as its impact on pathological processes suspected to be linked to GH (e.g., diabetic microvascular disease). The homogeneous genetic background, rapid breeding, short growth phase, and short life-span of this mouse model will accelerate the acquisition of knowledge that may take decades to accumulate in human Laron syndrome. Furthermore, the model will permit a detailed study of the absence of the GHR and GHBP on body composition and tissue characteristics at the histological, histochemical, and molecular levels—information that cannot be obtained in humans for ethical and technical reasons. Finally, the Laron mouse will serve as a useful null background for studies on the impact of gene replacement (e.g., GHBP vs. GHR vs. both) in a global or tissue-specific manner for physiological studies of the turnover and metabolic fate of GHBP and for dissecting the relative contributions of endocrine vs. paracrine IGF-I to growth and development through systemic IGF-I administration.
Some new insights already have been gained at this early stage. The birth weight of GHR/BP-deficient mice born of heterozygous parents was identical to that of wild-type mice. The role of GH in fetal growth and development, albeit minor, has been a matter of controversy because both GHR and GH are expressed in the fetus (36, 37). Our present data do not support a role of fetal GH in intrauterine growth up to the relatively “immature” stage at which mice are born. Inspection of the growth curves indicates that the GH dependence of postnatal growth commences at or shortly after birth rather than after a delay, as is sometimes suggested. There is a GHR gene dosage effect on the biochemical phenotype in that heterozygotes have GHR and GHBP levels intermediate between wild-type and homozygous knockout animals. The effect of heterozygosity on the physical phenotype is minimal but discernible within the first 12 weeks; growth in females appears more affected by gene dosage than in males. Visceral organs of Laron mice are small in size, with no other gross abnormality. The male/female ratio of homozygous GHR/BP knockout mice (1.37) is indistinguishable from that of wild-type mice (1.21), as would be expected for an autosomally transmitted disorder. This is a reassuring observation in view of the striking but unexplained female preponderance of Laron syndrome in the Ecuadorian population of Loja (2, 38). The normal sex ratio of Laron mice is concordant with the worldwide male/female ratio for Laron syndrome, which is close to unity (2, 8, 10); it suggests that deviations from unity in specific populations are due to either chance or factors independent of the GHR.
As mentioned above, an avian model for GHR deficiency exists in the form of the genetically heterogeneous sex-linked dwarf chicken (11). Although that model shares some features with mammalian GHR/BP deficiency, it is not an ideal model for human Laron syndrome for several reasons. Taxonomic and anatomic differences between mammals and birds render direct comparisons difficult. IGF regulation and IGF action may be different in birds and mammals (39, 40). The reproductive and perinatal biology of birds deviates greatly from that in mammals.
The growth curves of our Laron mice are very similar to that of the little (lit/lit) mouse that has isolated GH deficiency (41). In contrast, the Snell and Ames dwarf mice are more growth-retarded, presumably because they are hypothyroid in addition to being GH-deficient (42, 43).
Fertility appears to be preserved in homozygous GHR/BP knockout mice of both genders, indicating that GH action is not essential for fertility. This is consistent with several reported instances of fertility in both men and women affected with Laron syndrome. However, for mice born of two GHR/BP−/− parents, fetal outcome is not normal in that they have fewer siblings and decreased perinatal survival. It is presently not clear what is responsible for this phenomenon, but fetal–maternal size mismatch in utero and during birth or abnormal lactation or suckling are potential explanations. No corresponding information exists in humans because, to our knowledge, no union of two patients affected with Laron syndrome has been reported.
The residual GH binding in livers (and kidneys; not shown) of homozygous knockout mice is of interest. Although we did not detect GHR mRNA, we consistently found a small amount of GH binding activity above background. This may suggest that another GHR exists, a notion that has been postulated (22). If extant, this putative GHR does not appear to contribute to somatic growth as the animals are dwarfed, but it is an intriguing possibility that it may be involved in other GH-specific activities, such as metabolic and/or diabetogenic action. Further work will have to be performed to determine whether the residual GH binding activity represents a receptor.
The Laron mouse will perhaps be most useful in two areas of long term development in which human data are still limited or lacking. One is aging and longevity. It recently has been reported that Ames dwarf mice live significantly longer than their normal siblings, and GH deficiency has been implicated in this process (44). The life-span of GHR/BP knockout mice may yield new insights into a possible role of GH and IGF-I in senescence. Second, the Laron mouse may help answer a long-standing question in this field, namely whether IGF-I can promote long term linear growth and normal adult size in the absence of GH action. Isaksson et al. (45) have proposed that epiphyseal prechondrocytes must first differentiate under the influence of GH and only when differentiated undergo IGF-I-mediated clonal expansion, which leads to linear bone growth. The differentiated chondrocyte pool is limited and cannot be replenished by recruiting new chondrocytes except through continued GH action. Hence, they have suggested that IGF-I therapy of Laron syndrome may be effective for only a limited time, namely until the differentiated chondrocyte pool is depleted. Trials with chronic IGF-I therapy of Laron patients have been going on for ≈3–4 years, and IGF-I effectiveness is indeed declining with time (46, 47). The same is true, however, for GH therapy in GH deficiency, although perhaps to a lesser degree. It will take many years to determine the long term efficacy of IGF-I in human Laron syndrome. The same experiment, performed in the Laron mouse, will yield an answer regarding final size in 6 months. Thus, we hope that the present GHR/BP knockout mouse will provide the keystone in the proof of the somatomedin hypothesis.
In summary, we report the generation and initial data on the dwarf phenotype of a mouse with a GHR/BP gene knockout (the Laron mouse). In its homozygous and heterozygous form, the Laron mouse exhibits a phenotype that greatly resembles the human genetic disease known as Laron syndrome. Therefore, this GHR/BP-deficient mouse will be a useful model in the study of many unresolved aspects of GH-IGF-I function, such as aging, longevity, tumorigenesis, and diabetes and its complications.
Acknowledgments
We thank Dr. M. M. Gottesman for providing plasmid pSSC9, which contains the neo and tk genes, and Dr. J. Duffy for ES cells. This work was supported in part by the State of Ohio’s Eminent Scholar program, which includes a grant from the Milton and Lawrence Goll family, and by grants from the Sensus Corporation and the Northwestern Memorial Foundation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: GH, growth hormone; GHBP, GH binding protein; GHR, GH receptor; IGF-I, insulin-like growth factor I; ES, embryonic stem.
References
- 1.Laron Z, Pertzelan A, Mannheimer S. Isr J Med. 1966;2:152–155. [PubMed] [Google Scholar]
- 2.Rosenfeld R G, Rosenbloom A L, Guevara-Aguirre J. Endocr Rev. 1994;15:369–390. doi: 10.1210/edrv-15-3-369. [DOI] [PubMed] [Google Scholar]
- 3.Eshet R, Laron Z, Pertzelan A, Arnon R, Dintzman M. Isr J Med Sci. 1984;20:8–11. [PubMed] [Google Scholar]
- 4.Godowski P J, Leung D W, Meacham L R, Galgani J P, Hellmiss R, Keret R, Rotwein P S, Parks J S, Laron Z, Wood W I. Proc Natl Acad Sci USA. 1989;86:8083–8087. doi: 10.1073/pnas.86.20.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amselem S, Duquesnoy P, Duriez B, Dastot F, Sobrier M L, Valleix S, Goossens M. Hum Mol Genet. 1993;2:355–359. doi: 10.1093/hmg/2.4.355. [DOI] [PubMed] [Google Scholar]
- 6.Berg M A, Argente J, Chernausek S, Gracia R, Guevara-Aguirre J, Hopp M, Perez-Jurado L, Rosenbloom A, Toledo S P A, Francke U. Am J Hum Genet. 1993;52:998–1005. [PMC free article] [PubMed] [Google Scholar]
- 7.Sobrier M-L, Dastot F, Duquesnoy P, Kandemir N, Yordam N, Goossens M, Amselem S. J Clin Endocrinol Metab. 1997;82:435–437. doi: 10.1210/jcem.82.2.3725. [DOI] [PubMed] [Google Scholar]
- 8.Baumbach L, Schiavi A, Bartlett R, Perera E, Day J, Brown M R, Stein S, Eidson M, Parks J S, Cleveland W. J Clin Endocrinol Metab. 1997;82:444–451. doi: 10.1210/jcem.82.2.3784. [DOI] [PubMed] [Google Scholar]
- 9.Duquesnoy P, Sobrier M L, Duriez B, Dastot F, Buchanan C R, Savage M O, Preece M A, Craescu C T, Blouquit Y, Goossens M, Amselem S. EMBO J. 1994;13:1386–1395. doi: 10.1002/j.1460-2075.1994.tb06392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woods K A, Fraser N C, Postel-Vinay M C, Savage M O, Clark A J. J Clin Endocrinol Metab. 1996;81:1686–1690. doi: 10.1210/jcem.81.5.8626815. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal S K, Cogburn L A, Burnside J. J Endocrinol. 1994;142:427–434. doi: 10.1677/joe.0.1420427. [DOI] [PubMed] [Google Scholar]
- 12.Leung D W, Spencer S A, Cachianes G, Hammonds G R, Collins C, Henzel W J, Barnard R, Waters M J, Wood W I. Nature (London) 1987;330:537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, He L, Kopchick J J. Receptor. 1994;4:223–227. [PubMed] [Google Scholar]
- 14.Trivedi B, Daughaday W H. Endocrinology. 1988;123:2201–2206. doi: 10.1210/endo-123-5-2201. [DOI] [PubMed] [Google Scholar]
- 15.Sotiropoulos A, Goujon L, Simonin G, Kelly P A, Postel-Vinay M C, Finidori J. Endocrinology. 1993;132:1863–1865. doi: 10.1210/endo.132.4.8462483. [DOI] [PubMed] [Google Scholar]
- 16.Smith W C, Kuniyoshi J, Talamantes F. Mol Endocrinol. 1989;3:984–990. doi: 10.1210/mend-3-6-984. [DOI] [PubMed] [Google Scholar]
- 17.Baumbach W R, Horner D L, Logan J S. Genes Dev. 1989;3:1199–1205. doi: 10.1101/gad.3.8.1199. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, He L, Kopchick J J. Gene. 1996;177:257–259. doi: 10.1016/0378-1119(96)00277-6. [DOI] [PubMed] [Google Scholar]
- 19.Herington A C, Ymer S I, Tiong T S. Acta Ecdocrinol. 1991;124:14–20. [PubMed] [Google Scholar]
- 20.Lobie P E, Garcia-Aragon J, Wang B S, Baumbach W R, Waters M J. Endocrinology. 1992;130:3057–3065. doi: 10.1210/endo.130.5.1374020. [DOI] [PubMed] [Google Scholar]
- 21.Mercado M, Davila N, McLeod J F, Baumann G. J Clin Endocrinol Metab. 1994;78:731–735. doi: 10.1210/jcem.78.3.8126150. [DOI] [PubMed] [Google Scholar]
- 22.Mathews L S. Trends Endocrinol Metab. 1991;2:176–180. doi: 10.1016/1043-2760(91)90015-f. [DOI] [PubMed] [Google Scholar]
- 23.De Vos A M, Ultsch M, Kossiakoff T. Science. 1992;255:306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]
- 24.Mansour S L, Thomas K R, Capecchi M R. Nature (London) 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 25.Chauhan S S, Gottesman M M. Gene. 1992;120:281–285. doi: 10.1016/0378-1119(92)90106-y. [DOI] [PubMed] [Google Scholar]
- 26.Wurst W, Joyner A L. In: Gene Targeting: A Practical Approach. Joyner A L, editor. New York: Oxford Univ. Press; 1993. pp. 33–61. [Google Scholar]
- 27.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd Ed. Plainview, New York: Cold Spring Harbor Lab. Press; 1994. pp. 255–264. [Google Scholar]
- 28.Hooper M, Hardy K, Handyside A, Hunter S, Monk M. Nature (London) 1987;326:292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- 29.Chen W Y, Wight M E, Wagner T E, Kopchick J J. Endocrinology. 1991;129:1402–1408. doi: 10.1210/endo-129-3-1402. [DOI] [PubMed] [Google Scholar]
- 30.Baumann G, Shaw M A, Amburn K. Metabolism. 1989;38:683–689. doi: 10.1016/0026-0495(89)90108-x. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, He L, Baumann G, Kopchick J J. J Mol Endocrinol. 1997;19:1–13. doi: 10.1677/jme.0.0190001. [DOI] [PubMed] [Google Scholar]
- 32.Bell G I, Stempien M M, Fong N M, Rall L B. Nucleic Acids Res. 1986;14:7873–7882. doi: 10.1093/nar/14.20.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee P D K, Baker B K, Liu F, Kwan E Y, Hintz R L. J Clin Endocrinol Metab. 1996;81:2002–2005. doi: 10.1210/jcem.81.5.8626873. [DOI] [PubMed] [Google Scholar]
- 34.Daughaday W H, Trivedi B. Proc Natl Acad Sci USA. 1987;84:4636–4640. doi: 10.1073/pnas.84.13.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumann G, Shaw M A, Winter R J. J Clin Endocrinol Metab. 1987;65:814–816. doi: 10.1210/jcem-65-4-814. [DOI] [PubMed] [Google Scholar]
- 36.Nicoll C S, Liu L, Alarid E, Chiang M, Russell S M. Growth Regul. 1991;1:133–144. [PubMed] [Google Scholar]
- 37.Garcia-Aragon J, Lobie P E, Muscat G E O, Gobius K S, Norstedt G, Waters M J. Development. 1992;114:869–876. doi: 10.1242/dev.114.4.869. [DOI] [PubMed] [Google Scholar]
- 38.Rosenbloom A L, Guevara-Aguirre J, Rosenfeld R G, Fielder P J. N Engl J Med. 1990;323:1367–1374. doi: 10.1056/NEJM199011153232002. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka M, Hayashida Y, Sakaguchi K, Ohkubo T, Wakita M, Hoshino S, Nakashima K. Endocrinology. 1996;137:30–34. doi: 10.1210/endo.137.1.8536628. [DOI] [PubMed] [Google Scholar]
- 40.Tixier-Boichard M, Huybrechts L M, Decuypere E, Kuhn E R, Monvoisin J L, Coquerelle G, Charrier J, Simon J. J Endocrinol. 1992;133:101–110. doi: 10.1677/joe.0.1330101. [DOI] [PubMed] [Google Scholar]
- 41.Donahue L R, Beamer W G. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- 42.Van Buul S, Van den Brande J L. Acta Endocrinol. 1978;89:632–645. [PubMed] [Google Scholar]
- 43.Bartke A. Gen Comp Endocrinol. 1965;5:418–426. doi: 10.1016/0016-6480(65)90102-4. [DOI] [PubMed] [Google Scholar]
- 44.Brown-Borg H M, Borg K E, Meliska C J, Bartke A. Nature (London) 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 45.Ohlsson C, Nilsson A, Isaksson O, Lindahl A. Proc Natl Acad Sci USA. 1992;89:9826–9830. doi: 10.1073/pnas.89.20.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klinger B, Laron Z. J Pediatr Endocrinol Metab. 1995;8:149–158. doi: 10.1515/jpem.1995.8.3.149. [DOI] [PubMed] [Google Scholar]
- 47.Guevara-Aguirre J, Rosenbloom A L, Vasconez O, Martinez V, Gargosky S E, Allen L, Rosenfeld R G. J Clin Endocrinol Metab. 1997;82:629–633. doi: 10.1210/jcem.82.2.3743. [DOI] [PubMed] [Google Scholar]