Abstract
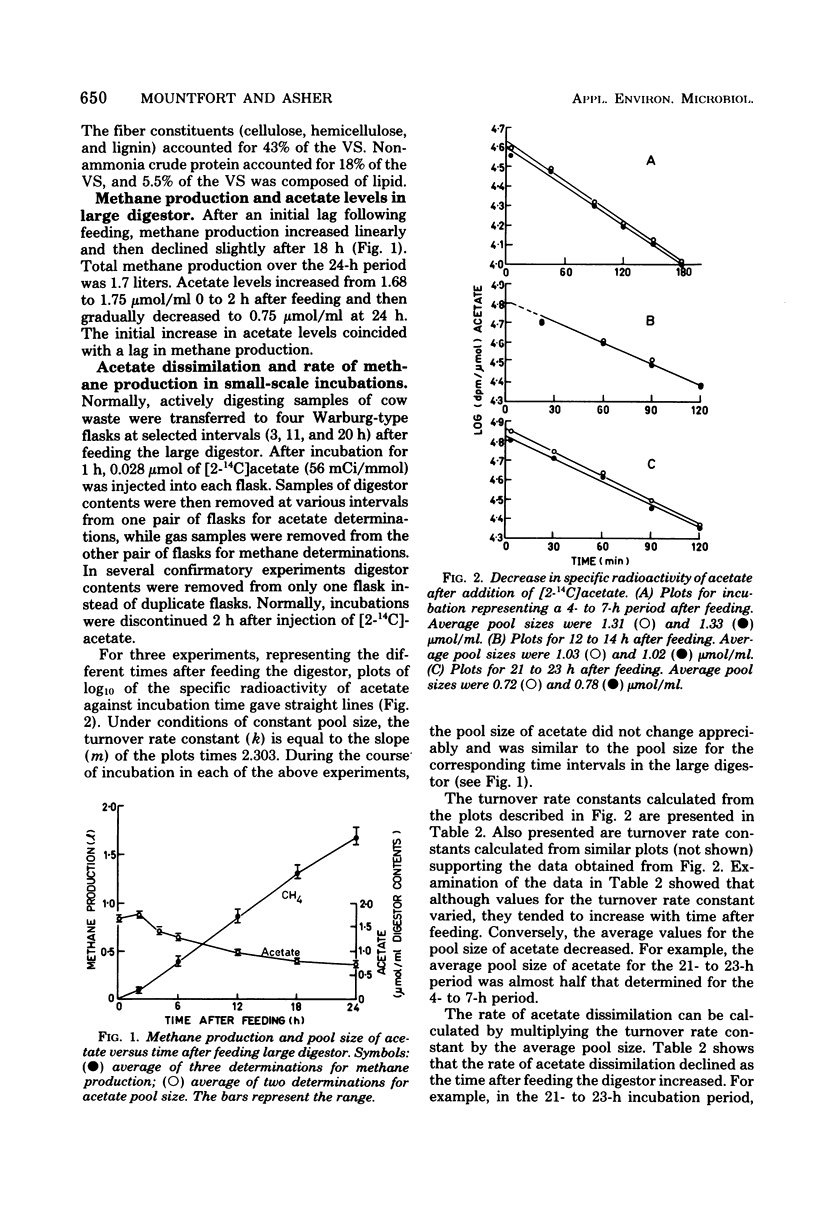
In an anaerobic digestor which was fed daily with bovine waste, during the early stages after feeding (4 to 7 h) acetate (via the methyl group) accounted for almost 90% of the methane produced. As time after feeding increased, acetate declined as a precursor so that in the 12- to 14-h and 21- to 23-h periods, after feeding the methyl group accounted for 80 and 73% of the methane produced, respectively. Measurements of methane production from CO2 reduction showed that in the 2- to 12-h period after feeding, CO2 accounted for 14% of the methane produced, whereas in the 12- to 24-h period it accounted for 27-5%. These results show that the percentages of methane accounted for by acetate and CO2 vary with time after feeding the digestor.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Cappenberg T. E., Prins R. A. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. 3. Experiments with 14C-labeled substrates. Antonie Van Leeuwenhoek. 1974;40(3):457–469. doi: 10.1007/BF00399358. [DOI] [PubMed] [Google Scholar]
- Mahadevan V., Stenroos L. Quantitative analysis of volatile fatty acids in aqueous solution by gas chromatography. Anal Chem. 1967 Nov;39(13):1652–1654. doi: 10.1021/ac50156a046. [DOI] [PubMed] [Google Scholar]
- STADTMAN T. C., BARKER H. A. Studies on the methane fermentation. IX. The origin of methane in the acetate and methanol fermentations by methanosarcina. J Bacteriol. 1951 Jan;61(1):81–86. doi: 10.1128/jb.61.1.81-86.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. C. Triton X-100 scintillant for carbon-14 labelled materials. Int J Appl Radiat Isot. 1968 Jul;19(7):557–563. doi: 10.1016/0020-708x(68)90065-3. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol. 1977 Feb 4;112(1):119–122. doi: 10.1007/BF00446665. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


