Abstract
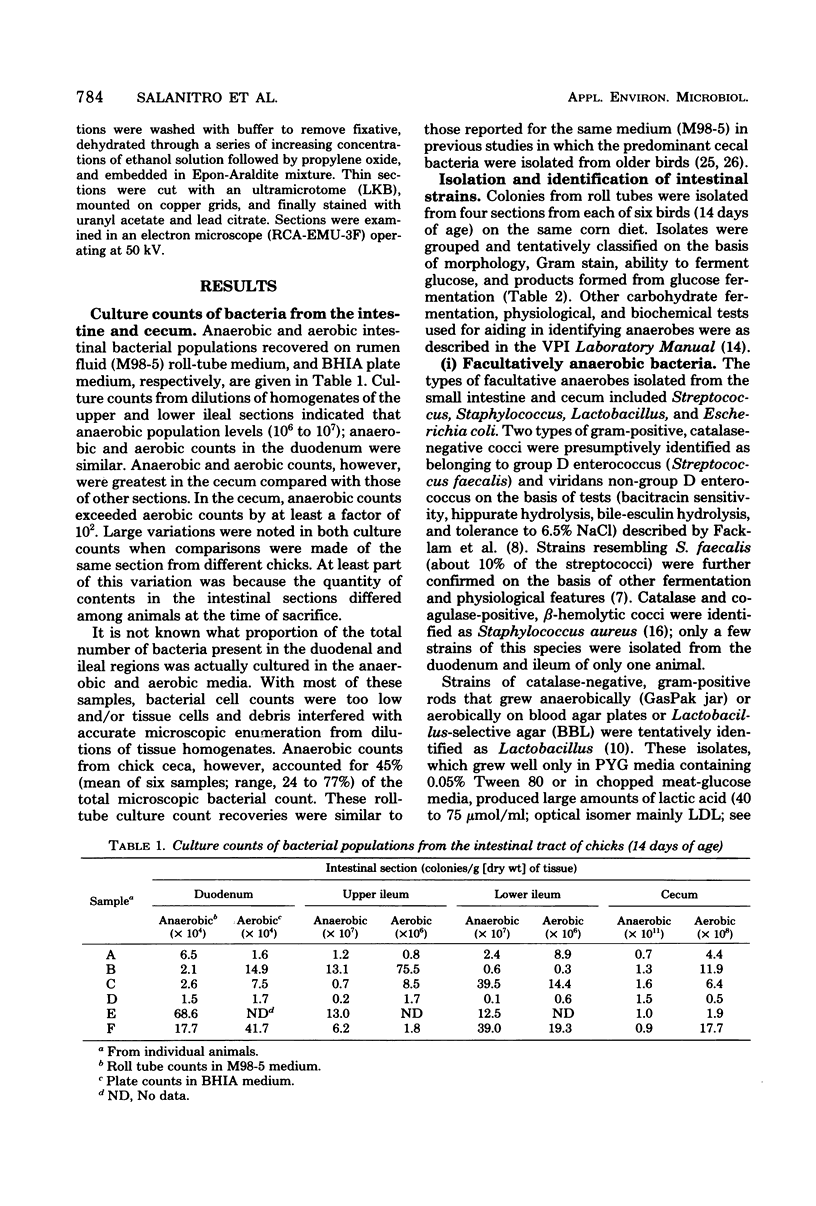
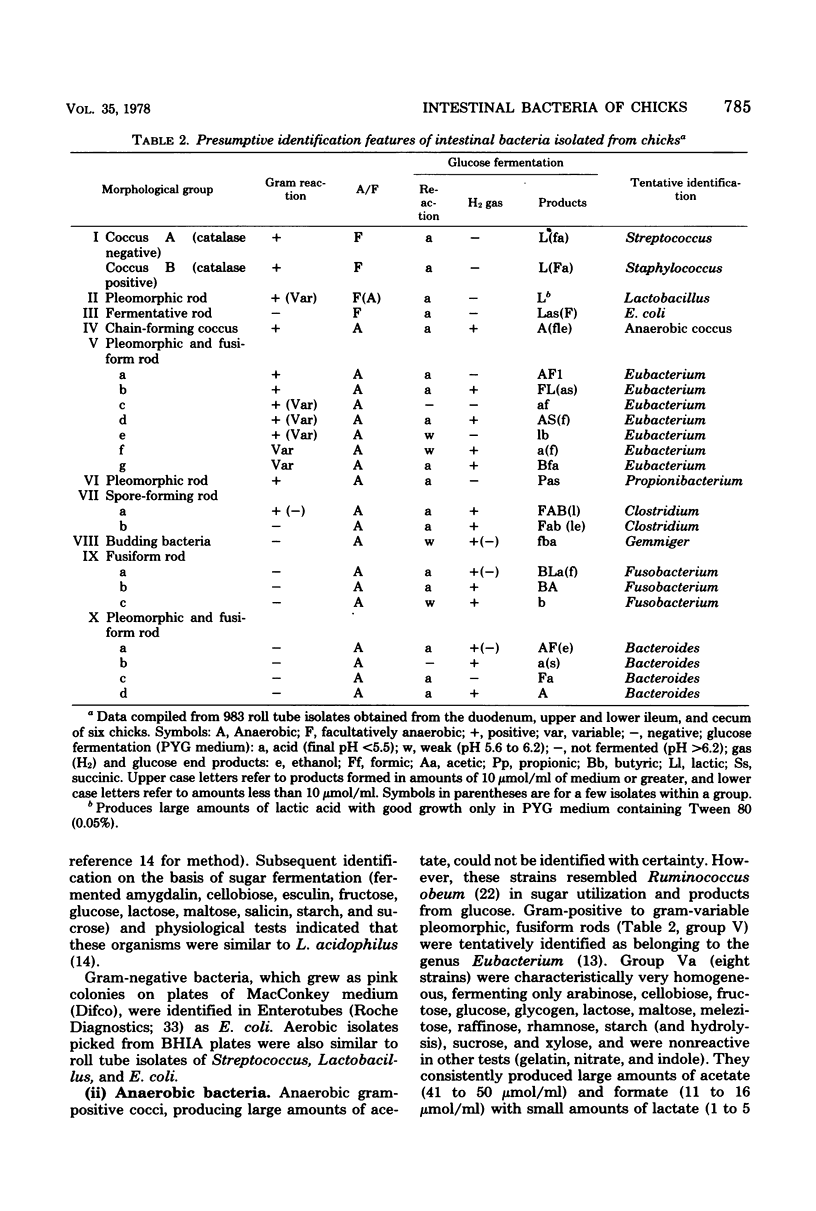
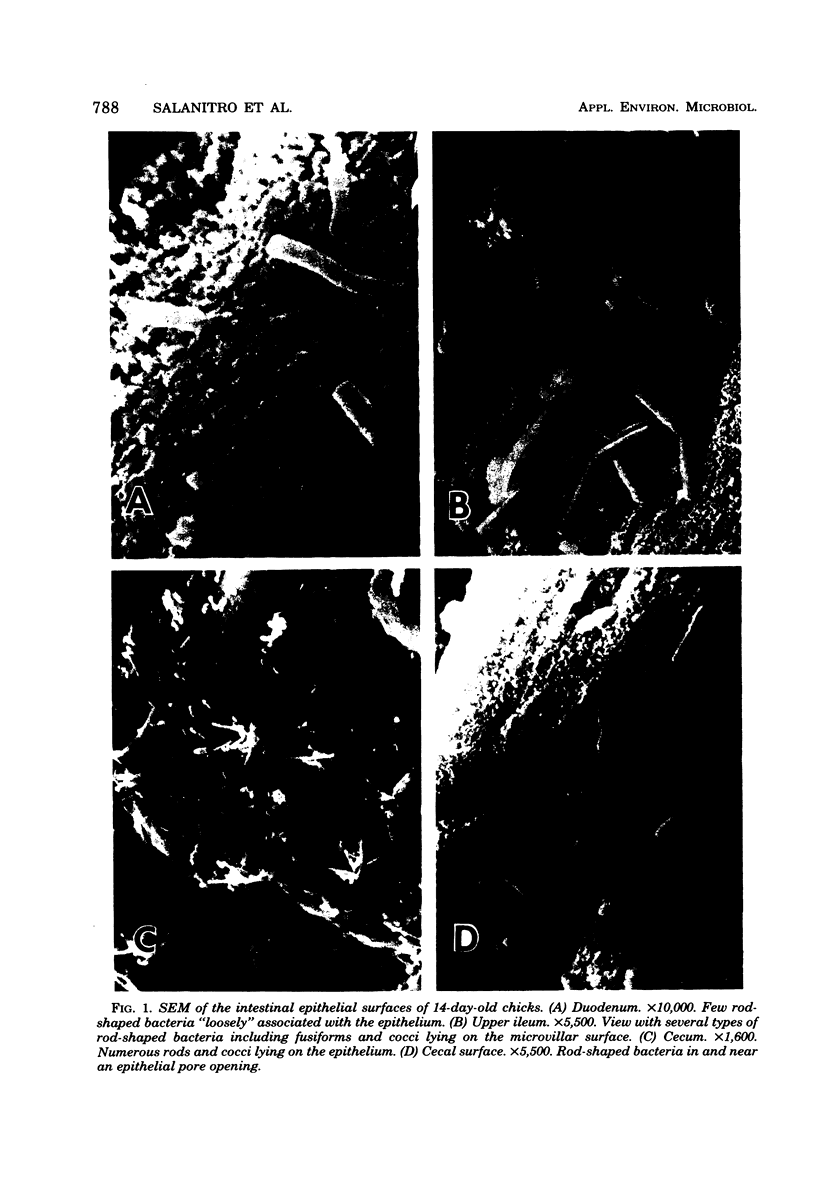
Facultatively anaerobic and strictly anaerobic bacteria colonizing the intestinal tracts of 14-day-old chicks fed a corn-based diet were enumerated, isolated, and identified. Colony counts from anaerobic roll tubes (rumen fluid medium) or aerobic plates (brain heart infusion agar) recovered from homogenates of the duodenum, upper and lower ileum, and cecum varied appreciably among samples from individual birds. Anaerobic and aerobic counts from the duodenum and ileum were similar. Anaerobic counts were highest from the cecum (0.7 X 10(11) to 1.6 X 10(11)/g of dry tissue) and exceeded aerobic plate counts by a factor of at least 10(2). Facultatively anaerobic groups (Streptococcus, Staphylococcus, Lactobacillus, and Escherichia coli) comprised the predominant flora of the duodenum and ileum, although large numbers of anaerobes (9 to 39% of the small intestine isolates), represented by species of Eubacterium, Propionibacterium, Clostridium, Gemmiger, and Fusobacterium, were also recovered. Strict anaerobes (anaerobic gram-positive cocci, Eubacterium, Clostridium Gemmiger, Fusobacterium, and Bacteriodes) made up nearly the entire microbial population of the cecum. Scanning electron microscopy of the intestinal epithelia of chicks revealed populations of microbes on the duodenal, ileal, and cecal mucosal surfaces.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Impey C. S. Some properties of the nonsporing anaerobes from poultry caeca. J Appl Bacteriol. 1972 Jun;35(2):241–251. doi: 10.1111/j.1365-2672.1972.tb03696.x. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Mead G. C., Barnum D. A., Harry E. G. The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. Br Poult Sci. 1972 May;13(3):311–326. doi: 10.1080/00071667208415953. [DOI] [PubMed] [Google Scholar]
- Brooker B. E., Fuller R. Adhesion of Lactobacilli to the chicken crop epithelium. J Ultrastruct Res. 1975 Jul;52(1):21–31. doi: 10.1016/s0022-5320(75)80019-0. [DOI] [PubMed] [Google Scholar]
- Davis C. P. Preservation of gastrointestinal bacteria and their microenvironmental associations in rats by freezing. Appl Environ Microbiol. 1976 Feb;31(2):304–312. doi: 10.1128/aem.31.2.304-312.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Padula J. F., Thacker L. G., Wortham E. C., Sconyers B. J. Presumptive identification of group A, B, and D streptococci. Appl Microbiol. 1974 Jan;27(1):107–113. doi: 10.1128/am.27.1.107-113.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl Microbiol. 1972 Jun;23(6):1131–1139. doi: 10.1128/am.23.6.1131-1139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R., Brooker B. E. Lactobacilli which attach to the crop epithelium of the fowl. Am J Clin Nutr. 1974 Nov;27(11):1305–1312. doi: 10.1093/ajcn/27.11.1305. [DOI] [PubMed] [Google Scholar]
- Gilliland S. E., Speck M. L., Morgan C. G. Detection of Lactobacillus acidophilus in feces of humans, pigs, and chickens. Appl Microbiol. 1975 Oct;30(4):541–545. doi: 10.1128/am.30.4.541-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUHTANEN C. N., PENSACK J. M. THE DEVELOPMENT OF THE INTESTINAL FLORA OF THE YOUNG CHICK. Poult Sci. 1965 May;44:825–830. doi: 10.3382/ps.0440825. [DOI] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead G. C., Adams B. W. Some observations on the caecal microflora of the chick during the first two weeks of life. Br Poult Sci. 1975 Mar;16(2):169–176. doi: 10.1080/00071667508416174. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotomi M., Watanabe T., Suegara N., Kawai Y., Mutai M. Distribution of indigenous bacteria in the digestive tract of conventional and gnotobiotic rats. Infect Immun. 1975 May;11(5):962–968. doi: 10.1128/iai.11.5.962-968.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi Y., Mitsuoka T., Sega T. Untersuchungen über die Darmflora des Huhnes. III. Die Entwicklung der Darmflora von Küken bis zum Huhn. Zentralbl Bakteriol Orig. 1964 Jun;193(1):80–95. [PubMed] [Google Scholar]
- Salanitro J. P., Blake I. G., Muirhead P. A. Studies on the cecal microflora of commercial broiler chickens. Appl Microbiol. 1974 Sep;28(3):439–447. doi: 10.1128/am.28.3.439-447.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Fairchilds I. G., Zgornicki Y. D. Isolation, culture characteristics, and identification of anaerobic bacteria from the chicken cecum. Appl Microbiol. 1974 Apr;27(4):678–687. doi: 10.1128/am.27.4.678-687.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Muirhead P. A. Quantitative method for the gas chromatographic analysis of short-chain monocarboxylic and dicarboxylic acids in fermentation media. Appl Microbiol. 1975 Mar;29(3):374–381. doi: 10.1128/am.29.3.374-381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Blumershine R. V. Surface-surface associations in microbial communities populating epithelial habitats in the murine gastrointestinal ecosystem: scanning electron microscopy. Infect Immun. 1974 Jul;10(1):240–250. doi: 10.1128/iai.10.1.240-250.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Microbial interference between indigenous yeast and lactobacilli in the rodent stomach. J Bacteriol. 1969 Jun;98(3):1278–1283. doi: 10.1128/jb.98.3.1278-1283.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. The development of the flora of the alimentary tract in young animals. J Pathol Bacteriol. 1965 Oct;90(2):495–513. [PubMed] [Google Scholar]
- Timms L. Observations on the bacterial flora of the alimentary tract in three age groups of normal chickens. Br Vet J. 1968 Oct;124(10):470–477. doi: 10.1016/s0007-1935(17)39155-8. [DOI] [PubMed] [Google Scholar]
- Tomfohrde K. M., Rhoden D. L., Smith P. B., Balows A. Evaluation of the redesigned enterotube--a system for the identification of Enterobacteriaceae. Appl Microbiol. 1973 Feb;25(2):301–304. doi: 10.1128/am.25.2.301-304.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]