Abstract
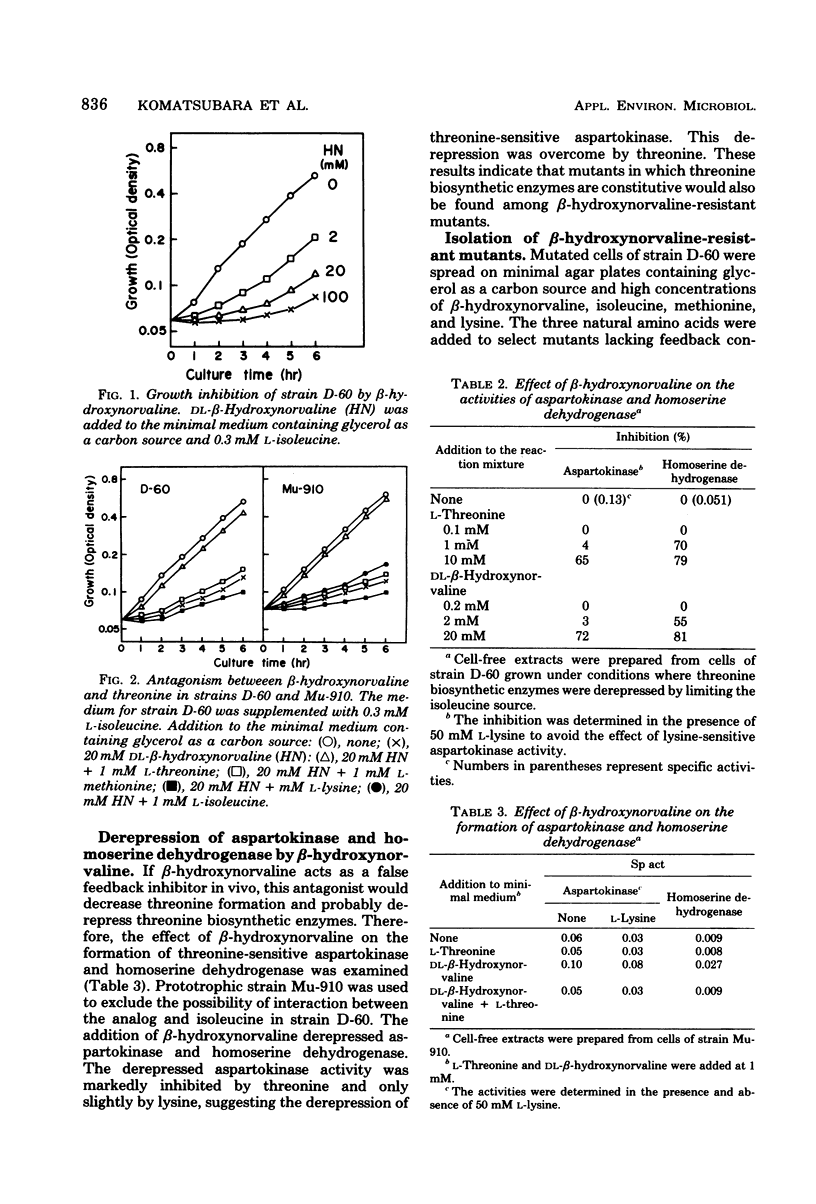
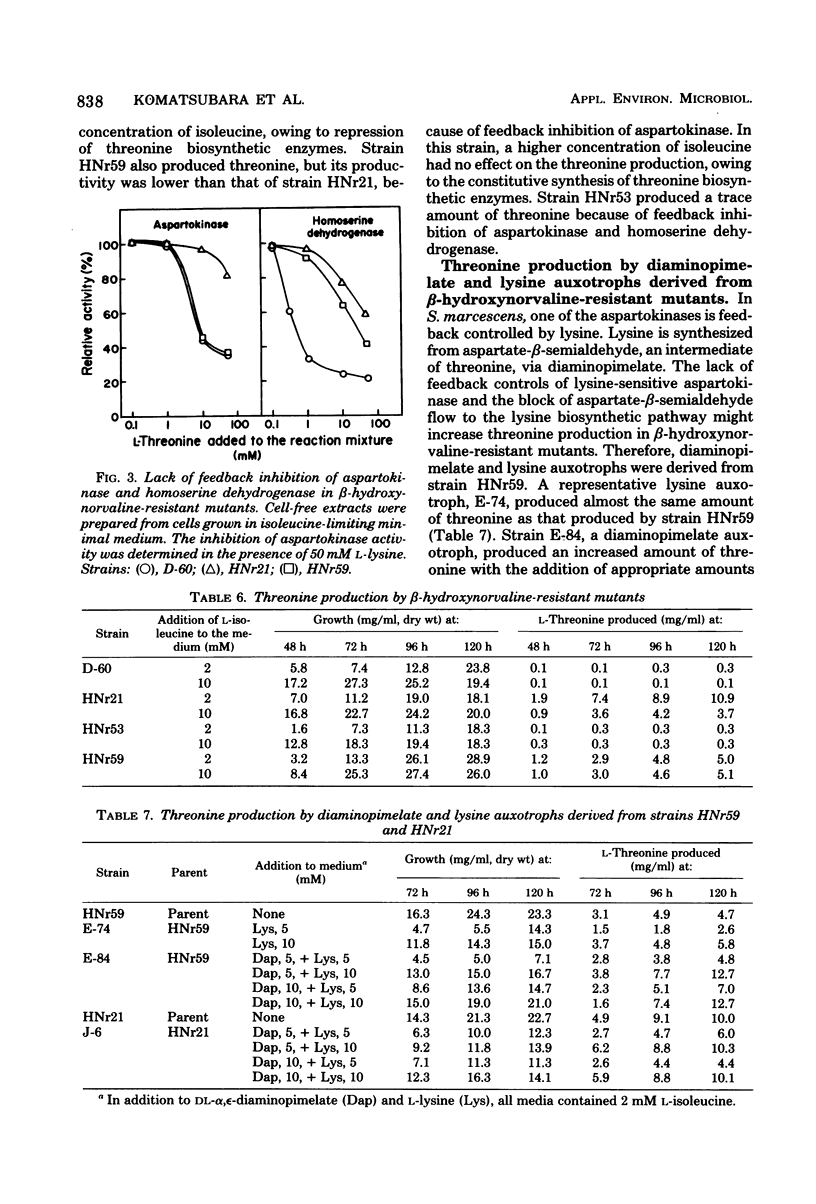
beta-Hydroxynorvaline (alpha-amino-beta-hydroxyvaleric acid)-resistant mutants of Serratia marcescens deficient in both threonine dehydrogenase and threonine deaminase were isolated and characterized. One of the mutants, strain HNr21, lacked feedback inhibition of threonine-sensitive aspartokinase and homoserine dehydrogenase, was repressed for the two enzymes, and produced 11 mg of threonine per ml of medium containing a limiting amount of isoleucine. The other mutant, strain HNr59, was constitutively derepressed for aspartokinase and homoserine dehydrogenase. Its kinase was sensitive to feedback inhibition, but its dehydrogenase was insensitive to feedback inhibition. This strain produced 5 mg of threonine per ml of medium containing either a limiting or an excess amount of isoleucine. Diaminopimelate auxotrophs derived from strain HNr59 produced more threonine (13 mg/ml) than the parent strain. However, similar auxotrophs derived from strain HNr21 produced the same amount of threonine as that produced by the parent strain.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUSTON H. W., CHURCHMAN J., BISHOP J. Synthetic alpha-amino-beta-hydroxyvaleric acids. J Biol Chem. 1953 Oct;204(2):665–668. [PubMed] [Google Scholar]
- Cafferata R. L., Freundlich M. Evidence for channeling of homoserine in Salmonella typhimurium. Biochim Biophys Acta. 1970 Dec 29;222(3):671–674. doi: 10.1016/0304-4165(70)90196-0. [DOI] [PubMed] [Google Scholar]
- KISUMI M. Studies on the isoleucine fermentation. I. Screening of organisms and investigation of cultural conditions. J Biochem. 1962 Dec;52:390–399. doi: 10.1093/oxfordjournals.jbchem.a127635. [DOI] [PubMed] [Google Scholar]
- Kisumi M., Kato J., Komatsubara S., Chibata I. Increase in isoleucine accumulation by alpha-aminobutyric acid-resistant mutants of Serratia marcescens. Appl Microbiol. 1971 Apr;21(4):569–574. doi: 10.1128/am.21.4.569-574.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Enhancement of isoleucine hydroxamate-mediated growth inhibition and improvement of isoleucine-producing strains of Serratia marcescens. Appl Environ Microbiol. 1977 Dec;34(6):647–653. doi: 10.1128/aem.34.6.647-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Valine accumulation by alpha-aminobutyric acid-resistant mutants of Serratia marcescens. J Bacteriol. 1971 May;106(2):493–499. doi: 10.1128/jb.106.2.493-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Isoleucine accumulation by regulatory mutants of Serratia marcescens: lack of both feedback inhibition and repression. J Bacteriol. 1972 May;110(2):761–763. doi: 10.1128/jb.110.2.761-763.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Isoleucine hydroxamate, an isoleucine antagonist. J Bacteriol. 1971 Sep;107(3):741–745. doi: 10.1128/jb.107.3.741-745.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Sugiura M., Chibata I. Properties of isoleucine hydroxamate-resistant mutants of Serratia marcescens. J Gen Microbiol. 1971 Dec;69(3):291–297. doi: 10.1099/00221287-69-3-291. [DOI] [PubMed] [Google Scholar]
- Kisumi M., Nakanishi N., Takagi T., Chibata I. L-Histidine production by histidase-less regulatory mutants of Serratia marcescens constructed by transduction. Appl Environ Microbiol. 1977 Nov;34(5):465–472. doi: 10.1128/aem.34.5.465-472.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotre A. M., Sullivan S. J., Savageau M. A. Metabolic regulation by homoserine in Escherichia coli B-r. J Bacteriol. 1973 Nov;116(2):663–672. doi: 10.1128/jb.116.2.663-672.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsumoto H., Hosogaya S., Suzuki K., Tazaki T. Arginine gene cluster of Serratia marcescens. Jpn J Microbiol. 1975 Feb;19(1):35–44. doi: 10.1111/j.1348-0421.1975.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Margarita D. Operator-constitutive mutants in the threonine operon of Escherichia coli K-12. J Bacteriol. 1975 Dec;124(3):1137–1141. doi: 10.1128/jb.124.3.1137-1141.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailaja M. S., Raghavendra Rao M. R. Methionine-repressible homoserine dehydrogenase of Serratia marcescens: purification and properties. Mol Cell Biochem. 1976 Jul 30;12(1):15–22. doi: 10.1007/BF01731899. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]
- Weiner R. M., Voll M. J., Cook T. M. Nalidixic acid for enrichment of auxotrophs in cultures of Salmonella typhimurium. Appl Microbiol. 1974 Oct;28(4):579–581. doi: 10.1128/am.28.4.579-581.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


