Abstract
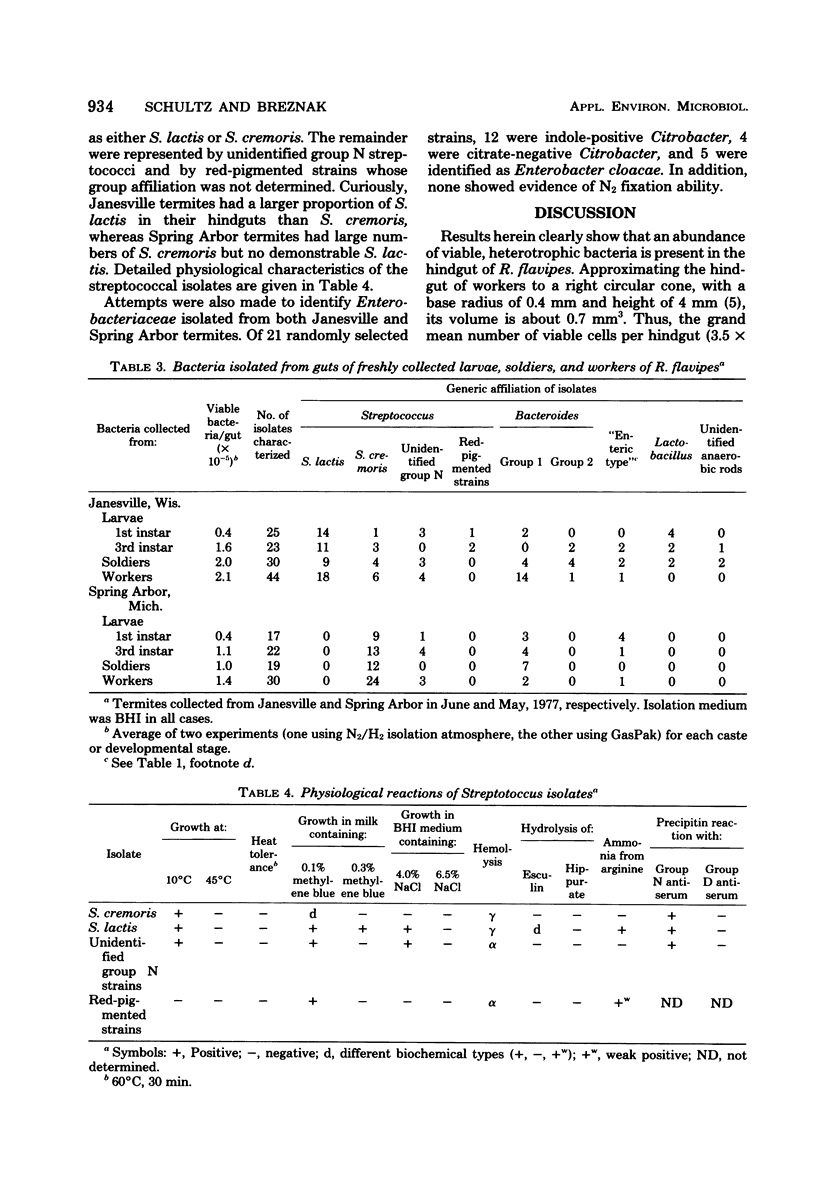
Strict anaerobic culture techniques were used to quantitate heterotrophic bacteria present in hindguts of Reticulitermes flavipes. The grand mean number of viable cells per hindgut was 0.4 X 10(5) (first-instar larvae), 1.3 X 10(5) (third-instar larvae), 3.5 X 10(5) (workers), and 1.5 X 10(5) (soldiers). Of a total of 344 isolates, 66.3% were streptococci that were always obtained regardless of the origin of termites, their developmental stage or caste, or their length of captivity. Most of the remaining isolates were strains of Bacteroides and Enterobacteriaceae. A small percentage were strains of Lactobacillus, Fusobacterium, and unidentified anaerobic gram-positive rods. Recovery of bacteria from worker hindguts was 13.0% of the direct microscopic count. Isolations performed aerobically failed to reveal strict aerobes. Attempts to isolate cellulolytic bacteria were uniformly unsuccessful. Of 145 streptococcal strains isolated from freshly collected termites, almost all were Streptococcus lactis and S. cremoris. Enterobacteriaceae isolates from the same termite specimens were indole-positive Citrobacter, citrate-negative Citrobacter, and Enterobacter cloacae. The possibility of in situ interspecies lactate transfer, between lactate producers (e.g., streptococci) and lactate fermenters (Bacteroides), is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A., Brill W. J., Mertins J. W., Coppel H. C. Nitrogen fixation in termites. Nature. 1973 Aug 31;244(5418):577–580. doi: 10.1038/244577a0. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Pankratz H. S. In situ morphology of the gut microbiota of wood-eating termites [Reticulitermes flavipes (Kollar) and Coptotermes formosanus Shiraki]. Appl Environ Microbiol. 1977 Feb;33(2):406–426. doi: 10.1128/aem.33.2.406-426.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A. Symbiotic relationships between termites and their intestinal microbiota. Symp Soc Exp Biol. 1975;(29):559–580. [PubMed] [Google Scholar]
- Bryant M. P. Nutritional features and ecology of predominant anaerobic bacteria of the intestinal tract. Am J Clin Nutr. 1974 Nov;27(11):1313–1319. doi: 10.1093/ajcn/27.11.1313. [DOI] [PubMed] [Google Scholar]
- DWORKIN M., FOSTER J. W. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 1958 May;75(5):592–603. doi: 10.1128/jb.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibel R. H. Utilization of arginine as an energy source for the growth of Streptococcus faecalis. J Bacteriol. 1964 May;87(5):988–992. doi: 10.1128/jb.87.5.988-992.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco D. E., Mah R. A., Rabin A. C. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Trans Am Microsc Soc. 1973 Jul;92(3):416–421. [PubMed] [Google Scholar]
- French J. R., Turner G. L., Bradbury J. F. Nitrogen fixation by bacteria from the hindgut of termites. J Gen Microbiol. 1976 Aug;96(2):202–206. doi: 10.1099/00221287-95-2-202. [DOI] [PubMed] [Google Scholar]
- Hungate R. E. Studies on Cellulose Fermentation: II. An Anaerobic Cellulose-decomposing Actinomycete, Micromonospora propionici, N. Sp. J Bacteriol. 1946 Jan;51(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- KENNER B. A., CLARK H. F., KABLER P. W. Fecal Streptococci. I. Cultivation and enumeration of Streptococci in surface waters. Appl Microbiol. 1961 Jan;9:15–20. doi: 10.1128/am.9.1.15-20.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. D., Mundt J. O. Enterococci in insects. Appl Microbiol. 1972 Oct;24(4):575–580. doi: 10.1128/am.24.4.575-580.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POCHON J., DE BARJAC H., ROCHE A. Recherches sur la digestion de la cellulose chez le termite Sphaerotermes sphaerothorax. Ann Inst Pasteur (Paris) 1959 Mar;96(3):352–355. [PubMed] [Google Scholar]
- Potrikus C. J., Breznak J. A. Nitrogen-fixing Enterobacter agglomerans isolated from guts of wood-eating termites. Appl Environ Microbiol. 1977 Feb;33(2):392–399. doi: 10.1128/aem.33.2.392-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Thayer D. W. Facultative wood-digesting bacteria from the hind-gut of the termite Reticulitermes hesperus. J Gen Microbiol. 1976 Aug;96(2):287–296. doi: 10.1099/00221287-95-2-287. [DOI] [PubMed] [Google Scholar]


