Abstract
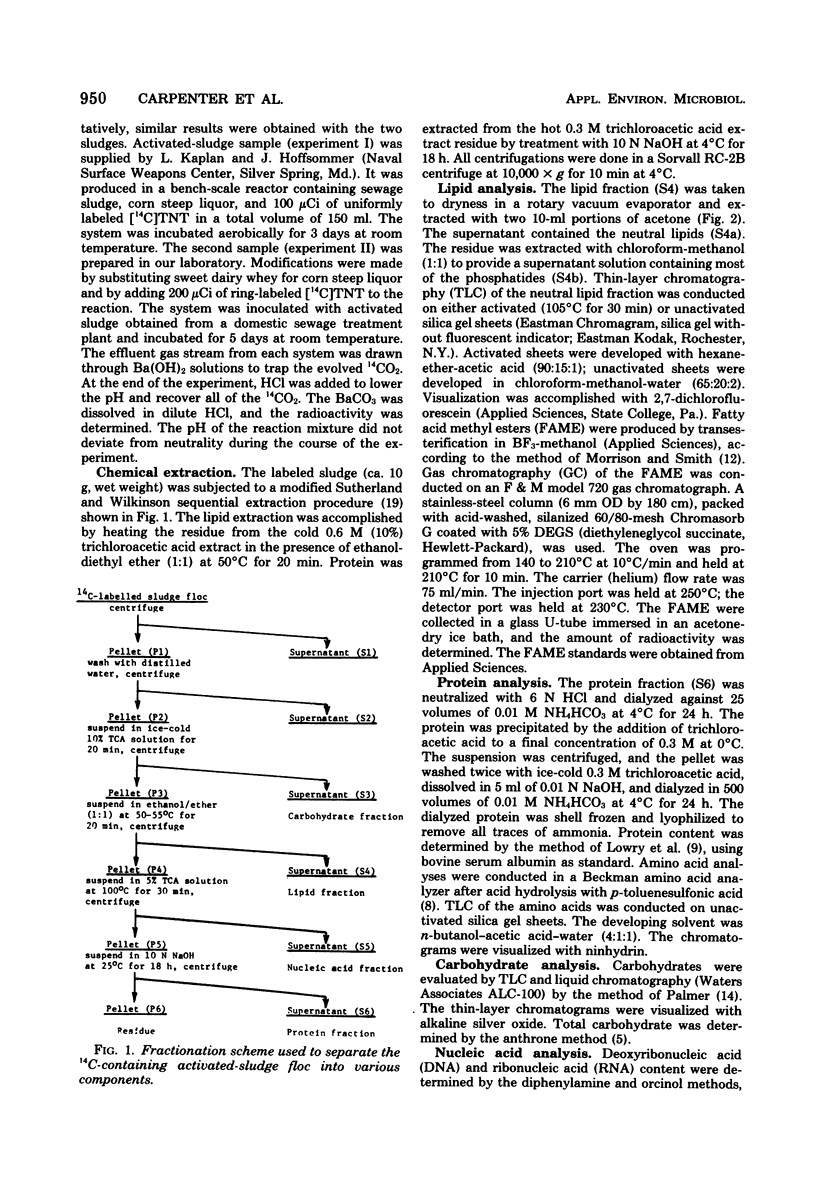
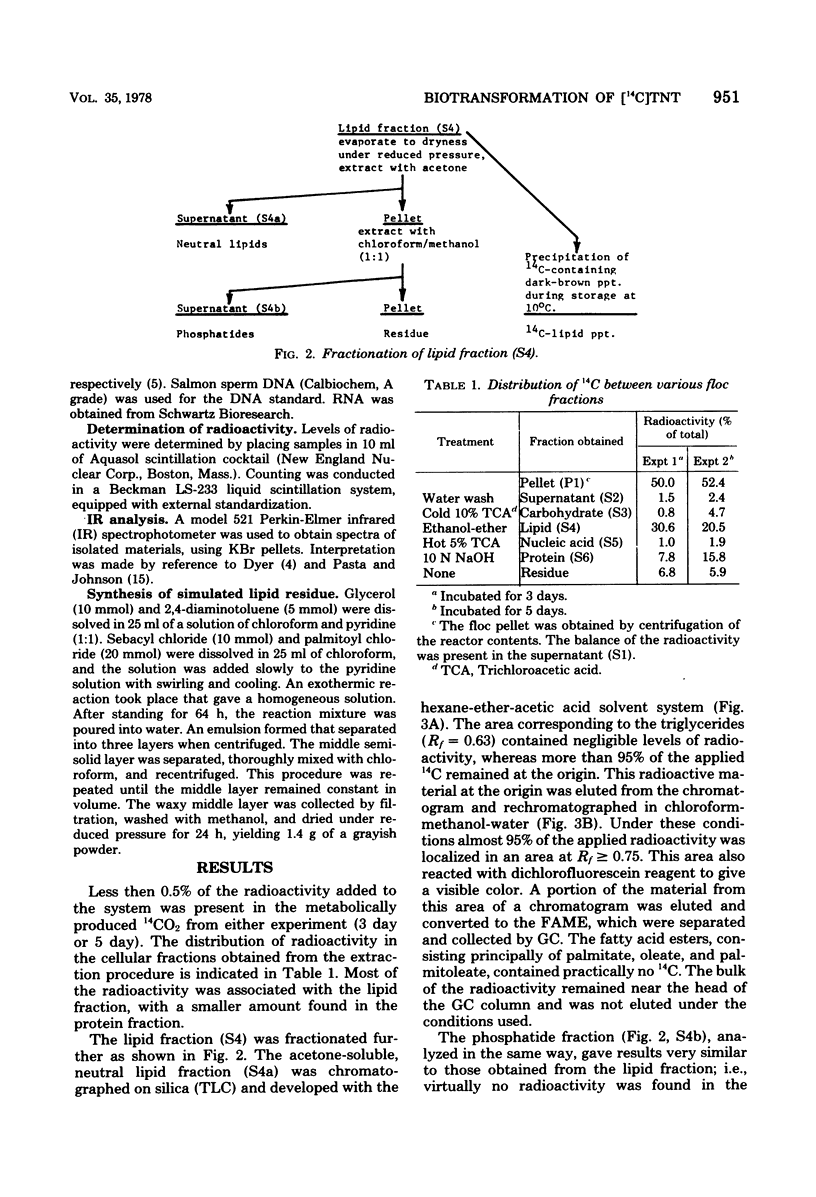
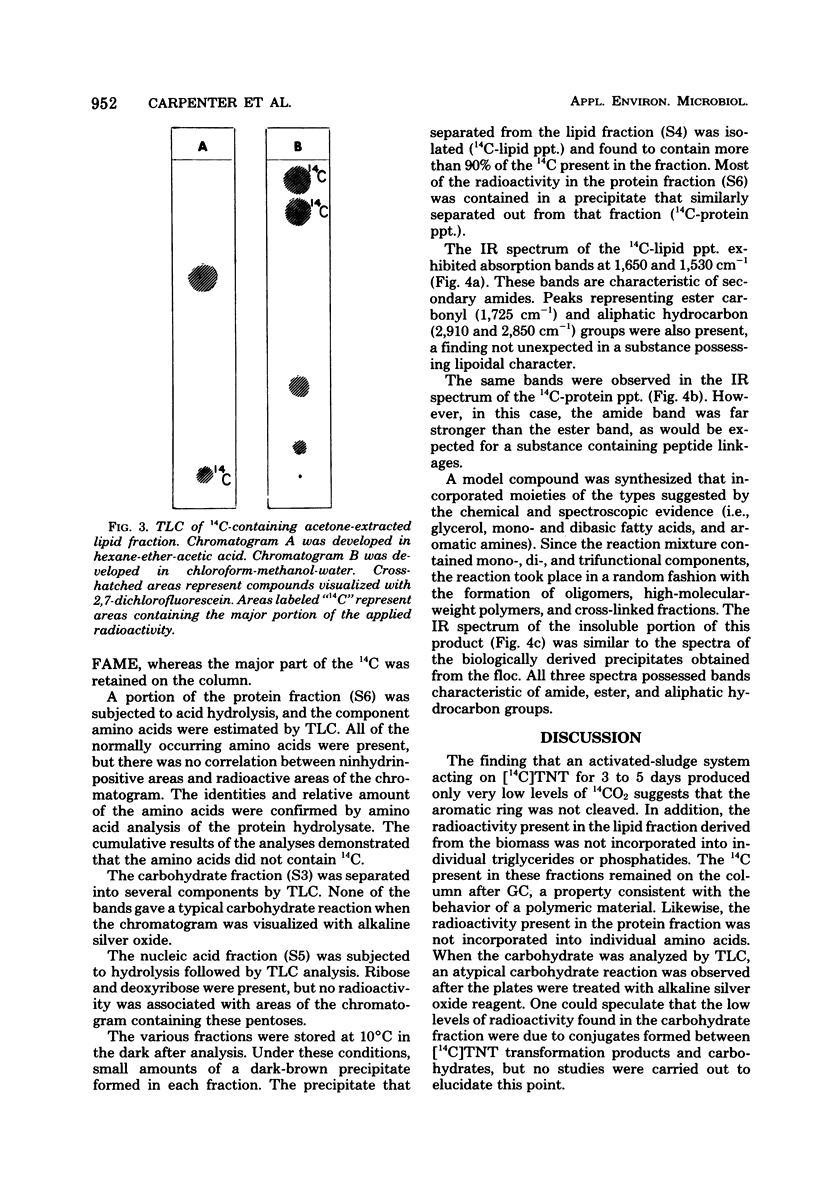
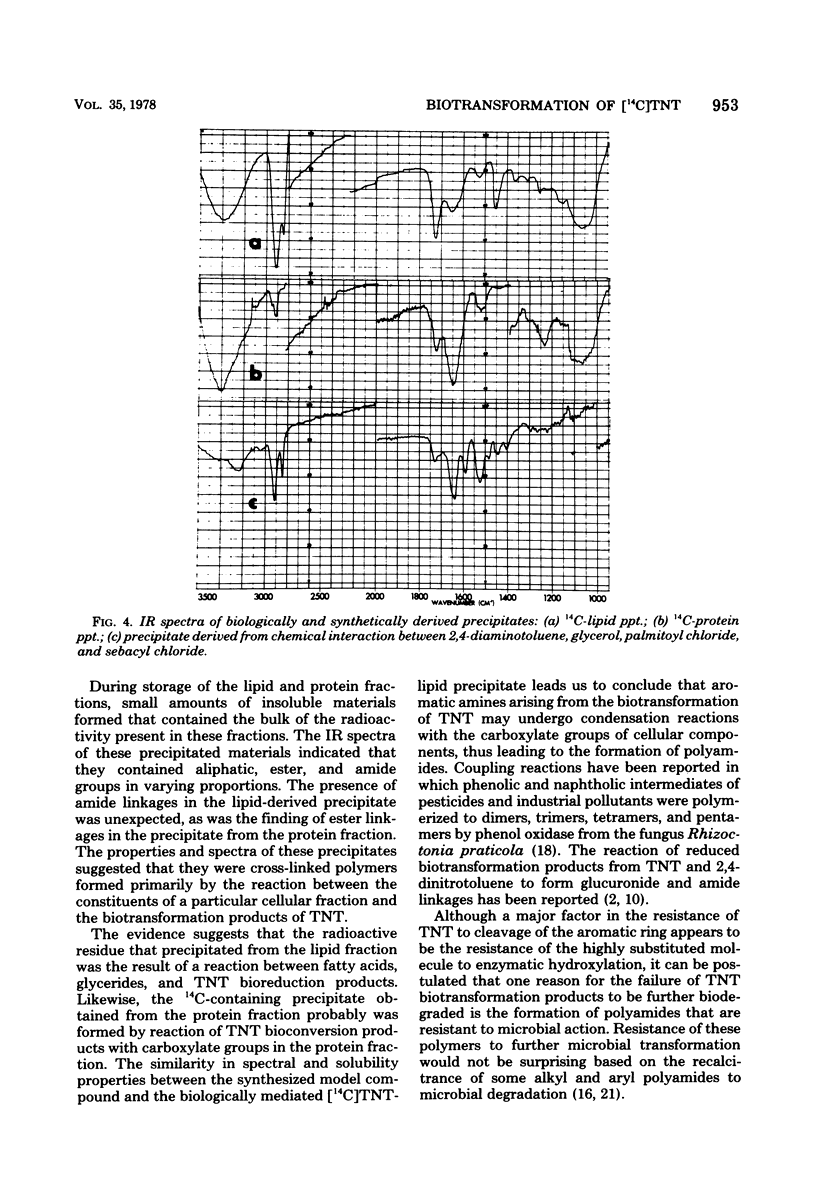
The fate of 14C-labeled 2,4,6-trinitrotoluene (TNT) in an activated-sludge system was investigated. No [14C]TNT could be detected in the contents of an aerated reactor after 3 to 5 days of incubation. No significant 14CO2 was formed, and the radioactivity was about equally divided between the floc and the supernatant. The radioactive carbon present in the microflora was mainly associated with the lipid and protein components, but the characteristic constituents of these compounds (e.g., fatty acids and amino acids) were not radioactive. The major part of the 14C present in the lipid and protein fractions was found in precipitates that formed in both fractions. The solubility properties and infrared spectra of these precipitates suggested that they are macromolecular structures of the polyamide type formed by the reaction of TNT biotransformation products with lipids, fatty acids, and protein constituents of the microbial flora. This hypothesis is further supported by the correspondence of the infrared spectrum of the lipid precipitate with that of a model compound synthesized from TNT transformation products and lipid precursors. The resistance of these macromolecules to further biodegradation was paralleled by the reported resistance to microbial attack of polyamides containing similar linkages.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Channon H. J., Mills G. T., Williams R. T. The metabolism of 2:4:6-trinitrotoluene (alpha-T.N.T.). Biochem J. 1944;38(1):70–85. doi: 10.1042/bj0380070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- McCormick N. G., Cornell J. H., Kaplan A. M. Identification of biotransformation products from 2,4-dinitrotoluene. Appl Environ Microbiol. 1978 May;35(5):945–948. doi: 10.1128/aem.35.5.945-948.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick N. G., Feeherry F. E., Levinson H. S. Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl Environ Microbiol. 1976 Jun;31(6):949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAZ A. K., SLIE R. B. The inhibition of organiz nitro reductase by aureomycin in cell-free extracts. II. Cofactor requirements for the nitro reductase enzyme complex. Arch Biochem Biophys. 1954 Jul;51(1):5–16. doi: 10.1016/0003-9861(54)90447-6. [DOI] [PubMed] [Google Scholar]
- Sjoblad R. D., Bollag J. M. Oxidative coupling of aromatic pesticide intermediates by a fungal phenol oxidase. Appl Environ Microbiol. 1977 Apr;33(4):906–910. doi: 10.1128/aem.33.4.906-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won W. D., Heckly R. J., Glover D. J., Hoffsommer J. C. Metabolic disposition of 2,4,6-trinitrotoluene. Appl Microbiol. 1974 Mar;27(3):513–516. doi: 10.1128/am.27.3.513-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


