Abstract
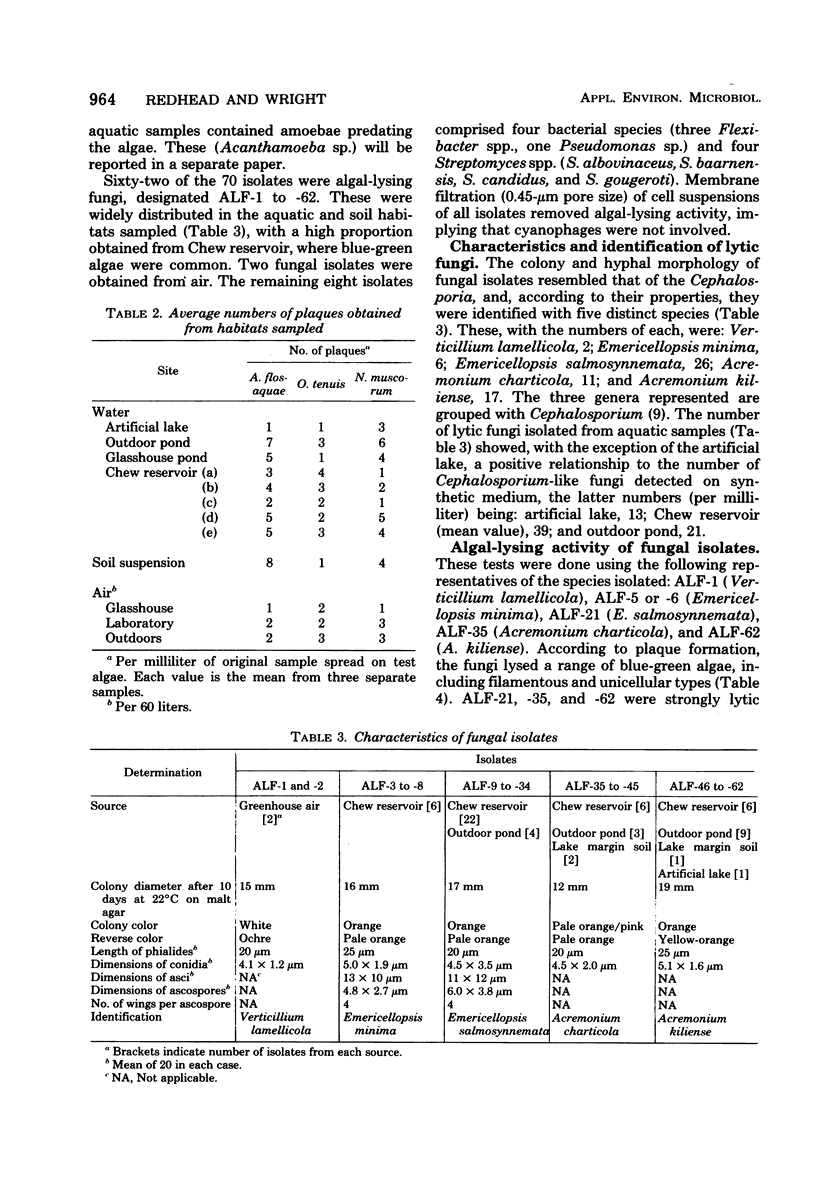
Of 70 pure microbial cultures isolated from aquatic habitats, soil, and air according to the ability to lyse live blue-green algae, 62 were fungi representing the genera Acremonium, Emericellopsis, and Verticillium. Algal-lysing fungi were isolated from all habitat types sampled. The remaining isolates comprised four bacteria and four streptomycetes. All isolates lysed Anabaena flos-aquae and, in most cases, several other filamentous and unicellular blue-green algae. The fungi generally showed greater activity than most other isolates towards a wider range of susceptible algae, including green algae in some cases. Acremonium and Emericellopsis isolates, but not Verticillium, also inhibited the growth of blue-green algae and gram-positive bacteria, but did not lyse the latter. Lysis of blue green algae by Acremonium and Emericellopsis spp. was associated with the formation of diffusible heat-stable extracellular factors which, evidence suggests, could be cephalosporin antibiotic(s). Blue-green algae were also lysed by pure cephalosporin C. The frequent isolation of lytic fungi from algal habitats suggests a possible natural algal-destroying role for such fungi, which might be exploitable for algal bloom control.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Granhall U., Berg B. Antimicrobial effects of Cellvibrio on blue-green algae. Arch Mikrobiol. 1972;84(3):234–242. doi: 10.1007/BF00425201. [DOI] [PubMed] [Google Scholar]
- Gromov B. V., Ivanov O. G., Mamkaeva K. A., Avilov I. A. Fleksibakteriia, liziruiushchaia sinezelenye vodorosli. Mikrobiologiia. 1972 Nov-Dec;41(6):1074–1079. [PubMed] [Google Scholar]
- Gunnison D., Alexander M. Basis for the susceptibility of several algae to microbial decomposition. Can J Microbiol. 1975 May;21(5):619–628. doi: 10.1139/m75-089. [DOI] [PubMed] [Google Scholar]
- Lewin R. A. A classification of flexibacteria. J Gen Microbiol. 1969 Oct;58(2):189–206. doi: 10.1099/00221287-58-2-189. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H. Classification of cephalosporins by their antibacterial activity and pharmacokinetic properties. J Antimicrob Chemother. 1975;1(3 Suppl):1–12. doi: 10.1093/jac/1.suppl_3.1. [DOI] [PubMed] [Google Scholar]
- SAFFERMAN R. S., MORRIS M. E. Algal virus: isolation. Science. 1963 May 10;140(3567):679–680. doi: 10.1126/science.140.3567.679. [DOI] [PubMed] [Google Scholar]
- SAFFERMAN R. S., MORRIS M. E. Evaluation of natural products for algicidal properties. Appl Microbiol. 1962 Jul;10:289–292. doi: 10.1128/am.10.4.289-292.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMEJIMA H., MYERS J. On the heterotrophic growth of Chlorella pyrenoidosa. J Gen Microbiol. 1958 Feb;18(1):107–117. doi: 10.1099/00221287-18-1-107. [DOI] [PubMed] [Google Scholar]
- Shilo M. Lysis of blue-green algae by myxobacter. J Bacteriol. 1970 Oct;104(1):453–461. doi: 10.1128/jb.104.1.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. R., Brown R. M., Jr Cytophaga that kills or lyses algae. Science. 1969 Jun 27;164(3887):1523–1524. doi: 10.1126/science.164.3887.1523. [DOI] [PubMed] [Google Scholar]



