Abstract
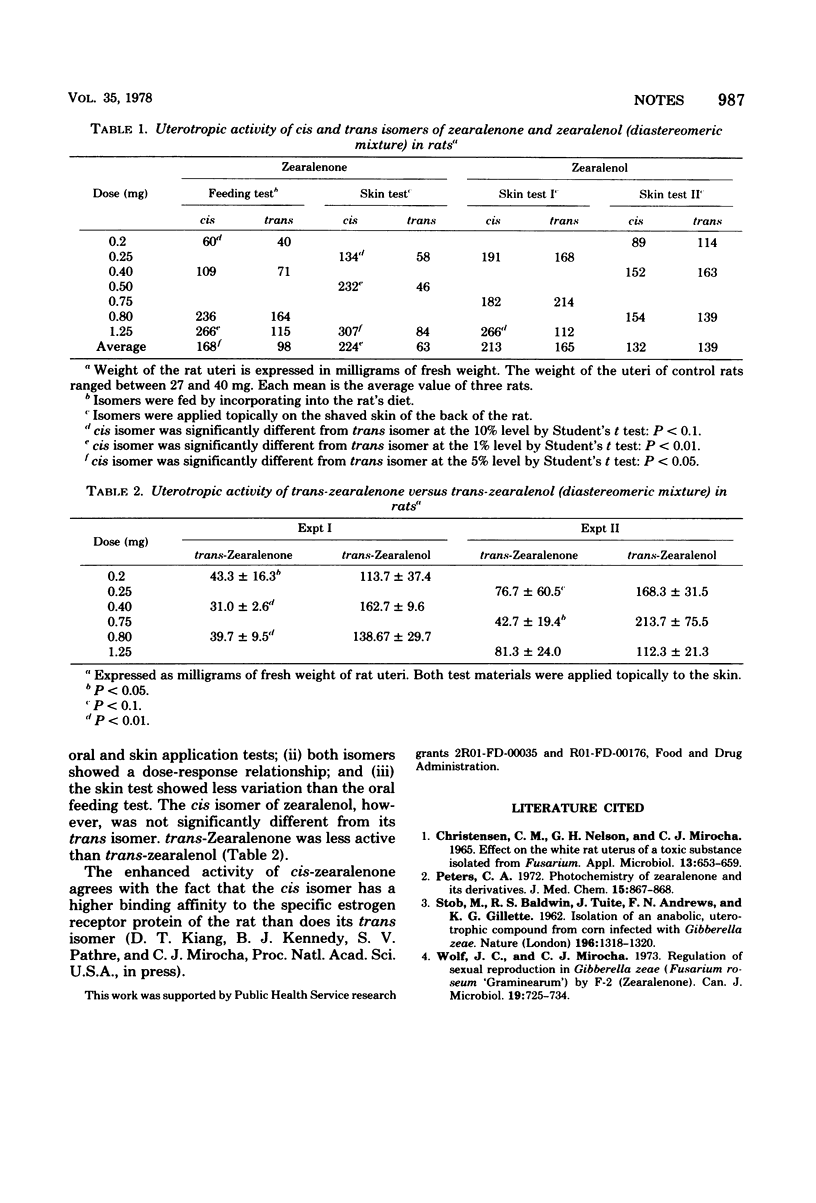
The cis and trans isomers of zearalenone [2,4-dihyroxy-6-(10-hydroxy-6-oxo-1-undecenyl)-benzoic acid mu-lactone] and zearalenol [2,4-dihydroxy-6-(6,10-dihydroxy-1-undecenyl)-benzoic acid mu-lactone] were tested for uterotropic activity in the white rat. The metabolites were administered through the oral route (per os) and by topical application to the freshly shaven skin on the back. cis-Zearalenone was significantly more active than trans when fed orally to the rats in the diet or when applied topically by skin application. However, the cis isomer of zearalenol was not significantly different than its trans isomer. trans-Zearalenone was less active than trans-zearalenol.
Full text
PDF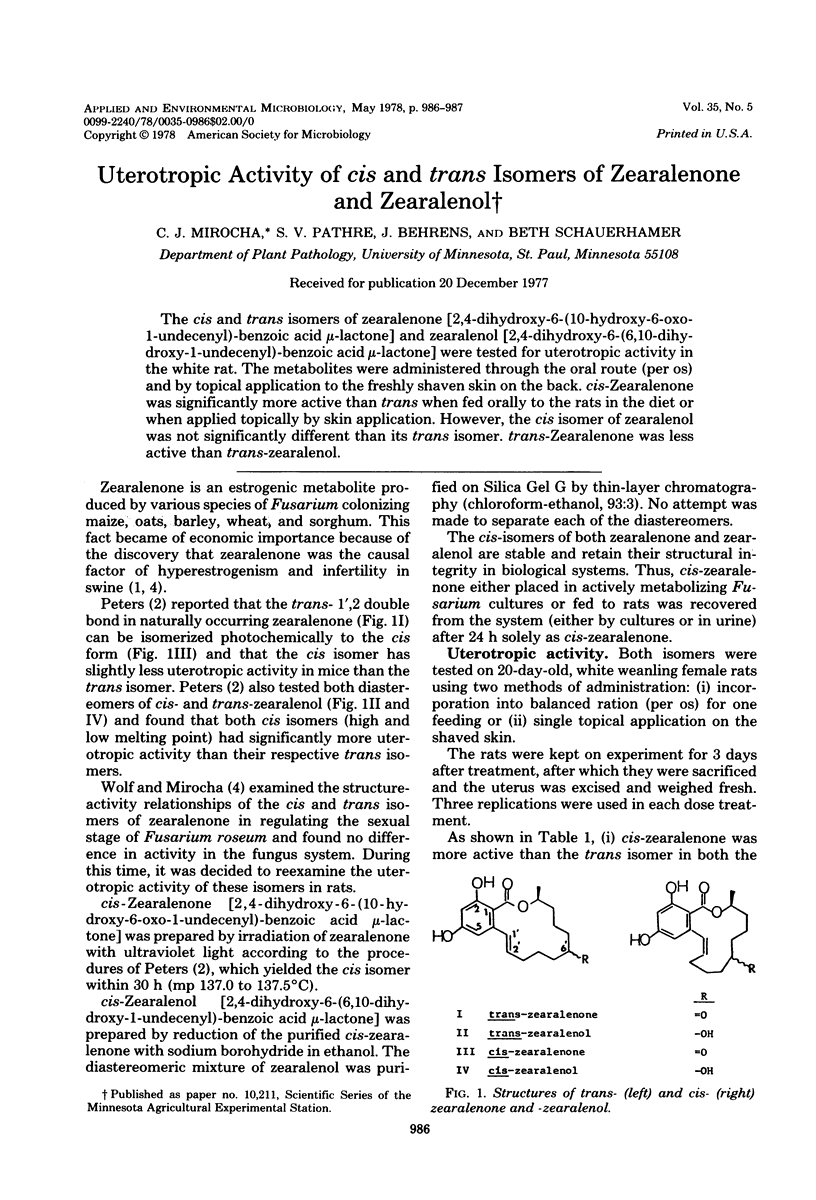
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen C. M., Nelson G. H., Mirocha C. J. Effect on the white rat uterus of a toxic substance isolated from Fusarium. Appl Microbiol. 1965 Sep;13(5):653–659. doi: 10.1128/am.13.5.653-659.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C. A. Photochemistry of zearalenone and its derivatives. J Med Chem. 1972 Aug;15(8):867–868. doi: 10.1021/jm00278a028. [DOI] [PubMed] [Google Scholar]
- STOB M., BALDWIN R. S., TUITE J., ANDREWS F. N., GILLETTE K. G. Isolation of an anabolic, uterotrophic compound from corn infected with Gibberella zeae. Nature. 1962 Dec 29;196:1318–1318. doi: 10.1038/1961318a0. [DOI] [PubMed] [Google Scholar]
- Wolf J. C., Mirocha C. J. Regulation of sexual reproduction in Gibberella zeae (Fusarium roxeum "graminearum") by F-2 (Zearalenone). Can J Microbiol. 1973 Jun;19(6):725–734. doi: 10.1139/m73-117. [DOI] [PubMed] [Google Scholar]


