Abstract
Triose phosphate isomerase (TPI) deficiency glycolytic enzymopathy is a progressive neurodegenerative condition that remains poorly understood. The disease is caused exclusively by specific missense mutations affecting the TPI protein and clinically features hemolytic anemia, adult-onset neurological impairment, degeneration, and reduced longevity. TPI has a well-characterized role in glycolysis, catalyzing the isomerization of dihydroxyacetone phosphate (DHAP) to glyceraldehyde-3-phosphate (G3P); however, little is known mechanistically about the pathogenesis associated with specific recessive mutations that cause progressive neurodegeneration. Here, we describe key aspects of TPI pathogenesis identified using the TPIsugarkill mutation, a Drosophila model of human TPI deficiency. Specifically, we demonstrate that the mutant protein is expressed, capable of forming a homodimer, and is functional. However, the mutant protein is degraded by the 20S proteasome core leading to loss-of-function pathogenesis.
GLYCOLYTIC enzymopathies are devastating human diseases that result from heritable mutations in genes encoding certain glycolytic enzymes. The underlying pathogenesis of these diseases remains poorly understood. Triose phosphate isomerase (TPI) deficiency is a severe glycolytic enzymopathy caused by specific missense mutations in the TPI gene that are associated with hemolytic anemia, progressive neuromuscular degeneration, increased susceptibility to infection, and premature death (Schneider et al. 1965; Valentine 1966). The TPI protein exists as a homodimer of ∼26.5-kDa monomers that functions to convert dihydroxyacetone phosphate (DHAP) to glyceraldehyde-3-phosphate (G3P) in glycolysis (Rieder and Rose 1959; Mande et al. 1994). Human TPI deficiency results when specific missense mutations are homozygous or are heterozygous with a null allele resulting in insufficient TPI function (Schneider 2000). TPI's well-characterized role in glycolysis suggests that bioenergetic impairment may underlie pathogenesis.
Alternatively, others have hypothesized that DHAP toxicity or its catabolite methylglyoxal may be integral to pathogenesis independent of a bioenergetic deficit (Karg et al. 2000; Ahmed et al. 2003; Ramasamy et al. 2005). Indeed, data are not consistent from existing reports to establish whether reduced protein levels, instability of the protein homodimer, aberrant protein complexes, and/or a decrease in isomerase activity contribute to pathogenesis of the disease (Olah et al. 2002; Orosz et al. 2006). In part, the difficulty arises from the fact that the disease has a strong neurological component, yet patients' erythrocytes are often used for studies due to their accessibility. Although these studies have produced important information about the disease, they have not provided much insight into neurological and degenerative aspects of TPI deficiency. Such studies have measured reduced isomerase activity from patients' erythrocytes, platelets, and lympocytes (Schneider et al. 1965; Zanella et al. 1985; Orosz et al. 2001; Olah et al. 2002, 2005), as well as brain and muscle tissue (Ationu et al. 1999). Studies have also documented markedly elevated DHAP levels, suggesting that the anemia may be caused by toxicity of DHAP or one of its catabolites (Zanella et al. 1985; Eber et al. 1991; Hollan et al. 1997; Karg et al. 2000; Olah et al. 2002, 2005). Interestingly, DHAP levels in nonerythrocytes are only modestly increased, presumably owing to it being a substrate for phospholipid biosynthesis in other cell types, and a yeast model of TPI deficiency reports that proteins bearing the human disease mutations maintain catalytic activity (Orosz et al. 2001, 2006; Ralser et al. 2006).
We have previously identified a recessive, hypomorphic, missense (M80T) mutation (sugarkill or sgk) in the TPI gene of Drosophila featuring reduced longevity, neurodegenration, and locomotor impairment (Celotto et al. 2006). These mutants, referred to as TPIsugarkill, serve as an amendable genetic model for elucidating the mechanism of TPI deficiency in vivo (Celotto et al. 2006; Gnerer et al. 2006; Orosz et al. 2006). The TPIsugarkill locomotor impairment is conditional with mechanical stress or elevated temperatures inducing acute paralysis. The mutation was originally identified as a temperature-sensitive paralytic mutant (Palladino et al. 2002). Each phenotype examined—including onset of neuropathology, locomotor impairment, and reduced longevity—is significantly worsened by modest elevations in temperature between 20° and 29° (Celotto et al. 2006). This suggests that TPIsugarkill animals may serve as a useful model of TPI impairment where varying temperatures can be utilized to study a series of hypomorphic conditions from mild to severe. Furthermore, in vitro temperature sensitivity has also been reported for the most common homozygous human mutant allele, Glu104Asp (Daar et al. 1986; Arya et al. 1997), suggesting a shared mechanism of pathogenesis exists with at least one of the common disease-causing missense mutations.
There are many proposed mechanisms of TPI deficiency pathogenesis, including (1) protein misfolding and toxic aggregates (Olah et al. 2002; Ovadi et al. 2004), (2) accumulation of a DHAP catabolite methylglyoxal, a potentially toxic metabolite (Karg et al. 2000; Ahmed et al. 2003; Ramasamy et al. 2005), (3) instability of the homodimer via thermoliablilty (Schneider 2000), and (4) bioenergetic defects resulting from reduced enzyme activity. Our model allows us to directly test the role of these mechanisms in pathogenesis resulting from a specific pathogenic missense mutation affecting TPI. Previously we have shown TPIsugarkill markedly reduces glycolytic flux, but this does not result in a bioenergetic crisis, suggesting a compensatory mechanism and an alternative cause of pathogenesis (Celotto et al. 2006). Our data utilizing the TPIsugarkill model of TPI deficiency reveal that the mutant protein is capable of forming a dimer and retains function; however, pathogenesis results when the protein is targeted for proteasome degradation.
MATERIALS AND METHODS
Drosophila stocks and culture:
The TPIsugarkill mutation, unless noted otherwise, is maintained as a homozygous viable ve e TPIsugarkill strain. The TPIsugarkill mutation, also known as sugarkill, TPIsgk, or TPIsgk1, results from a missense mutation predicted to cause an M80T. This numbering uses the established nomenclature for TPI mutations, where the start methionine is assumed to be removed following translation (see the recent review by Orosz et al. 2006). TPI transgenic strains were previously published (Celotto et al. 2006). All other strains used were obtained from the Bloomington Stock Center. Wild-type controls are ve e TPIsugarkill heterozygotes, ve e, Canton-S, or appropriate Gal4 controls, as noted.
Life span and behavioral analysis:
Life span analysis was performed as previously described (Palladino et al. 2003). Stress sensitivity (a.k.a. bang sensitivity) was assayed as previously described, and the time to recovery from paralysis was measured (Ganetzky and Wu 1982).
Size-exclusion chromatography:
Drosophila extract:
Two milliliters of flies were homogenized in 5 ml of mobile phase with an electric homogenizer (IKA Ultra-TURAX T8) on the highest setting. Protein complexes >100 kDa were removed from the extract using Amicon Ultra-4 Ultracel 100K columns (Millipore, Bedford, MA) to prevent damage to the HPLC column. The extract was then concentrated using an Amicon Ultra-4 Ultracel 5K column (Millipore). One hundred microliters was injected into the HPLC column, using an auto-injector. Each genotype was performed in duplicate at each temperature.
Nondenaturing size-exclusion chromatography:
Protein fractions were collected using a Shimadzu HPLC system and a Superdex 75 10/300 GL column at a flow rate of 0.4 ml/min. The temperature of the column was maintained at 22°, 30°, or 40°. The mobile phase consisted of 0.025 m NaH2PO4, 0.025 m Na2HPO4 pH 7.0, and 0.15 m NaCl. One hundred microliters of protein standard (Amersham Biosciences, Arlington Heights, IL) (at 5 mg/ml: ribonuclease A, 14.6 kDa; chymotrypsinogen A, 20.3 kDa; ovalbumin, 46.7 kDa; albumin, 62.9 kDa) or Drosophila extract were injected and monitored at 280 nm for 40 min. Four fractions were collected: (1) 1–23 min, >67-kDa proteins and complexes; (2) 23–28 min, corresponding to 31–67 kDa (53-kDa TPI dimer); (3) 28–34 min, corresponding to 12–31 kDa (26.5-kDa TPI monomer); and (4) 34–40 min, corresponding to 5- to 12-kDa proteins.
Western:
Once fractions were collected, they were concentrated using Amicon Ultra-4 Ultracel 5K columns (Millipore) down to 100 μl, 1 ml H2O was added, and the samples were reconcentrated to 50 μl. Sixteen microliters of loading dye were added to each fraction and were loaded onto a 12% protein gel. The gel was transferred to a nitrocellulose membrane and blocked with 1% milk. TPI primary antibody (Protein Tech rabbit anti-humanTPI) was added at a concentration of 1:1000 and incubated at room temperature overnight. The blot was washed extensively with PBST, secondary antibody (HRP goat anti-rabbit IgG) was added at a concentration of 1:4000, and the blot was incubated at room temperature for 1 hr. The blot was washed again with PBST and enhanced chemiluminescence (ECL) reaction (Pierce, Rockford, IL) was performed. Film was exposed for ∼1 min before developing.
Western blot analysis:
Fly lysates were obtained from flies aged for 24 or 48 hr as indicated and ground with a pestle in 100 μl of a standard SDS/Bromophenol Blue cell lysis buffer. After boiling for 1 min at 100°, 20 μl of extract were added per lane in a 12% SDS–PAGE gel at denaturing conditions. Signal detection was performed as above. ImageJ Version 10.2 software was used to analyze subsaturation images. Protein intensity represents the normalized TPI protein level (either to actin or β-tubulin, as indicated) in arbitrary pixel units (intensity). In all cases independent protein extracts were used (n ≥ 3).
RESULTS
Recessive nature of the sugarkill mutation:
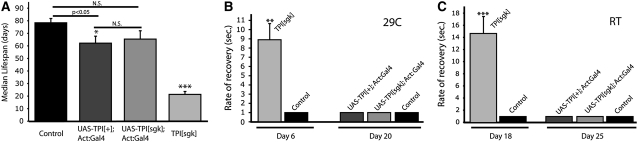
The sugarkill mutation was identified as a recessive temperature-sensitive paralytic mutant that causes progressive neurodegeneration (Palladino et al. 2002). The mutation was positionally cloned and found to be a missense mutation in the TPI (triose phosphate isomerase) gene (Celotto et al. 2006). TPIsugarkill (sgk) animals have progressive stress-sensitive locomotor impairment and reduced longevity. Surprisingly, given its well-characterized role in glycolysis, biochemical analyses revealed that bioenergic impairment was not a likely proximate cause of these phenotypes (Celotto et al. 2006; Gnerer et al. 2006). This surprising result led us to investigate alternative hypotheses to explain neuropathogenesis associated with TPI impairment, including the possibility that pathogenesis of the M80T missense mutation in TPI may in part result from a semidominant gain-of-function. Although previous data suggested that TPIsugarkill was fully recessive (Celotto et al. 2006), we examined a transgenic overexpression assay to test for semidominance effects. Utilizing the UAS-GAL4 system, we generated animals that overexpressed either the TPIsugarkill or the wild-type TPI protein in a wild-type genetic background and examined the effect on longevity and locomotor function. Specifically, we analyzed longevity of both UAS-TPIsgk;actin∷GAL4;+/+ and UAS-TPI+;actin∷GAL4;+/+ animals compared to actin∷GAL4;+/+ controls at 25° (Figure 1A). There was no significant decrease in life span of the UAS-TPIsgk;actin∷GAL4 animals compared to the control or the UAS-TPI+;actin∷GAL4 animals. There was a modest but significant decrease in median life span of the UAS-TPI+;actin∷GAL4 animals compared to that of the GAL4 controls. This is likely the result of transgenic overexpression toxicity or a background strain effect shared between the transgenic animal strains.
Figure 1.—
Life span and behavioral analysis of animals overexpressing wild-type and mutant TPI transgenes. (A) Survival of transgenic animals at 25° overexpressing mutant and wild-type TPI. A modest reduction in longevity is observed, associated with overexpression of wild-type TPI protein. Flies overexpressing the mutant TPI, UAS-TPI[sgk];Actin∷GAL4;+/+, do not show a significant life span deficit relative to either the control strain or animals overexpressing wild-type TPI. Longevity of TPIsgk mutant flies is shown for comparison and is significantly reduced compared to all genotypes. N = 80–120 animals in four to six independent populations. Locomotor function in response to stress was examined in transgenic flies at 29° (B) and room temperature (C). TPIsugarkill mutants exhibit a significantly longer recovery rate from mechanical stress compared to controls when aged at 29° (day 6) or room temperature (day 18). Transgenic overexpression of wild-type or mutant TPI protein did not result in stress-induced locomotor impairment when aged at either temperature. A total of 10–20 animals per genotype were tested. In A–C the error given is SEM and statistics were calculated using Student's t-test (**P < 0.01 and ***P < 0.001).
Further studies examined the effect of mechanical stress on locomotion of these transgenic animals at 29° or room temperature (∼22°). This revealed that overexpression of either mutant or wild-type TPI protein in a wild-type background does not result in a detectable stress-induced locomotor phenotype, such as is prominent in TPIsugarkill animals (Figure 1, B and C; Celotto et al. 2006). Importantly, the transgenes used in these overexpression experiments are capable of rescuing TPIsugarkill to a similar extent (see Figure 6). Together these transgenic experiments are consistent with the TPIsugarkill mutation being fully recessive and suggest a simple loss-of-function model of TPI pathogenesis.
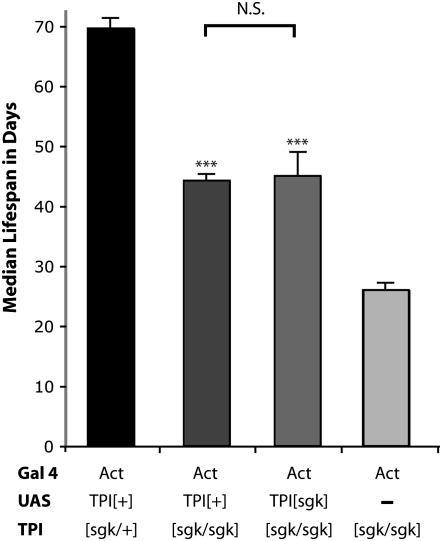
Figure 6.—
Rescue with TPIsugarkill overexpression. Transgenic animals expressing wild-type and mutant TPI protein were examined for longevity at 25° in a TPIsgk background. Both UAS-TPI+;Act∷Gal4; TPIsgk/sgk and UAS-TPIsgk;Act∷Gal4; TPIsgk/sgk animals show a significant increase in life span compared to Act∷Gal4; TPIsgk/sgk mutants (***P < 0.001, Student's t-test). Neither transgene was able to fully rescue the longevity to that of heterozygous control animals. Error given is SEM, and n = 120 animals in six independent populations.
Dimer instability of TPI protein in sugarkill mutants:
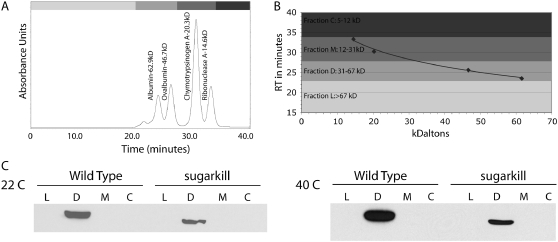
Several disease-associated TPI mutations and the TPIsugarkill mutation result in an amino acid substitution near the dimer interface of the protein, which has led us and others to hypothesize that dimer instability may underlie pathogenesis (Celotto et al. 2006; Orosz et al. 2006). Human TPI deficiency has previously been reported to be associated with the destabilization of the dimer state (Olah et al. 2002). To determine if TPIsugarkill pathogenesis may be associated with structural defects in the TPI protein that destabilize the dimer, we directly examined the stability of the TPI homodimer, using a nondenaturing size-exclusion chromatography assay (Figure 2). TPI is ∼26.5 kDa and exists physiologically as an ∼53-kDa homodimer (Rieder and Rose 1959; Mande et al. 1994). Native size-exclusion chromatography was standardized, whole fly extracts from wild-type controls and TPIsugarkill were fractionated, and Westerns were performed to determine which fraction contained TPI. At both 22° and 40° the mutant and wild-type TPI were observed only in the dimer fraction (31–67 kDa). No protein was detected in monomer or large fractions corresponding to 12–31 kDa and >67 kDa, respectively. We have noted that increases in temperature from 20° to 37° exacerbate all described phenotypes in TPIsugarkill (Celotto et al. 2006), yet even at 40° the dimer remained stable in this assay. These data demonstrate that TPIsugarkill is capable of forming a dimer and that the protein is not observed as a monomer in fly extracts under native conditions. Additional studies were performed with more extensive fractionation, which corroborate these findings (supplemental Figure 1). The TPIsugarkill protein exists as a dimer in the <100-kDa protein fraction, as is the case for wild-type TPI protein.
Figure 2.—
Size-exclusion chromatography: chromatography demonstrating that both the wild-type TPI and mutant TPIsugarkill form a dimer. (A) HPLC chromatograph using four standards (albumin, ovalbumin, chymotrypsinogen, and ribonuclease A) to determine size retention. (B) Standard curve used to determine time of elution from the column: fraction L (>67-kDa molecules), fraction D (TPI dimer that is 53 kDa), fraction M (TPI monomer that is 26.5 kDa), and fraction C (molecules between 5 and 12 kDa). (C) Western blots using TPI antibody: each fraction from wild-type and TPIsugarkill extracts was separated at 22° and 40°.
Protein stability and expression in sugarkill mutants:
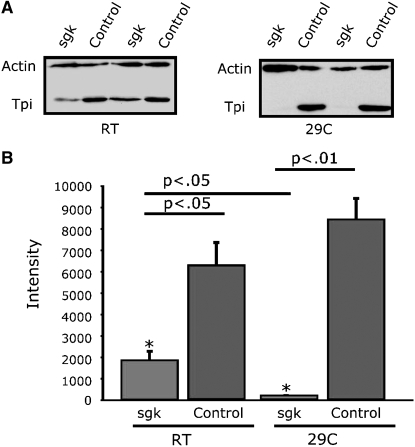
We have examined TPI mRNA expression in TPIsugarkill mutants at room temperature and at 29°. Expression of mRNA is not reduced, suggesting that the pathogenesis is associated with expression of TPI protein bearing the M80T missense mutation (Celotto et al. 2006 and data not shown). Surprisingly, we discovered that TPI protein in TPIsugarkill mutants is reduced and that the extent of the reduction in protein is temperature dependent (Figure 3). At room temperature there is a significant (73%) reduction in total TPI protein, whereas age-matched flies shifted to 29° for 48 hr reveal a marked (98%) reduction in TPI protein (Figure 3). The severity of this reduction is antagonized by temperature, consistent with other phenotypes observed in TPIsugarkill mutants. TPI protein being reduced in the absence of a reduction in TPI mRNA strongly suggests that TPI protein is unstable as a result of the TPIsugarkill mutation.
Figure 3.—
Western blot analysis of TPI protein levels. (A) TPIsugarkill mutants show a reduced level of TPI protein when aged for 48 hr at room temperature and 29° compared to actin controls. (B) Quantification of Western blot shows an ∼3.5-fold decrease in TPI protein levels at room temperature and an ∼48-fold decrease at 29°. *P < 0.05 (Student's t-test). Error reported is SEM. N = 3 at each temperature. Intensity of TPI was measured from a subsaturation blot and normalized to the intensity of the actin control using ImageJ 10.2.
Proteasomal degradation of TPIsugarkill protein:
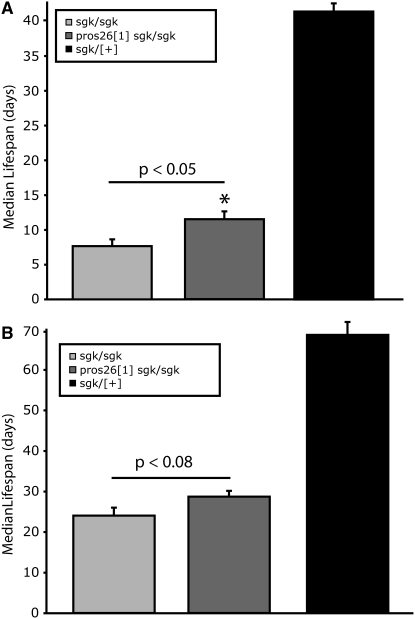
Pros261 is a dominant, temperature-sensitive lethal mutation, formerly known as DTS5, which results in larval stage-three lethality at nonpermissive temperatures ≥25° (Saville and Belote 1993). Pros261 was shown to be a hypomorphic missense mutation in the Pros26 gene that encodes an altered B6 subunit of the 20S proteasome core (Saville and Belote 1993; Smyth and Belote 1999). In Drosophila, this mutation has been shown to be a useful genetic inhibitor of proteasome function (Fernandez-Funez et al. 2000; Shulman and Feany 2003; Speese et al. 2003; Neuburger et al. 2006). We generated Pros261 TPIsgk animals to investigate the effect of proteasome inhibition on TPIsugarkill mutants, utilizing longevity as our most sensitive and quantitative phenotype. Proteasome impairment is itself toxic with respect to aging (Keller et al. 2002; Chondrogianni and Gonos 2005). Impairment also dramatically affects protein synthesis (Ding et al. 2006) and has been implicated in numerous disease states, including Alzheimer's (Keller et al. 2000), Parkinson's (McNaught and Olanow 2006; Olanow and Mcnaught 2006), and Huntington's (Seo et al. 2004) diseases. Surprisingly, Pros261 TPIsgk double mutants did not have reduced longevity compared to that of TPIsugarkill mutants. In fact, there is a modest but significant increase in longevity in the double-mutant strain (Figure 4). These data suggest that the TPIsugarkill protein is targeted for degradation by the 20S proteasome core and that impairment of the proteasome, though normally toxic, can partially suppress the TPIsugarkill life span phenotype.
Figure 4.—
Survivorship of TPIsugarkill Pros261 double mutants: statistical analysis of median life span of mutants aged at (A) 29° and (B) 25°. (A) Pros261 TPIsgk/sgk have a modest but significant increase in longevity compared to TPIsgk/sgk animals at 29° (P < 0.05). (B) Flies aged at 25° show a nonsignificant improvement in longevity. The median life span of Pros261 TPIsgk/sgk double mutants is still much reduced from that of heterozygous controls at both temperatures examined (P < 0.0001). In A and B, the error given is SEM, statistical comparison uses Student's t-test, and n = 120 animals in six independent populations for each genotype.
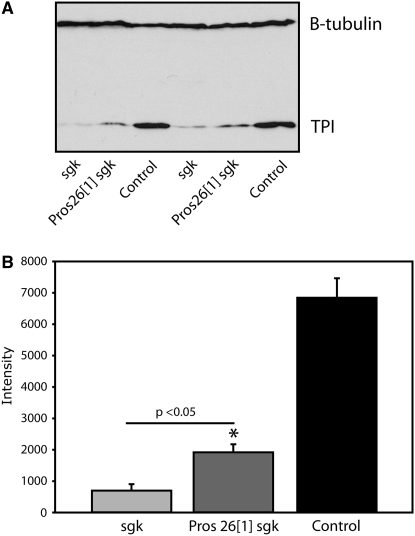
To examine this more directly, we measured TPI protein levels from the double mutant compared to control animals (Figure 5). This analysis revealed a significant increase in TPIsugarkill protein levels in the proteasome-inhibited double mutant compared to TPIsugarkill animals. Partial rescue of TPIsugarkill protein levels is consistent with the modest improvement observed in longevity (Figure 4). This also indicates that the 20S proteasome core is implicated in TPIsugarkill degradation. Further experiments have shown that the Pros261 mutation does not have a significant effect on wild-type TPI protein levels (supplemental Figure 2). These data imply either that the proteasome does not regulate wild-type TPI protein or that wild-type protein turnover is insufficient to detect a difference with these methods. The finding that a loss-of-function proteasome mutation reduces TPIsugarkill degradation and improves the longevity phenotype also strongly suggests that the TPIsugarkill protein is functional and that degradation of functional protein underlies pathogenesis in TPIsugarkill mutants.
Figure 5.—
Proteasome mutation increases TPI[sgk] protein levels. (A) Western blot of TPI and β-tubulin proteins from flies aged at 29° for 24 hr. Pros261 TPIsgk/sgk animals show an increase in TPI protein compared to TPIsgk/sgk mutants. (B) Quantitative analysis of TPI protein normalized to the tubulin loading control indicates a greater than twofold increase in TPI protein in the Pros261 TPIsgk/sgk double mutants (*P < 0.05, n = 3 each genotype, Student's t-test). Both TPIsgk/sgk and Pros261 TPIsgk/sgk animals show a marked reduction in TPI protein when compared to wild-type controls, consistent with previous data. Error reported is SEM. Protein intensity is measured from a subsaturation image with ImageJ 10.2.
Transgenic rescue with overexpression of TPIsugarkill protein:
If pathogenesis in TPIsugarkill results from a loss-of-function due to enhanced proteasomal degradation of functional protein, one would predict that overexpression of the mutant protein may rescue or partially rescue the TPIsugarkill mutation. Previous data demonstrated that this is not the case at 29° (Celotto et al. 2006). This is likely due to the marked instability of TPIsugarkill protein at 29° (Figure 3). We examined whether transgenic overexpression of TPIsugarkill protein could rescue the TPIsugarkill mutation at 25°, where the reduction in protein stability was not as severe. Both UAS-TPIsgk;actin∷GAL4; TPIsgk/sgk and UAS-TPI+;actin∷GAL4; TPIsgk/sgk animals were examined for longevity and found to dramatically improve longevity of TPIsugarkill mutants (Figure 6). Interestingly, the improvement was similar for each transgene, and neither transgenic strain fully rescued longevity compared to heterozygous controls. These data demonstrate that proteasome degradation of TPIsugarkill protein underlies pathogenesis and opens up the possibility that one or more of the human pathogenic mutations that cause TPI deficiency may result from a decrease in protein stability.
DISCUSSION
Despite the well-characterized role of TPI in glycolysis, TPI-deficiency pathogenesis remains poorly understood. Considerable attention has been focused on the fact that only specific missense mutations are pathogenic; TPI enzymopathy results when these missense mutations are homozygous or are heterozygous with a known null allele (Schneider 2000; Orosz et al. 2006). Numerous lines of evidence demonstrate that homozygous TPI null mutations do not result in disease and are developmentally lethal. Most notably, the high frequency of heterozygous null mutations in the population and lack of homozygous null observations, as well as the stage of lethality observed in mouse and fly null animals, are all consistent with this assertion (Mohrenweiser and Fielek 1982; Mohrenweiser 1987; Merkle and Pretsch 1989; Celotto et al. 2006). Several hypotheses have been proposed to explain TPI-deficiency pathogenesis associated with specific missense mutations. We have utilized a Drosophila model of human TPI-deficiency disease to examine several of these hypotheses in vivo. The Drosophila TPIsugarkill missense mutation recapitulates numerous key features of this progressive neurodegenerative disease and provides a unique system for investigating pathogenesis (Celotto et al. 2006; Orosz et al. 2006).
Independently, two laboratories studying the same genetic model of glycolytic enzymopathy demonstrated that TPI pathogenesis was not simply the result of bioenergetic impairment (Celotto et al. 2006; Gnerer et al. 2006). TPI-deficiency pathogenesis has also been proposed to result from protein unfolding, which could result from a loss-of-function or a gain-of-function toxicity (Olah et al. 2002). We investigated the possibility of TPIsugarkill semidominance that may result from a gain-of-function protein toxicity. The TPIsugarkill mutation is completely recessive and expression of transgenic TPIsugarkill protein does not result in any detectable toxicity beyond that of wild-type overexpression. Native HPLC size exclusion chromatography assays demonstrate that TPIsugarkill protein is capable of forming a dimer, suggesting the protein is properly folded.
The TPIsugarkill strain has reduced life span, progressive locomotor impairment, and neurodegeneration at room temperature, 25°, and 29°, where phenotypic onset is accelerated with increasing temperature (Celotto et al. 2006). These data suggest that the dysfunction associated with the mutation is temperature sensitive and the extent of dysfunction increases within the physiologically relevant range for Drosophila (∼18°–30°). This interpretation is consistent with the fact that the mutation was initialy isolated as a temperature-sensitive paralytic mutant (Palladino et al. 2002). Western blot analysis has demonstrated that the TPIsugarkill protein is reduced in the mutants and that this decrease is enhanced with increases in temperature within the normal physiological temperatures for Drosophila. These findings suggest that temperature can be used to antagonize TPIsugarkill function and allow a range of phenotypic severity to be studied, analogous to an allelic series of mutations.
Our studies demonstrate a temperature-dependent reduction in TPI protein: even an acute temperature shift dramatically reduces cellular TPI protein (Figure 3). However, mRNA levels are not reduced in TPIsugarkill animals at any temperature examined (Celotto et al. 2006 and data not shown). These findings suggested active proteolytic degradation of TPIsugarkill protein. Indeed, genetic inhibition of the 20S proteasome core with the Pros261 mutation results in modest increases in TPIsugarkill protein levels (Figure 5). These data suggest the TPIsugarkill protein is functional. Transgenic overexpression of TPIsugarkill protein was able to partially rescue the longevity defect associated with TPIsugarkill, further supporting the assertion that TPIsugarkill protein is functional. The data are consistent with a model whereby the mutation results in the degradation of functional TPI protein. Further experiments will be needed to determine whether the protein is fully functional and to assess the relative contributions of protein degradation and hypomorphic function.
It is possible that the mutant protein has an essentially normal structure and retains function but that the methionine-to-threonine missense mutation destabilizes the protein by a mechanism independent of protein function and structure. This could occur if the mutation created a signal stimulating the protein's degradation via a regulatory or a cellular quality-control mechanism. Such signals could include (1) an exposed hydrophobic patch in the protein, (2) a degradation signal for an E3 ubiquitin ligase subunit, (3) a post-translational covalent modification, or (4) a functional PEST domain. Although the protein retains function and the ability to form a dimer, it remains possible that it is partially misfolded, stimulating known quality-control pathways and its degradation. Alternatively, the mutation could create a degradation signal. One such pathway known to increase the rate of proteolytic degradation is the PEST pathway. Amino acid sequences rich in proline (P), glutamic acid (E), serine (S), and threonine (T) residues form functional PEST domains that can result in rapid degradation of proteins in vivo (Rogers et al. 1986; Rechsteiner and Rogers 1996). These are assigned PEST-FIND scores on the basis of an established algorithm (Rogers et al. 1986). TPI proteins are not known to be regulated by the PEST pathway—consistent with this, the PEST algorithm does not find any PEST sequences in the protein (http://www.at.embnet.org/toolbox/pestfind/). The sequence KGAFTGEISPAMLK is identified as a “poor PEST domain” with a score of −22.8. Although the TPIsugarkill mutant adds an additional T within this sequence (KGAFTGEISPATLK), the algorithm still rates the region as a “poor PEST domain” with a marginally improved score of −16.55 (Rogers et al. 1986).
Although it is not clear what stimulates the degradation of TPIsugarkill protein, the efficient degradation of mutant protein suggests a regulated quality-control mechanism. Quality-control pathways that degrade nonfunctional, misfolded, and unfolded proteins are extensive and required for the health and longevity of the cell (Hartl and Hayer-Hartl 2002; Barral et al. 2004; Young et al. 2004; Zhang and Kaufman 2006; Outeiro and Tetzlaff 2007). Cytosolic protein quality-control pathways are often mediated by Hsp90 and various cochaperones that form extensive networks with chaperones specific for individual or groups of substrates (Zhao et al. 2005). Further experimentation will be needed to determine the basis of the TPIsugarkill protein degradation and whether the mutant protein is degraded in an Hsp90 and/or ubiquitin-dependent manner. The TPIsugarkill mutant may prove useful at elucidating cytosolic quality-control mechanisms required to prevent premature senescence and progressive neurodegenerative disease.
Together, the data suggest that TPIsugarkill pathogenesis does not result from bioenergetic impairment, the encoded protein forms a stable dimer and retains sufficient function to rescue the mutation upon transgenic overexpression, and that pathogenesis results from proteasomal degradation of the apparently functional protein. Alternatively, it is possible that mutations abrogating isomerase activity are lethal (do not cause disease) and that the specific mutations that result in disease disrupt a hitherto novel function of TPI. This interpretation is consistent with recent findings in a yeast TPI model, where transgenic expression of multiple human mutant TPI proteins in vivo demonstrated catalytic isomerase activity associated with the pathogenic mutant proteins (Ralser et al. 2006).
Acknowledgments
We thank Don DeFranco, Lesley Ashmore, and Stacy Hrizo for helpful comments; Emily Trostel for editorial suggestions; and the Bloomington Stock Center for fly strains. We thank the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (T32NS 07391-07) (A.M.C.), the American Heart Association (0630344N) (M.J.P.), the NIH National Institute on Aging (AG025046) (M.J.P.), The University of Pittsburgh Department of Pharmacology and Chemical Biology, and The University of Pittsburgh School of Medicine for financial support.
References
- Ahmed, N., S. Battah, N. Karachalias, R. Babaei-Jadidi, M. Horanyi et al., 2003. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim. Biophys. Acta 1639 121–132. [DOI] [PubMed] [Google Scholar]
- Arya, R., M. R. Lalloz, A. J. Bellingham and D. M. Layton, 1997. Evidence for founder effect of the Glu104Asp substitution and identification of new mutations in triosephosphate isomerase deficiency. Hum. Mutat. 10 290–294. [DOI] [PubMed] [Google Scholar]
- Ationu, A., A. Humphries, M. R. Lalloz, R. Arya, B. Wild et al., 1999. Reversal of metabolic block in glycolysis by enzyme replacement in triosephosphate isomerase-deficient cells. Blood 94 3193–3198. [PubMed] [Google Scholar]
- Barral, J. M., S. A. Broadley, G. Schaffar and F. U. Hartl, 2004. Roles of molecular chaperones in protein misfolding diseases. Semin. Cell Dev. Biol. 15 17–29. [DOI] [PubMed] [Google Scholar]
- Celotto, A. M., A. C. Frank, J. L. Seigle and M. J. Palladino, 2006. Drosophila model of human inherited triosephosphate isomerase deficiency glycolytic enzymopathy. Genetics 174 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni, N., and E. S. Gonos, 2005. Proteasome dysfunction in mammalian aging: steps and factors involved. Exp. Gerontol. 40 931–938. [DOI] [PubMed] [Google Scholar]
- Daar, I. O., P. J. Artymiuk, D. C. Phillips and L. E. Maquat, 1986. Human triose-phosphate isomerase deficiency: a single amino acid substitution results in a thermolabile enzyme. Proc. Natl. Acad. Sci. USA 83 7903–7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Q., E. Dimayuga, W. R. Markesbery and J. N. Keller, 2006. Proteasome inhibition induces reversible impairments in protein synthesis. FASEB J. 20 1055–1063. [DOI] [PubMed] [Google Scholar]
- Eber, S. W., A. Pekrun, A. Bardosi, M. Gahr, W. K. Krietsch et al., 1991. Triosephosphate isomerase deficiency: haemolytic anaemia, myopathy with altered mitochondria and mental retardation due to a new variant with accelerated enzyme catabolism and diminished specific activity. Eur. J. Pediatr. 150 761–766. [DOI] [PubMed] [Google Scholar]
- Fernandez-Funez, P., M. L. Nino-Rosales, B. de Gouyon, W. C. She, J. M. Luchak et al., 2000. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408 101–106. [DOI] [PubMed] [Google Scholar]
- Ganetzky, B., and C. F. Wu, 1982. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J. Neurophysiol. 47 501–514. [DOI] [PubMed] [Google Scholar]
- Gnerer, J. P., R. A. Kreber and B. Ganetzky, 2006. wasted away, a Drosophila mutation in triosephosphate isomerase, causes paralysis, neurodegeneration, and early death. Proc. Natl. Acad. Sci. USA 103 14987–14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl, F. U., and M. Hayer-Hartl, 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295 1852–1858. [DOI] [PubMed] [Google Scholar]
- Hollan, S., M. Magocsi, E. Fodor, M. Horanyi, V. Harsanyi et al., 1997. Search for the pathogenesis of the differing phenotype in two compound heterozygote Hungarian brothers with the same genotypic triosephosphate isomerase deficiency. Proc. Natl. Acad. Sci. USA 94 10362–10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg, E., I. Nemeth, M. Horanyi, S. Pinter, L. Vecsei et al., 2000. Diminished blood levels of reduced glutathione and alpha-tocopherol in two triosephosphate isomerase-deficient brothers. Blood Cells Mol. Dis. 26 91–100. [DOI] [PubMed] [Google Scholar]
- Keller, J. N., K. B. Hanni and W. R. Markesbery, 2000. Impaired proteasome function in Alzheimer's disease. J. Neurochem. 75 436–439. [DOI] [PubMed] [Google Scholar]
- Keller, J. N., J. Gee and Q. Ding, 2002. The proteasome in brain aging. Ageing Res. Rev. 1 279–293. [DOI] [PubMed] [Google Scholar]
- Mande, S. C., V. Mainfroid, K. H. Kalk, K. Goraj, J. A. Martial et al., 1994. Crystal structure of recombinant human triosephosphate isomerase at 2.8 A resolution. Triosephosphate isomerase-related human genetic disorders and comparison with the trypanosomal enzyme. Protein Sci. 3 810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught, K. S., and C. W. Olanow, 2006. Proteasome inhibitor-induced model of Parkinson's disease. Ann. Neurol. 60 243–247. [DOI] [PubMed] [Google Scholar]
- Merkle, S., and W. Pretsch, 1989. Characterization of triosephosphate isomerase mutants with reduced enzyme activity in Mus musculus. Genetics 123 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrenweiser, H. W., 1987. Functional hemizygosity in the human genome: direct estimate from twelve erythrocyte enzyme loci. Hum. Genet. 77 241–245. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser, H. W., and S. Fielek, 1982. Elevated frequency of carriers for triosephosphate isomerase deficiency in newborn infants. Pediatr. Res. 16 960–963. [DOI] [PubMed] [Google Scholar]
- Neuburger, P. J., K. J. Saville, J. Zeng, K. A. Smyth and J. M. Belote, 2006. A genetic suppressor of two dominant temperature-sensitive lethal proteasome mutants of Drosophila melanogaster is itself a mutated proteasome subunit gene. Genetics 173 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah, J., F. Orosz, G. M. Keseru, Z. Kovari, J. Kovacs et al., 2002. Triosephosphate isomerase deficiency: a neurodegenerative misfolding disease. Biochem. Soc. Trans. 30 30–38. [DOI] [PubMed] [Google Scholar]
- Olah, J., F. Orosz, L. G. Puskas, L. Hackler, Jr., M. Horanyi et al., 2005. Triosephosphate isomerase deficiency: consequences of an inherited mutation at mRNA, protein and metabolic levels. Biochem. J. 392 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow, C. W., and K. S. McNaught, 2006. Ubiquitin-proteasome system and Parkinson's disease. Mov. Disord. 21 1806–1823. [DOI] [PubMed] [Google Scholar]
- Orosz, F., J. Olah, M. Alvarez, G. M. Keseru, B. Szabo et al., 2001. Distinct behavior of mutant triosephosphate isomerase in hemolysate and in isolated form: molecular basis of enzyme deficiency. Blood 98 3106–3112. [DOI] [PubMed] [Google Scholar]
- Orosz, F., J. Olah and J. Ovadi, 2006. Triosephosphate isomerase deficiency: facts and doubts. IUBMB Life 58 703–715. [DOI] [PubMed] [Google Scholar]
- Outeiro, T. F., and J. Tetzlaff, 2007. Mechanisms of disease II: cellular protein quality control. Semin. Pediatr. Neurol. 14 15–25. [DOI] [PubMed] [Google Scholar]
- Ovadi, J., F. Orosz and S. Hollan, 2004. Functional aspects of cellular microcompartmentation in the development of neurodegeneration: mutation induced aberrant protein-protein associations. Mol. Cell. Biochem. 256–257 83–93. [DOI] [PubMed] [Google Scholar]
- Palladino, M. J., T. J. Hadley and B. Ganetzky, 2002. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics 161 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino, M. J., J. E. Bower, R. Kreber and B. Ganetzky, 2003. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J. Neurosci. 23 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralser, M., G. Heeren, M. Breitenbach, H. Lehrach and S. Krobitsch, 2006. Triose phosphate isomerase deficiency is caused by altered dimerization–not catalytic inactivity–of the mutant enzymes. PLoS ONE 1 e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy, R., S. J. Vannucci, S. S. Yan, K. Herold, S. F. Yan et al., 2005. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 15 16R–28R. [DOI] [PubMed] [Google Scholar]
- Rechsteiner, M., and S. W. Rogers, 1996. PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 21 267–271. [PubMed] [Google Scholar]
- Rieder, S. V., and I. A. Rose, 1959. The mechanism of the triosephosphate isomerase reaction. J. Biol. Chem. 234 1007–1010. [PubMed] [Google Scholar]
- Rogers, S., R. Wells and M. Rechsteiner, 1986. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234 364–368. [DOI] [PubMed] [Google Scholar]
- Saville, K. J., and J. M. Belote, 1993. Identification of an essential gene, l(3)73Ai, with a dominant temperature-sensitive lethal allele, encoding a Drosophila proteasome subunit. Proc. Natl. Acad. Sci. USA 90 8842–8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, A. S., 2000. Triosephosphate isomerase deficiency: historical perspectives and molecular aspects. Baillieres Best Pract. Res. Clin. Haematol. 13 119–140. [DOI] [PubMed] [Google Scholar]
- Schneider, A. S., W. N. Valentine, M. Hattori and H. L. Heins, Jr., 1965. Hereditary hemolytic anemia with triosephosphate isomerase deficiency. N. Engl. J. Med. 272 229–235. [DOI] [PubMed] [Google Scholar]
- Seo, H., K. C. Sonntag and O. Isacson, 2004. Generalized brain and skin proteasome inhibition in Huntington's disease. Ann. Neurol. 56 319–328. [DOI] [PubMed] [Google Scholar]
- Shulman, J. M., and M. B. Feany, 2003. Genetic modifiers of tauopathy in Drosophila. Genetics 165 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, K. A., and J. M. Belote, 1999. The dominant temperature-sensitive lethal DTS7 of Drosophila melanogaster encodes an altered 20S proteasome beta-type subunit. Genetics 151 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese, S. D., N. Trotta, C. K. Rodesch, B. Aravamudan and K. Broadie, 2003. The ubiquitin proteasome system acutely regulates presynaptic protein turnover and synaptic efficacy. Curr. Biol. 13 899–910. [DOI] [PubMed] [Google Scholar]
- Valentine, W. N., 1966. Hereditary hemolytic anemia with triosephosphate isomerase deficiency. Am. J. Med. 41 27–41. [DOI] [PubMed] [Google Scholar]
- Young, J. C., V. R. Agashe, K. Siegers and F. U. Hartl, 2004. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell. Biol. 5 781–791. [DOI] [PubMed] [Google Scholar]
- Zanella, A., M. Mariani, M. B. Colombo, C. Borgna-Pignatti, P. De Stefano et al., 1985. Triosephosphate isomerase deficiency: 2 new cases. Scand. J. Haematol. 34 417–424. [DOI] [PubMed] [Google Scholar]
- Zhang, K., and R. J. Kaufman, 2006. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66 S102–S109. [DOI] [PubMed] [Google Scholar]
- Zhao, R., M. Davey, Y. C. Hsu, P. Kaplanek, A. Tong et al., 2005. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120 715–727. [DOI] [PubMed] [Google Scholar]