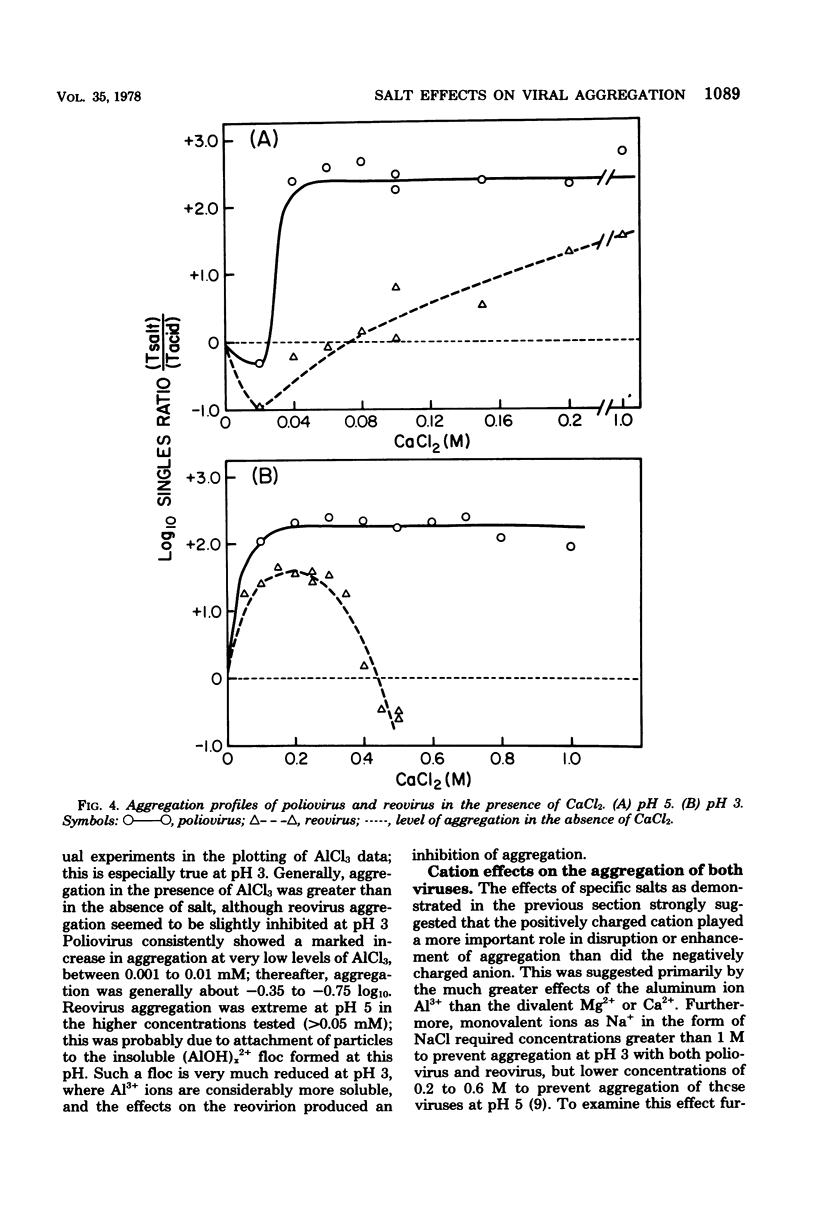
Abstract
As a first step toward the understanding of virus particle interactions in water, we have used the modified single particle analysis test to follow the aggregation of poliovirus and reovirus as induced by low pH in suspensions containing varying amounts of dissolved salts. Salts composed of mono-, di-, and trivalent cations and mono- and divalent anions were tested for their ability to reduce or increase the aggregation of these viruses in relation to that obtained by low pH alone. Mono- and divalent cations in concentrations covering those in natural waters were generally found to cause a decrease in aggregation, with the divalent cations having a much greater effectiveness than the monovalent cations. Trivalent ions (Al3+), in micromolar concentrations, were found to cause aggregation over that at low pH alone. Anions, whether monovalent or divalent, had little ability to produce inhibition of viral aggregation, and thus the overall effects were due almost exclusively to the cation. This was true regardless of whether the overall charge on the virus particle was positive or negative, as determined by the relation between the isoelectric point and the pH at which the tests were carried out. Thus, whereas virus particles conform to classical colloid theory in many respects, there are specific exceptions which must be taken into account in the design of any experiment in which viral aggregation is a factor.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chlumecka V., D'Obrenan P., Colter J. S. Electrophoretic studies on three variants of Mengo encephalomyelitis virus. Can J Biochem. 1973 Nov;51(11):1521–1526. doi: 10.1139/o73-202. [DOI] [PubMed] [Google Scholar]
- Dunlap R. C., Brown E. R., Barry D. W. Determination of the viral particle content of influenza vaccines by electron microxcopy. J Biol Stand. 1975;3(3):281–289. doi: 10.1016/0092-1157(75)90032-3. [DOI] [PubMed] [Google Scholar]
- Floyd R., Johnson J. D., Sharp D. G. Inactivation by bromine of single poliovirus particles in water. Appl Environ Microbiol. 1976 Feb;31(2):298–303. doi: 10.1128/aem.31.2.298-303.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Aggregation of poliovirus and reovirus by dilution in water. Appl Environ Microbiol. 1977 Jan;33(1):159–167. doi: 10.1128/aem.33.1.159-167.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: quantitation and kinetics of the aggregation of poliovirus and reovirus. Appl Environ Microbiol. 1978 Jun;35(6):1079–1083. doi: 10.1128/aem.35.6.1079-1083.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka R. S., Ackermann W. W. Evidence for conformational states of poliovirions: effects of cations on reactivity of poliovirions to guanidine. Proc Soc Exp Biol Med. 1975 Apr;148(4):1070–1074. doi: 10.3181/00379727-148-38690. [DOI] [PubMed] [Google Scholar]
- Fujioka R. S., Ackermann W. W. The inhibitory effects of MgCl2 on the inactivation kinetics of poliovirus by urea. Proc Soc Exp Biol Med. 1975 Apr;148(4):1063–1069. doi: 10.3181/00379727-148-38689. [DOI] [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- Mandel B. Neutralization of poliovirus: a hypothesis to explain the mechanism and the one-hit character of the neutralization reaction. Virology. 1976 Feb;69(2):500–510. doi: 10.1016/0042-6822(76)90480-3. [DOI] [PubMed] [Google Scholar]
- O'Brien R. T., Newman J. S. Inactivation of polioviruses and coxsackieviruses in surface water. Appl Environ Microbiol. 1977 Feb;33(2):334–340. doi: 10.1128/aem.33.2.334-340.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer P., Durham A. C. The cation binding associated with structural transitions in bromegrass mosaic virus. Virology. 1977 Sep;81(2):419–432. doi: 10.1016/0042-6822(77)90157-x. [DOI] [PubMed] [Google Scholar]
- Sebastian F. P. Purified wastewater--the untapped water resource. J Water Pollut Control Fed. 1974 Feb;46(2):239–246. [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Initial fast reaction of bromine on reovirus in turbulent flowing water. Appl Environ Microbiol. 1976 Feb;31(2):173–181. doi: 10.1128/aem.31.2.173-181.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Floyd R., Johnson J. D. Nature of the surviving plaque-forming unit of reovirus in water containing bromine. Appl Microbiol. 1975 Jan;29(1):94–101. doi: 10.1128/am.29.1.94-101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Kim K. S. Multiplicity reactivation and radiation survival of aggregated vaccinia virus. Calculation of plaque titer based on MR and particle aggregation seen in the electron microscope. Virology. 1966 Jul;29(3):359–366. doi: 10.1016/0042-6822(66)90211-x. [DOI] [PubMed] [Google Scholar]
- Sharp D. G. Multiplicity reactivation of animal viruses. Prog Med Virol. 1968;10:64–109. [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. Stabilization of poliovirus by cations. Tex Rep Biol Med. 1961;19:683–700. [PubMed] [Google Scholar]
- WALLIS C., MENICK J. L. Cationic stabilization--a new property of enteroviruses. Virology. 1962 Apr;16:504–506. doi: 10.1016/0042-6822(62)90234-9. [DOI] [PubMed] [Google Scholar]
- WALLIS C., SMITH K. O., MELNICH J. L. REOVIRUS ACTIVATION BY HEATING AND INACTIVATION BY COOLING IN MGC12 SOLUTIONS. Virology. 1964 Apr;22:608–619. doi: 10.1016/0042-6822(64)90083-2. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Rapp F. Different effects of MgC1-2 and MgSO-4 on the thermostability of viruses. Virology. 1965 Aug;26(4):694–699. doi: 10.1016/0042-6822(65)90332-6. [DOI] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Mountain C. W. Demonstration of solids-associated virus in wastewater and sludge. Appl Environ Microbiol. 1976 Mar;31(3):354–358. doi: 10.1128/aem.31.3.354-358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. C., Sharp D. G. Poliovirus aggregates and their survival in water. Appl Environ Microbiol. 1977 Jan;33(1):168–177. doi: 10.1128/aem.33.1.168-177.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



