Abstract
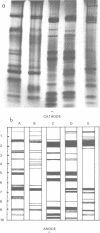
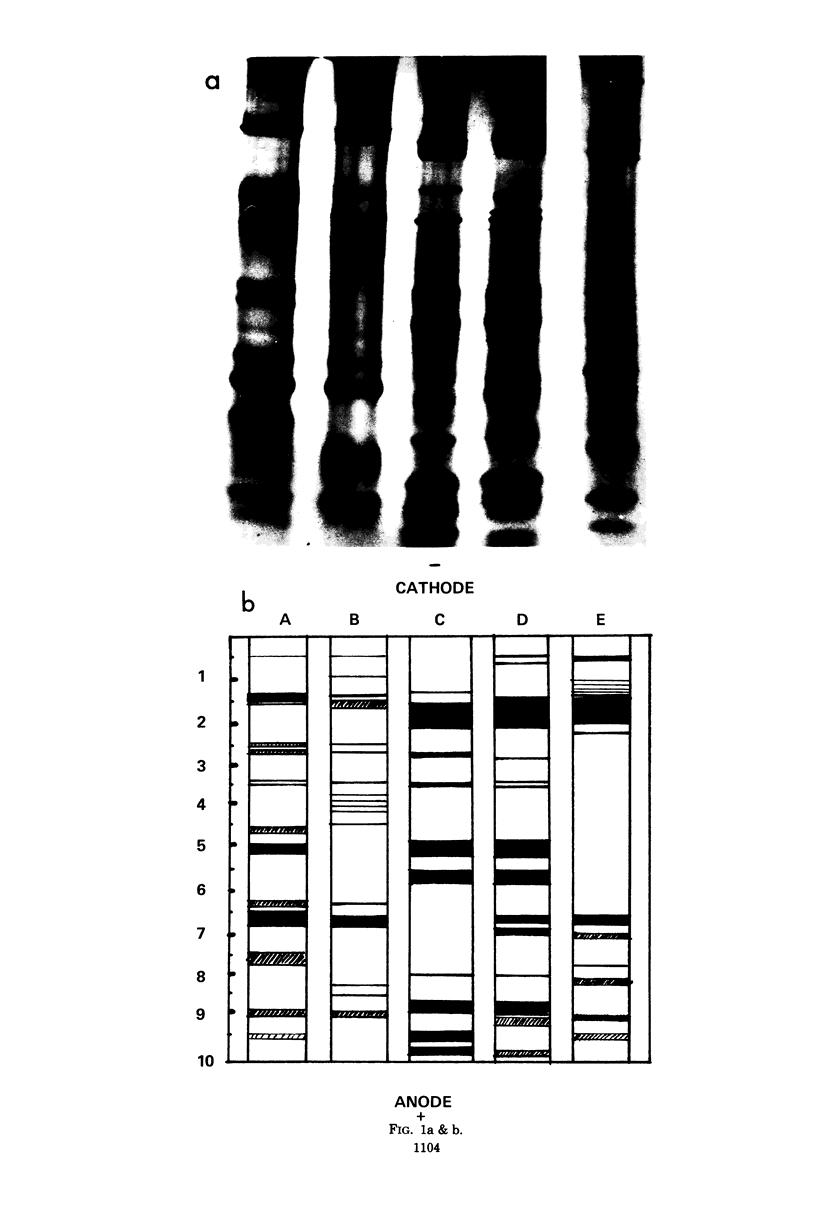
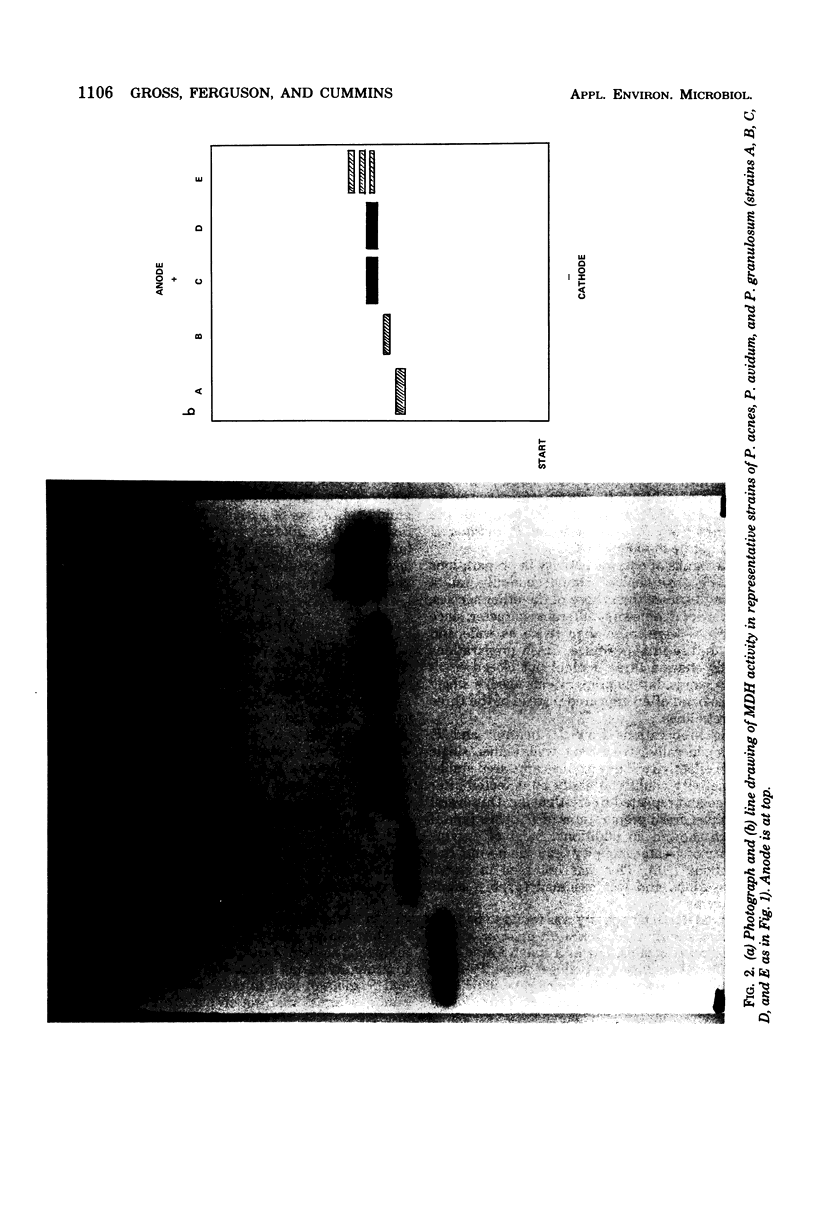
The soluble protein patterns and electrophoretic mobilities of malate and succinate dehydrogenases and catalase have been examined in 25 strains of Propionibacterium acnes, P. granulosum, and P. avidum. A distinctive protein pattern for each species was found, and it was possible also to distinguish the serotypes within P. acnes and P. avidum. Strains of P. acnes, P. granulosum, and P. avidum could be differentiated by the mobilities of their malate dehydrogenases. Catalase activity was detected in the soluble fractions of all strains. Catalases from P. acnes and P. avidum strains had the same mobility, whereas that from P. granulosum was slightly slower. Under the conditions used, succinate dehydrogenase activity could be detected, but the patterns were not distinctive.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Cummins C. S. Identification of Propionibacterium acnes and related organisms by precipitin tests with trichloroacetic acid extracts. J Clin Microbiol. 1976 Aug;2(2):104–110. [PMC free article] [PubMed] [Google Scholar]
- Cummins C. S., Johnson J. L. Corynebacterium parvum: a synonym for Propionibacterium acnes? J Gen Microbiol. 1974 Feb;80(2):433–442. doi: 10.1099/00221287-80-2-433. [DOI] [PubMed] [Google Scholar]
- Gasser F. Electrophoretic characterization of lactic dehydrogenases in the genus Lactobacillus. J Gen Microbiol. 1970 Aug;62(2):223–239. doi: 10.1099/00221287-62-2-223. [DOI] [PubMed] [Google Scholar]
- Green S. S., Goldberg H. S., Blenden D. C. Enzyme patterns in the study of leptospira. Appl Microbiol. 1967 Sep;15(5):1104–1113. doi: 10.1128/am.15.5.1104-1113.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Cummins C. S. Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. J Bacteriol. 1972 Mar;109(3):1047–1066. doi: 10.1128/jb.109.3.1047-1066.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keudell K. C., Goldberg H. S. Dehydrogenase patterns in the study of Bacteroidaceae. Appl Microbiol. 1970 Mar;19(3):505–511. doi: 10.1128/am.19.3.505-511.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- THORUP O. A., Jr, STROLE W. B., LEAVELL B. S. A method for the localization of catalase on starch gels. J Lab Clin Med. 1961 Jul;58:122–128. [PubMed] [Google Scholar]
- Worsfold V., Marshall M. J., Ellis E. B. Enzyme detection using phenazine methosulphate and tetrazolium salts: interference by oxygen. Anal Biochem. 1977 May 1;79(1-2):152–156. doi: 10.1016/0003-2697(77)90389-x. [DOI] [PubMed] [Google Scholar]