Abstract
Research on early renal function decline in diabetes is hampered by lack of simple tools for detecting trends (particularly systematic decreases) in renal function over time when GFR is normal or elevated. This study sought to assess how well serum cystatin C meets that need. Thirty participants with type 2 diabetes in the Diabetic Renal Disease Study met these three eligibility criteria: GFR >20 ml/min per 1.73 m2 at baseline (based on cold iothalamate clearance), 4 yr of follow-up, and yearly measurements of iothalamate clearance and serum cystatin C. With the use of linear regression, each individual’s trend in renal function over time, expressed as annual percentage change in iothalamate clearance, was determined. Serum cystatin C in mg/L was transformed to its reciprocal (100/cystatin C), and linear regression was used to determine each individual’s trend over time, expressed as annual percentage change. In paired comparisons of 100/cystatin C with iothalamate clearance at each examination, the two measures were numerically similar. More important, the trends in 100/cystatin C and iothalamate clearance were strongly correlated (Spearman r = 0.77). All 20 participants with negative trends in iothalamate clearance (declining renal function) also had negative trends for 100/cystatin C. Results were discordant for only three participants. In contrast, the trends for three commonly used creatinine-based estimates of GFR compared poorly with trends in iothalamate clearance (Spearman r < 0.35). Serial measures of serum cystatin C accurately detect trends in renal function in patients with normal or elevated GFR and provide means for studying early renal function decline in diabetes.
Assessment of a patient’s renal function may be used for two different purposes. One is to diagnose impaired renal function, and the other is to detect the presence of a progressive loss of renal function over time. The first is a cross-sectional assessment at a particular moment. Minimizing diagnostic errors is important, so accuracy of the GFR is critical, specifically in the neighborhood of the threshold for chronic renal failure (CRF; 60 ml/min per 1.73 m2). The second is a longitudinal assessment to detect systematic decreases in renal function (a negative trend) and requires repeated measurements over time. The most important result is whether a downward trend (slope) is present, regardless of the initial GFR value. Therefore, accuracy of the slope rather than the individual GFR measurements is the primary concern (1).
In patients with CRF, trends of renal function over time are determined using one of several approximations of GFR based on serum creatinine (2–4). These approximations, however, perform adequately only in advanced disease (GFR <60 ml/min per 1.73 m2) (4–6). In patients who have normal or elevated renal function but are suspected of losing renal function over time, creatinine-based measures are unreliable for detecting trends (2).
If CRF is to be prevented, a clinically applicable method is needed for detecting negative trends in renal function when the GFR is normal or elevated. The current methods for detecting trends in renal function in this range involve direct measurements of GFR based on intravenous infusion of an exogenous marker (e.g., iothalamate, the reference method used in this study), and none is simple enough for use in clinical or epidemiologic studies (2,3). The procedures are time-consuming, costly, and susceptible to measurement error (2). For routine clinical use, a simple measure that is based on an endogenous marker is needed—similar to serum creatinine but without its limitations (1–3).
The serum concentration of cystatin C has recently been proposed as an endogenous marker of renal function that is accurate even at the low concentrations found when GFR is normal or elevated. Cystatin C is a nonglycosylated basic protease inhibitor that is produced at a constant rate by all nucleated cells (7,8). It is freely filtered by the renal glomerulus and primarily catabolized in the renal tubules (8). Furthermore, levels are reported to be independent of gender, age, and body mass (9–12). Diurnal variation is insignificant, levels are not altered by inflammatory conditions, and the concentration is stable in stored serum (13–15). Automated particle-enhanced nephelometric immunoassays are well validated and commercially available (13). Criterion validity in cross-sectional studies of renal diseases of multiple causes including diabetes has been demonstrated extensively (15–21). However, the accuracy of serum cystatin C for detecting systematic changes in GFR (slope) over time in patients with normal or elevated GFR remains to be determined.
To test this issue, we examined serial GFR measurements in Pima Indians who had type 2 diabetes and participated in the Diabetic Renal Disease Study. Their onset of type 2 diabetes was at an early age, many have elevated GFR, and their risk for developing ESRD is high (22). These characteristics make this a suitable population for studying early renal function decline that is generalizable to many type 2 diabetes populations and to the type 1 diabetes population. This study sought to determine how accurately serial determinations of serum cystatin C detect trends (particularly systematic decreases) in renal function over time that have been documented by measurements of iothalamate clearance in patients with normal or elevated GFR. For comparison, we also report how accurately the trends obtained with three commonly used creatinine-based approximations of GFR reflect the trend in iothalamate clearance.
Materials and Methods
Patient Population
The study group was selected from among the participants in the Diabetic Renal Disease Study, sponsored by the National Institutes of Health and designed to investigate longitudinal changes in GFR in diabetes and diabetic renal disease. Participants were recruited between 1989 and 1994 from the Gila River Indian Community in Arizona (22). Urinary albumin excretion status was determined by the albumin to creatinine ratio (ACR) from four untimed urine samples collected at least 1 wk apart (23). Individuals with advanced renal disease (serum creatinine concentration >1.3 and >1.5 mg/dl for women and men, respectively) were ineligible, as were pregnant women and those with a chronic debilitating condition or evidence of nondiabetic renal disease. The protocol was approved by the institutional review boards of the National Institutes of Health and of Stanford University Medical Center and by the Gila River Indian Community Tribal Council (Sacaton, AZ).
GFR was measured by the urinary clearance of iothalamate, hereafter referred to as the direct measure of renal function. Measures of renal function that are based on serum creatinine or serum cystatin C are referred to as indirect measures. To have a study group with normal or elevated GFR, we selected participants with a baseline iothalamate clearance above the median for the whole Pima study group (120 ml/min per 1.73 m2). Of the 134 participants with diabetes included in the Diabetic Renal Disease Study, 30 met these eligibility criteria: Elevated baseline iothalamate clearance, 4 yr of follow-up with measurement of iothalamate clearance at least annually, and frozen serum samples available from the clearance measurements. Other characteristics of the eligible and ineligible participants did not differ.
Protocol for the Direct Measure of GFR by Iothalamate Clearance
On the day of the iothalamate clearance study, an indwelling plastic canula was inserted into the antecubital vein of each arm, one for the collection of blood samples and the other for infusing nonradiolabeled (“cold”) iothalamate (24). After the bladder was spontaneously voided, diuresis was initiated with an oral water load of 10 ml/kg (or 1500-ml maximal dose for participants >150 kg). Iothalamate (30% solution) was infused with a loading dose of 30% (300 mg plus 3 mg/kg for each kg >100 kg). Iothalamate then was delivered by an infusion pump to maintain a constant plasma concentration of 1.5 mg/dl. After a 60-min equilibration period, the bladder again was emptied by voiding, and four carefully timed urine collections, bracketed by the collection of blood samples, were made at 20-min intervals. Urinary clearance of iothalamate was estimated by the average of the four time intervals. The within-assay coefficient of variation (CV) based on the four 20-min collections is 12 ± 11% (n = 202), whereas the between-assay CV is 9 ± 8% (n = 29). In the baseline evaluation, iothalamate clearance was significantly correlated with body weight (Spearman correlation coefficient [rSp] = 0.63, P = 0.0002) but not significantly with height (rSp = 0.34, P = 0.07). Standardization of the clearance to a body surface area (BSA) of 1.73 m2 removed most of the variation caused by differences in body weight (rSp = 0.25, P = 0.19), so iothalamate clearance was analyzed after standardizing for BSA (ml/min per 1.73 m2). Given that the impact of standardization has not been examined in longitudinal studies, we analyzed both standardized and unstandardized values.
Laboratory Procedures and the Protocol for the Indirect Measures of GFR
All urine and serum samples were stored at −70°C until the day of assay, which for all measures except cystatin C were performed within 30 d of the sample collection. The concentration of urinary albumin was determined by immunonephelometry, whereas urine creatinine was measured by a modified picrate method of Jaffe. An HPLC system with a sensitive ultraviolet light detector was used to assay iothalamate at 236 nm (Instrumentation Shimadzu #6A, Kyoto, Japan). Ultrafiltrate of plasma and diluted urine were injected onto a reverse-phase column (#C18, 5μ Ultrasphere; Beckman, San Ramon, CA). The mobile phase was 3.5% acetonitrile in 10 mM triethylamine at a pH of 3.5, and the flow rate was 1.0 ml/min. Iothalamate concentration was determined from its solute peak, corresponding to column retention times of 14 min. Serum creatinine was measured by a modified picrate method of Jaffe on a Ciba Corning Express Plus Chemistry Analyzer. Serum creatinine was calibrated to Modification of Diet in Renal Disease (MDRD) laboratory values by comparing measurements in 186 paired specimens in the Phoenix and Cleveland laboratories. The between-assay CV in samples from the lowest and highest quartiles of the creatinine distribution were 2.76 and 1.76%, respectively. Serum cystatin C was measured by a single operator (B.E.P.O.) at the Joslin Diabetes Clinic using thawed samples by an immunoassay based on rabbit monospecific anti-human cystatin C antiserum (Dade Behring Diagnostics) conducted on a BN Prospec System nephelometer (Dade Behring Inc., Newark, DE). The between-assay CV in samples from the lowest and highest quartiles of the cystatin C distribution was 3.8 and 3.0%, respectively. As serum cystatin C concentration was independent of height and weight (rSp = −0.05, P = 0.78; and rSp = −0.2, P = 0.30, respectively), no standardization was required for this variable.
Statistical Analyses
Descriptive statistics and estimates of linear trends in renal function were obtained using SAS (SAS 8.02 for Windows; SAS Institute, Cary, NC). Serum cystatin C was transformed to the reciprocal multiplied by 100 (100/cystatin C in mg/L). Three serum creatinine-based estimates of GFR were calculated: The reciprocal multiplied by 100 (100/creatinine in mg/dl), the modified Cockcroft-Gault formula (2), and the MDRD equation (25). In studies of renal function, serum concentrations of creatinine and cystatin C are commonly transformed to their reciprocals for analysis (26). This serves to make changes over time have the same direction as the changes in iothalamate clearance, whereas untransformed serum concentrations change in the opposite direction. Pairwise comparisons between measures were made by the method of Bland and Altman (27). For longitudinal analysis, measures of renal function were transformed to the logarithmic scale. For each individual, five estimates of the trend in renal function over time were obtained by regression analysis: One for the direct measurement by iothalamate clearance and four for the indirect methods based on serum cystatin C and serum creatinine. A linear term adequately captured the temporal variability within the 4-yr span, so the trends could be represented as a slope and expressed as annual percentage change (the terms trend, slope, and annual percentage change are used interchangeably throughout this article). Error variance for each method of estimation of trend was calculated from the regression mean square error and expressed as a percentage of the mean (the within-individual residual SD). For evaluating the accuracy of trends based on the four indirect methods, each was compared with the trend based on iothalamate clearance (the reference method) using Spearman correlation coefficients and paired t test. Sensitivity and specificity of each measure for detecting declining renal function, defined as a negative annual percentage change in iothalamate clearance, were calculated.
Results
Clinical Characteristics of the Study Group and Pairwise Comparison of the Direct Measure of GFR with the Four Indirect Measures
The study population comprised 30 eligible Pima Indians with type 2 diabetes and GFR >120 ml/min per 1.73 m2 at baseline examination. The 30 participants (18 female, 12 male) were 40 ± 9 yr of age in the baseline period and were examined at least on a yearly basis (Table 1). The mean BMI for this cohort was in the obesity range (33 ± 7 kg/m2) and remained stable over the study periods. All participants had microalbuminuria or proteinuria at baseline, and the mean albumin to creatinine ratio increased over the study period. On average, direct measurements of GFR by iothalamate clearance decreased monotonically over the four time periods. Because the estimated body surface area of most participants was >1.73 m2, standardized clearance was less than unstandardized iothalamate clearance (Table 1). Serum cystatin C increased monotonically from one measurement to the next, so its reciprocal, 100/cystatin C, declined in parallel with iothalamate clearance, approximating the standardized more closely than unstandardized values. In contrast, changes in serum creatinine between follow-up examinations was not monotonic, although its concentration had increased above baseline by the final follow-up period. The three creatinine-based methods of GFR estimation differed substantially from each other. Although all three were close enough on average to mean iothalamate clearance to be of some use for comparisons of group means, their usefulness for classification of individuals according to renal status depends on the magnitude of the errors in individual estimates.
Table 1.
Clinical characteristics of the 30 Pima Indian participants with type 2 diabetes and elevated GFR at baseline,a according to period of follow-upb
| Clinical Characteristic | Period of Follow-Up
|
|||
|---|---|---|---|---|
| Baseline | First | Second | Third | |
| Female gender (%) | 19 (63%) | — | — | — |
| Age (yr) | 40 ± 9 | 41 ± 9 | 42 ± 9 | 43 ± 9 |
| BMI | 33.0 ± 7.0 | 32.4 ± 6.9 | 32.8 ± 7.4 | 32.2 ± 7.0 |
| ACR (mg/g)c | 103 (49 to 228) | 80.3 (44 to 601) | 109 (63 to 497) | 121 (36 to 992) |
| Iothalamate clearance | ||||
| Standardized (ml/min per 1.73 m2) | 153 ± 27 | 146 ± 33 | 137 ± 36 | 136 ± 42 |
| Unstandardized (ml/min) | 176 ± 40 | 163 ± 39 | 154 ± 44 | 152 ± 50 |
| Serum cystatin C | ||||
| Concentration (mg/L) | 0.66 ± 0.14 | 0.71 ± 0.28 | 0.78 ± 0.35 | 0.85 ± 0.58 |
| 100/cystatin C | 158 ± 34 | 156 ± 44 | 143 ± 39 | 139 ± 42 |
| Serum creatinine | ||||
| Concentration (mg/dl) | 0.72 ± 0.17 | 0.71 ± 0.21 | 0.75 ± 0.36 | 0.93 ± 1.00 |
| 100/creatinine | 146 ± 32 | 149 ± 34 | 148 ± 40 | 138 ± 39 |
| Cockroft-Gault (ml/min)d | 167 ± 60 | 164 ± 60 | 163 ± 66 | 152 ± 54 |
| MDRD (ml/min per 1.73 m2)e | 130 ± 32 | 130 ± 35 | 132 ± 42 | 117 ± 36 |
Defined as a GFR >120 ml/min as determined by iothalamate clearance standardized for BSA.
BMI, body mass index; ACR, albumin to creatinine ratio; MDRD, Modification of Diet in Renal Disease; BSA, body surface area. Data are mean ± SD.
Data are median (interquartile range).
The GFR based on the Cockcroft-Gault formula was estimated by the conventional modified formula [(140 − age in years) × (actual weight in kilograms)/(72 × serum creatinine in mg/dl) × 0.85 (if female)] [(see reference 3)].
The GFR based on the MDRD equation was estimated by the conventional formula for whites [186 × (SCr)−1.154 × (age)−0.203 × (0.742 if female)] [(see reference (25)].
To illustrate the magnitude of error in each of the four indirect estimates (100/cystatin C, 100/creatinine, the Cockcroft-Gault formula, and the MDRD equation) relative to direct measurement of GFR (standardized iothalamate clearance), we plotted all of the available data for the 30 participants in Figure 1, a cross-sectional comparison. A 30% error margin for an estimate of GFR at a point in time is considered tolerable agreement by current clinical practice guidelines (shaded regions in Figure 1) (2). All four indirect estimates were biased slightly upward with more values above than below the shaded areas. For 100/cystatin C, 14 of the 144 paired values were overestimates and none were underestimates (Figure 1A). The agreement was not as good for 100/creatinine and Cockcroft-Gault. For 100/creatinine, 34 values were overestimates and three were underestimates (Figure 1B), and for Cockcroft-Gault, 31 were overestimates and nine were underestimates (Figure 1C). For the MDRD estimates, 12 were overestimates and eight were underestimates (Figure 1D). The results of a Bland-Altman analysis of agreement were similar (see Figure 1 legend). Thus, 100/cystatin C and MDRD estimates agree more closely with standardized iothalamate clearance than 100/creatinine and Cockcroft-Gault estimates in these cross-sectional comparisons. However, if any of these indirect measures is used to assess a patient’s renal function at a single point in time, then allowance must be made for the occurrence of large errors (>30%) if GFR is normal or elevated. Such errors will be least frequent with 100/cystatin C.
Figure 1.
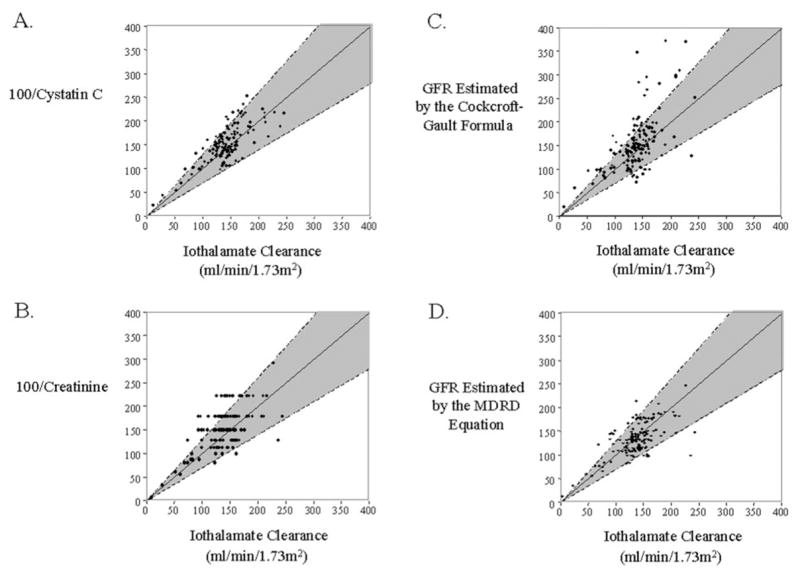
Cross-sectional comparison of standardized iothalamate clearance with four indirect methods of measuring renal function in 30 Pima Indian participants with type 2 diabetes. The 30 participants contributed a total of 144 paired measurements for which iothalamate clearance and serum for the determination of GFR by the indirect methods were collected simultaneously. The shaded regions represent error margins of ±30% for agreement between methods. The 95% distribution of differences between the methods of estimation and the reference method, expressed as percentages by the methods of Bland and Altman, were −30 to 32% for 100/cystatin C, −29 to 79% for 100/creatinine, −42 to 73% for the Cockcroft-Gault formula, and −43 to 39% for the Modification of Diet in Renal Disease (MDRD) equation.
Longitudinal Trends in Renal Function
Of the 30 eligible participants, seven contributed four to six direct GFR measurements, 10 contributed seven, and 13 contributed eight. Mean follow-up was 3.8 ± 0.3 yr.
For each individual, regression slopes (expressed as annual percentage change) were calculated for iothalamate clearance and for the four indirect measures of renal function. The slopes fitted to the standardized and unstandardized iothalamate clearance were identical (the regression models differed only in the intercepts), so the unstandardized values were not considered further. The annual percentage change in standardized iothalamate clearance was −4.4%. The annual percentage change in 100/cystatin C closely approximated this value and was not significantly different from it. The pairwise differences between the slopes for iothalamate clearance and each of the other indirect measures were also not statistically significant, but the magnitude of the differences was much larger (Table 2). In the subgroup of greatest interest, the 20 patients with declining renal function (negative slope for iothalamate clearance), the average slope for 100/cystatin C was still close to the slope for iothalamate clearance and not significantly different from it, whereas the average slope for 100/creatinine and the Cockcroft-Gault formula significantly underestimated it. The slope for estimates based on the MDRD equation also underestimated the slope for iothalamate clearance, but the difference was not quite significant.
Table 2.
Comparison of trends in renal function in 30 Pima Indian participants with type 2 diabetes and elevated GFR at baselinea
| Method of GFR Estimation | Mean Baseline Value | Annual Percentage Change ± SD | Mean Difference ± SD | P Valueb |
|---|---|---|---|---|
| Total cohort (n = 30) | ||||
| direct | ||||
| iothalamate clearance (ml/min per 1.73 m2) | 153 ± 27 | −4.4 ± 10.3% | ||
| indirect | ||||
| 100/cystatin Cc | 158 ± 34 | −4.3 ± 7.4% | −0.1 ± 4.0% | 0.88 |
| 100/creatininec | 149 ± 37 | −2.2 ± 8.6% | −2.2 ± 5.6% | 0.09 |
| Cockroft-Gault (ml/min) | 167 ± 60 | −3.4 ± 8.4% | −1.0 ± 6.1% | 0.50 |
| MDRD (ml/min per 1.73 m2) | 130 ± 32 | −2.8 ± 10.3% | −1.6 ± 7.9% | 0.29 |
| Declining renal functiond (n = 20) | ||||
| direct | ||||
| iothalamate clearance (ml/min per 1.73 m2) | 156 ± 30 | −8.1 ± 10.9% | ||
| indirect | ||||
| 100/cystatin Cc | 163 ± 34 | −6.9 ± 7.7% | −1.2 ± 4.1% | 0.21 |
| 100/creatininec | 148 ± 42 | −3.8 ± 9.5% | −4.3 ± 7.1% | 0.01 |
| Cockroft-Gault (ml/min) | 166 ± 66 | −4.5 ± 9.5% | −3.6 ± 7.5% | 0.04 |
| MDRD (ml/min per 1.73 m2) | 127 ± 35 | −4.4 ± 11.2% | −3.7 ± 7.9% | 0.07 |
| Stable renal function (n = 10) | ||||
| direct | ||||
| iothalamate clearance (ml/min per 1.73 m2) | 148 ± 18 | 2.9 ± 2.0% | ||
| indirect | ||||
| 100/cystatin Cc | 149 ± 34 | 0.8 ± 2.8% | 2.1 ± 3.0% | 0.06 |
| 100/creatininec | 153 ± 25 | 1.1 ± 5.3% | 1.8 ± 5.2% | 0.29 |
| Cockroft-Gault (ml/min) | 171 ± 48 | −1.4 ± 5.4% | 4.3 ± 5.1% | 0.03 |
| MDRD (ml/min per 1.73 m2) | 137 ± 21 | 0.7 ± 7.1% | 2.2 ± 6.7% | 0.30 |
GFR >120 ml/min per 1.73 m2 as determined by iothalamate clearance standardized for BSA.
Paired t test statistic.
Arbitrary units.
Defined as a negative annual change in GFR as determined by iothalamate clearance standardized for BSA.
To illustrate the magnitude of error in an individual’s slope (annual percentage change) based on each of the four indirect estimates of renal function relative to the slope for direct measurements of GFR (standardized iothalamate clearance), we plotted the slopes against the annual percentage change for iothalamate clearance (Figure 2). The annual percentage change in 100/cystatin C was strongly correlated with the annual percentage change in iothalamate clearance (rSp = 0.77, P < 0.0001; Figure 2A). If declining renal function is defined as a negative annual percentage change, then the three individuals plotted in the lower right quadrant represent false-positive diagnoses, that is, declining renal function according to 100/cystatin C but stable iothalamate clearance. Conversely, the empty upper left quadrant indicates that none of the individuals with declining iothalamate clearance failed to have declining renal function according to 100/cystatin C. Consequently, the false-positive and false-negative rates for the annual percentage change in 100/cystatin C were 10% (3 of 30) and 0% (0 of 30), respectively.
Figure 2.
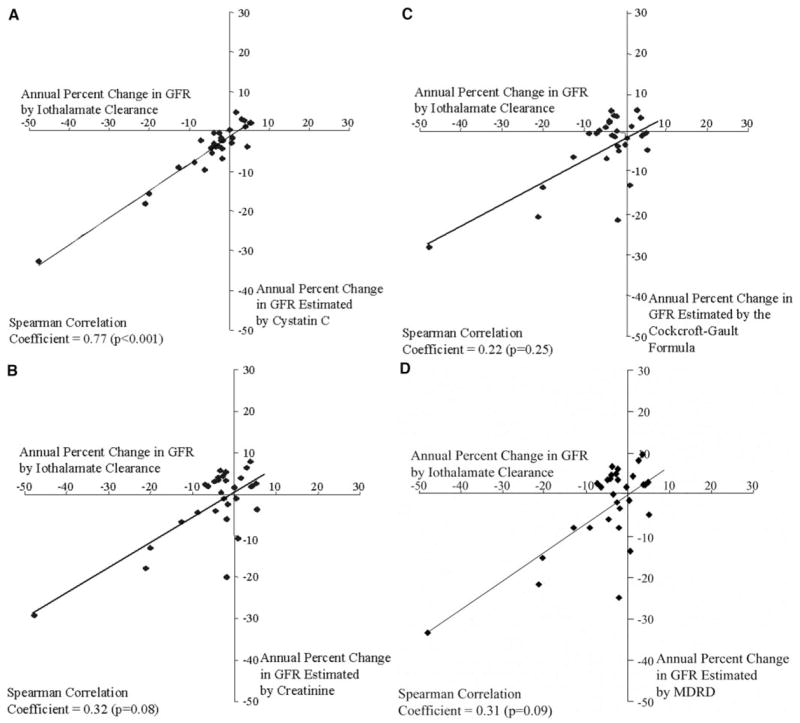
Correlation between estimates of the annual percentage change in renal function as determined from serial measurements of standardized iothalamate clearance and four indirect measures in 30 Pima Indian participants. Points in the lower right quadrant of a plot represent false-positive results for declining renal function (defined by an annual percentage change in iothalamate clearance <0 ml/min per 1.73 m2). Points in the upper left quadrant of a plot represent false-negative results for declining renal function (defined by an annual percentage change in iothalamate clearance ≥0 ml/min per 1.73 m2). Operating characteristics for trends in renal function estimated by cystatin C (A) are described in the text. The operating characteristics (sensitivity and specificity) of trends estimated by 100/creatinine (B), Cockcroft-Gault formula (C), and MDRD equation (D) were 50% and 70%, 60% and 60%, and 50% and 70%, respectively.
In contrast to the results for slopes based on 100/cystatin C, the slopes for the creatinine-based measures of renal function were weakly correlated with the annual percentage change in iothalamate clearance. For 100/creatinine (rSp = 0.34, P = 0.06), there were three (10%) false-positive and 10 (33%) false-negative results (Figure 2B). For the Cockcroft-Gault formula (rSp = 0.22, P = 0.25), the false-positive and false-negative rates were 20% (6 of 30) and 27% (8 of 30), respectively (Figure 2C). For the MDRD equation (rSp = 0.31, P = 0.09), the false-positive and false-negative rates were 10% (3 of 30) and 33% (10 of 30), respectively (Figure 2D).
The sensitivity and specificity of trends in serum cystatin C to detect declining renal function were 100 and 70%, respectively. The positive predictive value and negative predictive value were 87 and 100%, respectively. The overall accuracy of this method, defined as the measure of true findings (sum of the true-positive and true-negative results) divided by all test results, was 90%. The corresponding data for the creatinine-based estimates are detailed in the legend to Figure 2.
Analysis of the Precision of the Annual Percentage Change in GFR
To explain the observed advantage of cystatin C over the creatinine-based estimates for accurately reflecting trends in renal function, we sought to determine whether cystatin C is associated with less error variance than the other measures. The within-individual residual SD for the four methods of GFR estimation was smallest for 100/cystatin C. For the 30 participants, the mean residual SD for 100/cystatin C was 9.0% as compared with 13.8% for 100/creatinine (paired t test, P = 0.01), 14.2% for the Cockcroft-Gault formula (paired t test, P = 0.01), and 16.6% for the MDRD equation (paired t test, P = 0.002). Furthermore, the mean residual SD for standardized iothalamate clearance (12.1%) tended to be greater than that of cystatin C (paired t test, P = 0.10).
In Figure 3, we present four examples that illustrate the observation that trends in 100/cystatin C have a smaller error variance than direct measurements of iothalamate clearance. The first example is a clear illustration of the observation (Figure 3A). Although the annual percentage change in GFR based on iothalamate clearance and cystatin C are similar (−21 and −18%, respectively), the mean residual SD was larger for the trend in iothalamate clearance than for the trend in cystatin C (14 and 10%, respectively). Similarly, in the three participants in which the direction of change in 100/cystatin C was discrepant with iothalamate clearance (the “false-positives” in Figure 2A), the mean residual SD was larger for iothalamate clearance in all instances (Figure 3, B through D).
Figure 3.
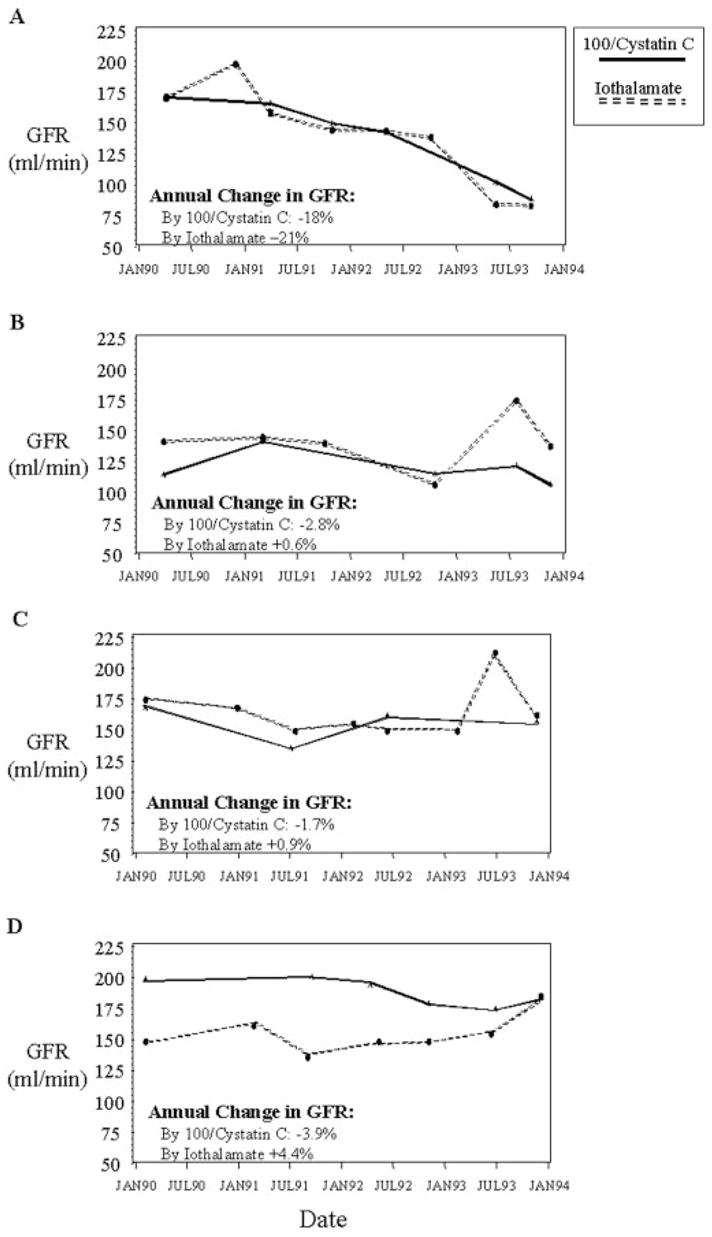
Serial determinations of renal function by standardized iothalamate clearance and by 100/cystatin C. The rate of change in renal function for each individual was estimated by linear regression of the determinations (transformed to the logarithmic scale) over time and expressed as the annual percentage change. (A) A participant with rapidly declining GFR. (B through D) Three individuals with disagreement between the trends in iothalamate clearance and 100/cystatin C for determining declining renal function. All examples illustrate the greater variability of standardized iothalamate clearance than 100/cystatin C. Within-individual SD for standardized iothalamate clearance was 14, 16, 16, and 9% for examples 1 through 4, respectively. For 100/cystatin C, it was 10, 11, 15, and 5%, respectively.
Discussion
This 4-yr study of renal function in type 2 diabetes demonstrates for the first time the close relationship between longitudinal trends in iothalamate clearance and the trends in renal function estimated from serum cystatin C. Previous reports on the performance of cystatin C have focused on its cross-sectional accuracy in identifying patients with existing CRF. Results of the current study, however, serve a different purpose: They demonstrate that serial measurements of serum cystatin C provide an accurate estimation of trends in renal function in patients with diabetes and normal or elevated GFR.
The implications of this feature of cystatin C are of great importance. First, it permits early identification of patients who are at risk for (but before) the development of CRF—at a stage of disease when interventions may be most effective. Second, it carries the important research implication that a practical tool now exists for studying renal function decline as a biologic end point. Before now, the available creatinine-based methods provided poor estimation of longitudinal trends in renal function in this range of GFR.
Two very important aspects of the performance of serum cystatin C were demonstrated in this study. First, it confirms cross-sectional correlation of cystatin C with iothalamate clearance (the reference method) across the full range of GFR values. Although this correlation has been reported extensively, the patients studied have tended to have renal function in the normal or impaired renal function ranges (15–21). The current results extend these previous findings by allowing generalization to the range of hyperfiltration, a range in which creatinine-based estimates are known to perform poorly. Second and of greater importance, this study demonstrates the predictive validity of serial measures of cystatin C for detecting declining renal function.
The findings of this study also suggest that measurement of serum cystatin C has scientific as well as practical advantages over direct measurement of GFR by one of the reference methods, such as iothalamate clearance. A difficulty with the direct measurement of GFR by one of the reference methods is the surprising variability inherent in replicate measurements (28–30). This variability in iothalamate clearance may arise from technical error or reflect short-term variability in true GFR. In the former case, variability may arise from both human and technical error during repeated timed urine collections and multiple assays for the calculation of iothalamate clearance (2,4,28,31). In this study, these factors were minimized by the skill and extensive training of the technical staff. In contrast, measurement of an endogenous marker such as cystatin C requires only a single assay and no critical timing in the sample collection.
Alternatively, the large variability in measurements of iothalamate clearance may be due to short-term excursions in true GFR. These excursions may result from physiologic variation related to dietary factors or glycemic control among other things (32,33). That similar variability is not seen with serum cystatin C suggests that the serum concentration reflects the cumulative effect of the GFR over a period of time, akin to glycated hemoglobin A1c as a time-averaged measure of plasma glucose. Rather than a shortcoming, this hypothesized feature would make serial measurements of cystatin C an attractive representation of persistent change for the purposes of evaluating long-term trends in renal function. This explanation for the superior reproducibility of estimates of GFR based on serum cystatin C requires further study.
There exists some controversy over the accuracy of cystatin C. Contrary to previous reports, a large cross-sectional study suggests that factors other than renal function (age, weight, gender, smoking, and levels of c-reactive protein) may influence serum cystatin C levels independent of GFR (34). This conclusion can be questioned, however, because GFR was estimated in that study by the creatinine clearance, a measure that is recognized to have its own biases and lack of precision relative to gold standard methods such as inulin or iothalamate clearance (2). Thus, the associations seen in that study may be the result of the performance of creatinine clearance rather than problems with cystatin C. It is important to recognize that factors associated with systematic differences between 100/cystatin C and GFR in cross-sectional comparisons may not affect the accuracy of determination of trends in renal function, given that the latter analysis depends on the change in cystatin C over time rather than its absolute value.
In conclusion, this first study of the longitudinal behavior of cystatin C provides convincing evidence that sequential measurements of cystatin C are an accurate and precise alternative to gold standard methods for measuring the urinary clearance of exogenous markers to quantify trends in renal function and detect declining renal function. This finding has major implications for clinical research because it demonstrates the existence of a practical, inexpensive, and accurate alternative for investigating trends in renal function in epidemiologic studies. Although creatinine-based estimates may provide sufficient accuracy for diagnosing the presence of CRF, unlike cystatin C, they do not have sufficient precision for detecting longitudinal trends in GFR in the normal and hyperfiltration ranges. Validation of cystatin C in this range of renal function permits epidemiologic research into the timing and determinants of the initiation of early renal function decline and the early intervention to prevent chronic kidney disease in patients with type 1 or type 2 diabetes, for whom hyperfiltration is a common feature of the early stages of kidney complications.
Acknowledgments
This study was supported by grants (DK41526, DK58549, and DK67638 to A.S.K.) from the National Institutes of Health and by a Juvenile Diabetes Foundation International fellowship grant (3-2001-829 to B.A.P.) and a William Randolph Hearst Fellowship provided to B.A.P. by the William Randolph Hearst Foundation.
We are indebted to Linda H. Ficociello, MSc, for significant contributions to the analysis and presentation of the data and to Dr. Camille Jones for expert advice on manuscript content. We are also indebted to Lois Jones, RN, for performing the GFR measurements and to the members of the Gila River Indian Community for participating in this investigation.
References
- 1.Hsu C-Y, Chertow GM, Curhan GC. Methodological issues in studying the epidemiology of mild to moderate chronic renal insufficiency. Kidney Int. 2002;61:1567–1576. doi: 10.1046/j.1523-1755.2002.00299.x. [DOI] [PubMed] [Google Scholar]
- 2.K/DOQI clinical practice guidelines for chronic kidney disease. Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 3.Price CP, Finney H. Developments in the assessment of glomerular filtration rate. Clin Chim Acta. 2000;297:55–66. doi: 10.1016/s0009-8981(00)00233-3. [DOI] [PubMed] [Google Scholar]
- 4.Massey D. Commentary: Clinical diagnostic use of cystatin C. J Clin Lab Anal. 2004;18:55–60. doi: 10.1002/jcla.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viberti G, Walker JD. Natural history and pathogenesis of diabetic nephropathy. J Diabet Complications. 1991;5:72–75. doi: 10.1016/0891-6632(91)90022-h. [DOI] [PubMed] [Google Scholar]
- 6.Mogensen CE. How to protect the kidney in diabetic patients: With special reference to IDDM. Diabetes. 1997;46:S104–S111. doi: 10.2337/diab.46.2.s104. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin LD. Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens. 2001;10:551–553. doi: 10.1097/00041552-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Newman DJ. Cystatin C. Ann Clin Biochem. 2002;39:89–104. doi: 10.1258/0004563021901847. [DOI] [PubMed] [Google Scholar]
- 9.Laterza OF, Price CP, Scott MG. Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 10.Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function—A review. Clin Chem Lab Med. 1999;37:389–395. doi: 10.1515/CCLM.1999.064. [DOI] [PubMed] [Google Scholar]
- 11.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C—A new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–881. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 12.Vinge E, Lindergard B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest. 1999;59:587–592. doi: 10.1080/00365519950185076. [DOI] [PubMed] [Google Scholar]
- 13.Mussap M, Ruzzante N, Varagnolo M, Plebani M. Quantitative automated particle-enhanced immunonephelometric assay for the routine measurement of human cystatin C. Clin Chem Lab Med. 1998;36:859–865. doi: 10.1515/CCLM.1998.151. [DOI] [PubMed] [Google Scholar]
- 14.Galteau MM, Guyon M, Gueguen R, Siest G. Determination of serum cystatin C: Biological variation and reference values. Clin Chem Lab Med. 2001;39:850–857. doi: 10.1515/CCLM.2001.141. [DOI] [PubMed] [Google Scholar]
- 15.Kazama JJ, Kutsuwada K, Ataka K, Maruyama H, Gejyo F. Serum cystatin C reliably detects renal dysfunction in patients with various renal diseases. Nephron. 2002;91:13–20. doi: 10.1159/000057599. [DOI] [PubMed] [Google Scholar]
- 16.Randers E, Erlandsen EJ, Pedersen OL, Hasling C, Danielsen H. Serum cystatin C as an endogenous parameter of the renal function in patients with normal to moderately impaired kidney function. Clin Nephrol. 2000;54:203–209. [PubMed] [Google Scholar]
- 17.Mussap M, Dalla Vestra M, Fioretto P, Saller A, Varagnolo M, Nosadini R, Plebani M. Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int. 2002;61:1453–1461. doi: 10.1046/j.1523-1755.2002.00253.x. [DOI] [PubMed] [Google Scholar]
- 18.Tan GD, Lewis AV, James TJ, Altmann P, Taylor RP, Levy JC. Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: Reproducibility and accuracy compared with standard measures and iohexol clearance. Diabetes Care. 2002;25:2004–2009. doi: 10.2337/diacare.25.11.2004. [DOI] [PubMed] [Google Scholar]
- 19.Buysschaert M, Joudi I, Wallemacq P, Hermans MP. Comparative performance of serum cystatin-C versus serum creatinine in diabetic subjects. Diabetes Metab. 2003;29:377–383. doi: 10.1016/s1262-3636(07)70048-4. [DOI] [PubMed] [Google Scholar]
- 20.Hoek FJ, Kemperman FAW, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–2031. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- 21.Xia LH, Bing XG, An XT. Serum cystatin C assay for the detection of early renal impairment in diabetic patients. J Clin Lab Anal. 2004;18:31–35. doi: 10.1002/jcla.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, Hirschman GH, Myers BD. Development and progression of renal disease in Pima Indians with noninsulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335:1636–1642. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 23.Myers BD, Nelson RG, Tan M, Beck GJ, Bennett PH, Knowler WC, Blouch K, Mitch WE. Progression of overt nephropathy in non-insulin-dependent diabetes. Kidney Int. 1995;47:1781–1789. doi: 10.1038/ki.1995.246. [DOI] [PubMed] [Google Scholar]
- 24.Myers BD, Nelson RG, Williams GW, Bennett PH, Hardy SA, Berg RL, Loon N, Knowler WC, Mitch WE. Glomerular function in Pima Indians with noninsulin-dependent diabetes mellitus of recent onset. J Clin Invest. 1991;88:524–530. doi: 10.1172/JCI115335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinler SA, Nawarskas JJ, Boyce EG, Connors JE, Charland SL, Goldfarb S. Predictive performance of ten equations for estimating creatinine clearance in cardiac patients. Iohexol Cooperative Study Group. Ann Pharmacother. 1998;32:1275–1283. doi: 10.1345/aph.18122. [DOI] [PubMed] [Google Scholar]
- 26.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 28.Apperloo AJ, de Zeeuw D, Donker AJ, de Jong PE. Precision of glomerular filtration rate determinations for long-term slope calculations is improved by simultaneous infusion of 125I-iothalamate and 131I-hippuran. J Am Soc Nephrol. 1996;7:567–572. doi: 10.1681/ASN.V74567. [DOI] [PubMed] [Google Scholar]
- 29.Gaspari F, Perico N, Remuzzi G. Application of newer clearance techniques for the determination of glomerular filtration rate. Curr Opin Nephrol Hypertens. 1998;7:675–680. doi: 10.1097/00041552-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 31.Rahn KH, Heidenreich S, Bruckner D. How to assess glomerular function and damage in humans. J Hypertens. 1999;17:309–317. doi: 10.1097/00004872-199917030-00002. [DOI] [PubMed] [Google Scholar]
- 32.Nair KS, Pabico RC, Truglia JA, McKenna BA, Statt M, Lockwood DH. Mechanism of glomerular hyperfiltration after a protein meal in humans. Role of hormones and amino acids. Diabetes Care. 1994;17:711–715. doi: 10.2337/diacare.17.7.711. [DOI] [PubMed] [Google Scholar]
- 33.Wiseman MJ, Saunders AJ, Keen H, Viberti G. Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin-dependent diabetes. N Engl J Med. 1985;312:617–621. doi: 10.1056/NEJM198503073121004. [DOI] [PubMed] [Google Scholar]
- 34.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]


