Abstract
In haplochromine cichlids, female mate choice based on male nuptial coloration has played an important role in speciation. Recent studies suggest that male coloration strongly influences the distribution of these fishes based on male–male aggression; males direct more aggression towards similarly coloured opponents while tolerating differently coloured individuals. We explored the role of male nuptial colour in aggression among the mbuna of Lake Malawi, examining aggression by male Metriaclima mbenjii, the red top cobalt zebra, towards conspecific opponents, similarly coloured heterospecific opponents and differently coloured heterospecifics. In trials in which focal males were offered a single opponent, while the total number of aggressive behaviours did not vary among opponent species, the types of behaviours did; focal males directed more lateral displays towards conspecifics than towards the other opponent species. When focal males were offered two opponents simultaneously, M. mbenjii directed more aggressive behaviours and more lateral displays towards similarly coloured opponents, regardless of species. Furthermore, when offered a conspecific and a similarly coloured opponent simultaneously, there were no differences in behaviour towards either opponent. Thus, nuptial coloration is used by males to identify competitors, and it suggests that male–male aggression may have also been an important diversifying force in speciation in rock-dwelling Lake Malawi cichlids.
Keywords: haplochromine, cichlid, speciation, Lake Malawi, sexual selection, male–male aggression
1. Introduction
The explosive radiation of haplochromine cichlids in Lake Malawi has been attributed to sexual selection (Turner 1994; Barlow 2000). While female mate choice, based largely on male nuptial coloration, has played a prominent role in the rock-dwelling ‘mbuna’ (Jordan et al. 2003), an additional hypothesis is the possible role of male–male aggression in the diversification of cichlid flocks. Turner (1994), citing an unpublished paper by Kornfield et al., suggested this possibility in mbuna, but this idea went largely unexplored until Seehausen & Schluter (2004), who found that the distribution of Lake Victorian haplochromines was influenced by male nuptial coloration; similarly coloured congeners were rarely found in sympatry. This suggests that males direct a disproportionate amount of aggression towards similarly coloured individuals, and that differently coloured species may coexist.
Behavioural studies of Lake Victoria cichlids supported Seehausen & Schluter's (2004) hypotheses. Dijkstra et al. (2005, 2006) found that these males directed more aggression towards similarly coloured opponents, though this bias disappeared when colour differences among opponents were muted experimentally. This is contrary to what Korzan & Fernald (2006) found in a Lake Tanganyika haplochromine (Astatotilapia burtoni), in which the males of two differently coloured sympatric morphotypes directed more aggression towards the males of the opposite morphotypes.
Few examinations of male–male aggression in the mbuna exist, though a field study by Holzberg (1978) found the pattern suggested by Seehausen & Schluter (2004); aggression in male Metriaclima zebra is biased towards similarly coloured conspecifics. A more recent field study found that males direct more aggression towards conspecifics, but ignore other mbuna species, though no colour-based biases were noted (Genner et al. 1999). Given that male nuptial coloration is similar among mbuna species, and that similarly coloured congeners rarely co-occur (Konings 2001), we hypothesize that male–male aggression based on coloration is an important diversification mechanism in the rock-dwelling Lake Malawi cichlid fauna. Owing to the competition among similarly coloured male conspecifics for territory and mates (Turner 1994; Barlow 2000), we also hypothesize that male nuptial coloration, regardless of species, is likely to be a cue by which males recognize competitors.
2. Material and methods
(a) Experimental animals
We used seven wild-caught adult male Metriaclima mbenjii as the subjects of these experiments. This species features a red dorsal fin and a solid blue body, often called a red top (RT) colour pattern. We examined aggressive behaviour in M. mbenjii by presenting individuals with opponents of three species: M. zebra ‘Chilumba’ (n=5; blue dorsal fin, heavily barred blue–black body; a ‘BB’ coloration); Labeotropheus fuelleborni ‘Chidunga’ (n=5; RT coloration); or a conspecific. Metriaclima mbenjii co-occurs with these two species in the wild, though not with these exact populations (Konings 2001). Owing to limited numbers of fish, all M. mbenjii served as both focal and stimulus fish in the experiments (Dijkstra et al. 2006); the order in which they did was haphazard. These fish were housed in two groups, and never given tankmates as opponents.
Fish were kept and experiments were conducted at the Milwaukee County Zoo. Each group of fish was housed separately in 151 l aquaria; group housing ensured the maintenance of social behaviours. Barriers were placed between aquaria to prevent observations of behavioural interactions of neighbours and assessments of potential opponents. Subjects were fed a mixture of foods twice daily. Aquaria were filtered with box filters and water changes were performed weekly. Fish were allowed to acclimate for approximately 30 days prior to experimentation.
(b) Experimental procedures
Experiments were conducted in a 151 l aquarium in which an artificial coral provided territory for the focal male. Smaller aquaria (approx. 6 l; one for experiment 1 and two for experiment 2) were suspended in the arena to house the opponent fish. These containers provided only visual contact between subjects and allowed the focal fish to interact with the opponent, but eliminated aggression-related injuries.
A single focal male was placed in the arena and allowed to acclimate for approximately 24–48 hours prior to observation. We haphazardly selected opponents (one for experiment 1 and two for experiment 2), placed them into the suspended aquaria and videotaped the behavioural interactions for 10 min with no humans present. After experimentation, all subjects were returned to their respective holding tanks. Both focal and opponent males were used no more frequently than once every three experiments.
Aggressive behaviour was scored from the videotapes, and the numbers of behaviours recorded by observers averaged per experiment. We selected three types of aggressive behaviours from Baerends & Baerends-van Roon (1950), recording the number of each performed only by the focal male. These included lateral displays, frontal displays and butting or biting (see Baerends & Baerends-van Roon (1950) for further description).
In experiment 1, focal males were offered one opponent. We presented each focal male (n=7) with three opponent species in three separate trials, yielding a total of 21 experiments scored by three observers. In experiment 2, focal males (n=6) were simultaneously presented with two opponents, such that they were given all possible combinations of two opponent species (M. mbenjii and L. fuelleborni; M. mbenjii and M. zebra Chilumba; and L. fuelleborni and M. zebra Chilumba), yielding 18 experiments. Because the same focal fish were used in both the experiments, we added a fourth observer to score experiment 2, which ensured that the patterns of behaviour observed in experiment 1 were verifiable and not resulting from inter-observer influence.
(c) Statistical analyses
All data were square-root transformed for normality. For experiment 1, the numbers of individual behaviours and their sum (‘total aggression’) were dependent variables in a multivariate ANOVA to determine whether or not the opponent species had an effect on the behaviour of the focal fish. Any behaviours significantly impacted by opponent species, along with total aggression, were used in analysing experiment 2. In experiment 2, a series of paired t-tests was used to determine whether the focal males were more aggressive to one opponent species than the other. Since our sample size was small and fish reused, and owing to the total number (6) of t-tests performed, we used a two-tailed t-test and a Holm–Bonferroni correction of the p-values obtained from these t-tests.
Since focal males were offered the same opponent species in both experiments 1 and 2, we wanted to determine whether or not prior experience had an influence on focal fish behaviour in experiment 2. We used total aggression towards each opponent species in experiment 1 as the independent variable in an ANOVA; total aggression towards each opponent species in experiment 2 was the dependent variable. There was no effect of prior experience on a focal male's behaviour in experiment 2 (F2,12=0.385; p=0.689).
3. Results
(a) Experiment 1
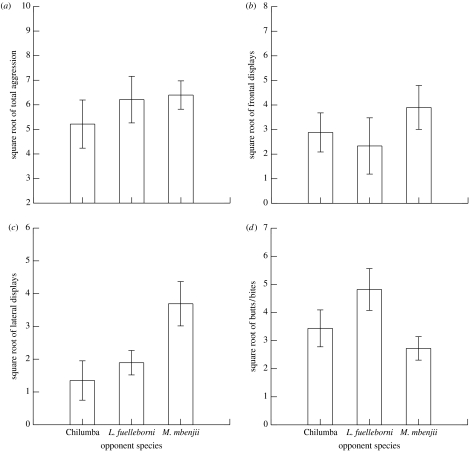
In experiment 1, male M. mbenjii behaved differently towards different opponent species. A multivariate ANOVA revealed that while there were no differences in the responses of M. mbenjii among opponents in either the total number of aggressive behaviours or the number of frontal displays performed, there were differences in the number of lateral displays and butts/bites performed (table 1; figure 1a,b). The focal male M. mbenjii performed significantly more lateral displays towards the opponent male M. mbenjii than they did towards the other two opponent species (figure 1c). Opponent male L. fuelleborni tended to receive more butts/bites than the other two species, though this difference was not significant (figure 1d).
Table 1.
Multivariate ANOVA demonstrating the effects of opponent species on behaviour in single-opponent trials (Wilks' Λ=0.362; F8,30=2.483, p=0.034).
| effect | SS | d.f. | MS | F | p |
|---|---|---|---|---|---|
| square root of total aggression | 5.646 | 2 | 2.823 | 0.658 | 0.530 |
| error | 77.254 | 18 | 4.292 | ||
| square root of frontal displays | 8.820 | 2 | 4.410 | 0.821 | 0.456 |
| error | 96.674 | 18 | 5.371 | ||
| square root of lateral displays | 21.022 | 2 | 10.511 | 5.603 | 0.013 |
| error | 33.767 | 18 | 1.876 | ||
| square root of butts/bites | 15.907 | 2 | 7.953 | 3.497 | 0.052 |
| error | 40.941 | 18 | 2.275 |
Figure 1.
Differences in the numbers of behaviours directed to single opponents by the focal male M. mbenjii. (a) Total aggression, (b) frontal displays, (c) lateral displays and (d) butts/bites. See table 1 for statistical tests.
(b) Experiment 2
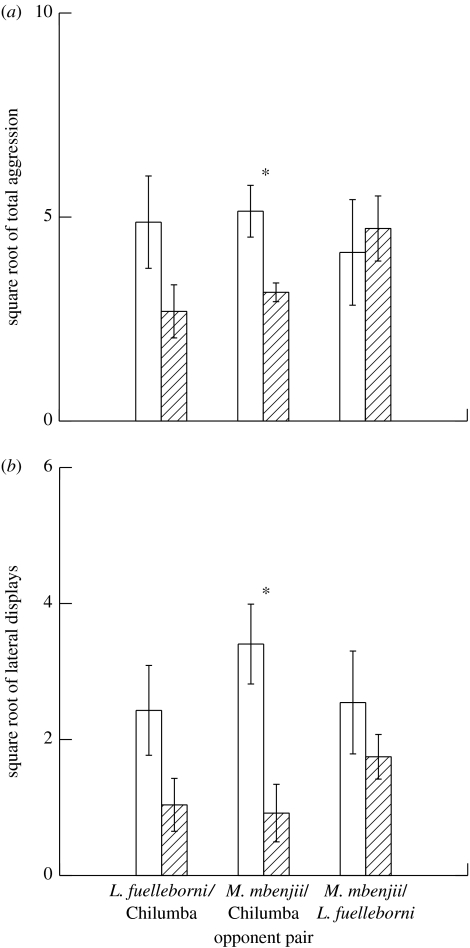
When offered two opponents, the behaviour of focal males depended upon the opponent pair offered. Because the only significant behaviour in experiment 1 was the number of lateral displays, we conducted our analyses on that and total aggression. When offered a pair of opponent males consisting of a M. mbenjii and a M. zebra Chilumba, the focal male directed a greater total number of aggressive behaviours (paired t-test: t5=4.077; p=0.010) and a greater number of lateral displays (paired t-test: t5=4.597; p=0.006) towards the opponent M. mbenjii. A similar trend was seen in opponent pairs consisting of a L. fuelleborni and a M. zebra Chilumba; more total aggression (paired t-test: t5=3.011; p=0.030; n.s. after Holm–Bonferroni correction) and more lateral displays (paired t-test: t5=2.411; p=0.061; n.s.) were directed towards the RT L. fuelleborni than were directed towards the M. zebra Chilumba. Finally, when offered an opponent pair consisting of a L. fuelleborni and a M. mbenjii, there was no difference in either total aggression (paired t-test: t5=−0.339; p=n.s.) or the number of lateral displays (paired t-test: t5=1.050; p=n.s.) performed to either species (figure 2).
Figure 2.
Differences in the numbers of behaviours directed to opponents by focal male M. mbenjii when two opponents (open bars, RT opponent; striped bars, other opponent) are simultaneously presented. (a) Total aggression and (b) lateral displays. For both, ‘RT opponent’ can refer to either M. mbenjii or L. fuelleborni, but refers to M. mbenjii specifically in mbenjii/fuelleborni opponent pairs (*p≤0.01).
4. Discussion
When offered single opponents, male M. mbenjii exhibited behavioural differences depending on the opponent species: opponent M. mbenjii received more lateral displays and opponent L. fuelleborni received more butts/bites. Thus, M. mbenjii do not change the overall number of aggressive behaviours performed towards different species, but instead change the types of behaviours used. When offered two opponents, the focal male M. mbenjii still makes a distinction, one based on opponent coloration. When the RT-coloured opponents (M. mbenjii or L. fuelleborni) were offered alongside a M. zebra Chilumba, these opponents received more lateral displays and more total aggression than the BB M. zebra Chilumba. When offered pairs of similarly coloured opponents (M. mbenjii and L. fuelleborni), the focal male M. mbenjii did not behave differently towards either the conspecific or heterospecific opponent.
Our findings that M. mbenjii males directed less intense (experiment 1) or fewer (experiment 2) aggressive behaviours towards M. zebra Chilumba agree with a field study by Genner et al. (1999), which found that males of the ‘zebra’ complex tolerated heterospecifics within their feeding territories. Our findings, however, differ from Genner et al.'s (1999) in that M. mbenjii directed intense and numerous aggressive behaviours towards L. fuelleborni; when offered M. mbenjii and L. fuelleborni concurrently in experiment 2, there was no difference in the aggression of focal males towards either species. We attribute this difference to the fact that the L. fuelleborni we used had the same nuptial colour pattern as our focal male M. mbenjii; this idea is supported by the aggressive bias displayed towards L. fuelleborni in opponent pairs consisting of L. fuelleborni and M. zebra Chilumba in experiment 2.
The behavioural differences in the treatment of opponents are very interesting. In aggressive encounters in cichlids, lateral displays intimidate opponents through exhibitions of size, strength and colour (Baerends & Baerends-van Roon 1950). Intimidation suggests that the individual views his opponent as a rival, and by performing these displays attempts to communicate his superiority, rather than simply doing physical harm (Barlow 2000). In experiment 1, focal male M. mbenjii performed significantly more lateral displays towards opponent M. mbenjii than they did towards the other two opponent species. This makes sense, given that conspecific males are rivals for territory and mating opportunities (Genner et al. 1999). The other two opponent species were typically bitten at or butted, which are attempts to chase them away (Barlow 2000). Given the treatment of the similarly coloured L. fuelleborni in experiment 2, opponent coloration probably plays a significant role in the decision to intimidate versus chase away opponents.
Our findings suggest a novel role for male nuptial coloration in the mbuna of Lake Malawi: the identification of opponents and the modulation of aggressive behaviour towards them. Although this has been demonstrated in Lake Victoria cichlids (Seehausen & Schluter 2004), our results are among the first, to the best of our knowledge, suggesting that aggression among the mbuna is modulated via male coloration. This implies that male–male aggression plays a role in the distribution of mbuna species throughout Lake Malawi, and explains why similarly coloured congeners are rarely found in sympatry (Konings 2001). Our results also offer further support for Seehausen & Schluter's (2004) hypothesis that male–male aggression may act as a diversifying force in haplochromine cichlids.
Acknowledgments
Funding was provided by a Ruth Walker grant. We thank C. Pelke and the ARC staff for their assistance. B. Harrison is thanked for project assistance. H. Bootsma, W. Leibel and P. Loiselle commented on the manuscript. This research was approved by and conducted under the auspices of the Milwaukee County Zoo Senior Animal Staff, and adheres to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research, and the legal requirements of the country in which the work was performed.
Supplementary Material
Identification image of L. fuelleborni Chidunga
Identification image of M. mbenjii
Identification image of M. zebra ‘Chilumba’
Identification image of L. fuelleborni Chidunga
References
- Baerends G.P, Baerends-van Roon J.M. An introduction to the ethology of cichlid fishes. Behav. Suppl. 1950;1:1–242. [Google Scholar]
- Barlow G.W. Perseus Publishing; Cambridge, MA: 2000. The cichlid fishes: nature's grand experiment in evolution. [Google Scholar]
- Dijkstra P.D, Seehausen O, Groothius T.G.G. Direct male–male competition can facilitate invasion of new colour types in Lake Victoria cichlids. Behav. Ecol. Sociobiol. 2005;58:136–143. doi:10.1007/s00265-005-0919-5 [Google Scholar]
- Dijkstra P.D, Seehausen O, Gricar B.L.A, Maan M.E, Groothius T.G.G. Can male–male competition stabilize speciation? A test in Lake Victoria haplochromine cichlid fish. Behav. Ecol. Sociobiol. 2006;59:704–713. doi:10.1007/s00265-005-0100-1 [Google Scholar]
- Genner M.J, Turner G.F, Hawkins S.J. Resource control by territorial male cichlid fish in Lake Malawi. J. Anim. Ecol. 1999;68:522–529. doi:10.1046/j.1365-2656.1999.00301.x [Google Scholar]
- Holzberg S. A field and laboratory study of the ecology of Pseudotropheus zebra (Boulenger), and endemic cichlid of Lake Malawi (Pisces: Cichlidae) Z. Zool. Syst. Evol. Forsch. 1978;16:171–187. [Google Scholar]
- Jordan R, Kellogg K, Juanes F, Stauffer J.R., Jr Evaluation of female mate choice cues in a group of Lake Malawi Mbuna (Cichlidae) Copeia. 2003;2003:181–186. doi:10.1643/0045-8511(2003)003[0181:EOFMCC]2.0.CO;2 [Google Scholar]
- Konings A.F. 3rd edn. Cichlid Press; El Paso, TX: 2001. Malawi cichlids in their natural habitat. [Google Scholar]
- Korzan W.J, Fernald R.D. Territorial male color predicts agonistic behavior of conspecifics in a color polymorphic species. Behav. Ecol. 2006;18:318–323. doi:10.1093/beheco/arl093 [Google Scholar]
- Seehausen O, Schluter D. Male–male competition and nuptial-colour displacement as a diversifying force in Lake Victoria cichlid fishes. Proc. R. Soc. B. 2004;271:1345–1353. doi: 10.1098/rspb.2004.2737. doi:10.1098/rspb.2004.2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G.F. Speciation in Lake Malawi cichlids: a critical review. Adv. Limnol. 1994;44:139–160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification image of L. fuelleborni Chidunga
Identification image of M. mbenjii
Identification image of M. zebra ‘Chilumba’
Identification image of L. fuelleborni Chidunga