Abstract
An individual's sex depends upon its genes (genotypic sex determination or GSD) in birds and mammals, but reptiles are more complex: some species have GSD whereas in others, nest temperatures determine offspring sex (temperature-dependent sex determination). Previous studies suggested that montane scincid lizards (Bassiana duperreyi, Scincidae) possess both of these systems simultaneously: offspring sex is determined by heteromorphic sex chromosomes (XX–XY system) in most natural nests, but sex ratio shifts suggest that temperatures override chromosomal sex in cool nests to generate phenotypically male offspring even from XX eggs. We now provide direct evidence that incubation temperatures can sex-reverse genotypically female offspring, using a DNA sex marker. Application of exogenous hormone to eggs also can sex-reverse offspring (oestradiol application produces XY as well as XX females). In conjunction with recent work on a distantly related lizard taxon, our study challenges the notion of a fundamental dichotomy between genetic and thermally determined sex determination, and hence the validity of current classification schemes for sex-determining systems in reptiles.
Keywords: discordant sex, reptile, sex chromosomes, temperature-dependent sex determination
1. Introduction
The phenotypic traits of any adult organism are the outcomes of a complex interplay between the individual's genetic constitution and the environments that it has experienced. Sex determination is of particular interest from this perspective, because a genetic switch (or in some cases, an environmental trigger) that determines sex early in life results in a profound cascade of changes that can massively modify the size, shape, physiology and behaviour of the adult animal. As a result, the processes by which sex is determined have received extensive study (Bull 1983, 2004; Mittwoch 2000). The literature on amniotic vertebrates suggests a broadly dichotomous view, whereby an individual organism's sex is determined either by its genes (as in the mammalian XX–XY system) or by its incubation environment (as in crocodiles, where nest temperature determines offspring sex: Bull 1983; Deeming 2004; Valenzuela 2004).
Although this classificatory scheme remains the dominant paradigm for vertebrates, recent studies challenge the clear-cut distinction between ‘genotypic’ and ‘environmental’ sex determination. First, some turtles exhibit significant among-clutch variance in threshold temperatures for sex determination (Ewert et al. 2004), such that offspring sex is a product of the interaction between genes and nest temperatures rather than simply one or the other. An even greater challenge comes from reports that some lizards possess heteromorphic sex chromosomes that determine offspring sex if the eggs are incubated at ‘normal’ temperatures, but are overridden by thermal factors if eggs are incubated under extreme conditions. One of these reports (on the agamid Pogona vitticeps) included tests for genotypic sex (Quinn et al. 2007), but the other (on the scincid Bassiana duperreyi: Shine et al. 2002) was based only on shifts in the numbers of phenotypically sexed male versus female offspring. We now provide genetic evidence of environmentally induced sex reversal in the latter species, and show that an individual embryo's genetic constitution (XX or XY) can be overridden, not only by thermal regimes but also by hormone application to the eggshell.
2. Material and methods
Adult female lizards (B. duperreyi, Scincidae) from the Brindabella Range (148°50′ E, 35°21′ S) of southeastern Australia produce a single clutch of 3–11 eggs each year (Shine et al. 1997, 2002). We collected females a week prior to laying, allowed them to oviposit in captivity at the University of Sydney, then incubated their eggs on moist vermiculite (water potential—200 kPa) at a diel cycle of either 16.0±7.5°C (cold nest conditions, n=40 eggs) or 22±7.5°C (hot nest conditions, n=40 eggs) to mimic regimes measured in natural nests at high versus low elevations, respectively (Shine et al. 2002). In another experiment, we applied 17β-oestradiol to eggshells to disrupt the endocrine environment within the egg. Using a split-clutch design, we topically applied 5 μg of 17β-oestradiol in 5 μl of ethanol to 112 eggs less than 12 hours after oviposition; 112 control eggs from the same clutches received only 5 μl of ethanol. All eggs were then incubated separately as above, split between hot and cold incubation treatments.
We assessed the phenotypic sex of offspring by eversion of hemipenes in males (Harlow 1996), verified by histological examination of gonads at 10 weeks of age (n=12 hatchlings). To identify chromosomal sex of 137 hatchlings, we applied PCR-based tests for a Y chromosome sequence of B. duperreyi (A. E. Quinn and R. S. Radder 2006, unpublished data; accession no. EU259191). Genomic DNA was extracted from tail-tip tissue by two methods: (i) proteinase K and SDS digestion, followed by high salt and ethanol precipitation and (ii) proteinase K digestion in a 10% (w/v) suspension of Chelex-100 beads. For each individual, we performed two separate PCRs to identify genotypic sex. The first PCR amplified a 185 bp Y chromosome fragment (males only), and the second PCR amplified a 92 bp fragment (males only), nested within the 185 bp fragment (figure 1). Both PCRs were duplex reactions; a second set of primers in each PCR amplified a 356 bp fragment of the single-copy nuclear gene C-mos, in both males and females, and this served as a positive control for successful amplification (thus avoiding false identification of genotypic females in case of PCR failure). The PCR conditions were optimized to favour amplification of the Y-chromosome fragments over the C-mos fragment, to avoid false identification of genotypic females through amplification of C-mos only in genotypic males. We performed these PCRs on the two separate DNA extractions for each individual, to check consistency of genotypic sex identification. Phenotypic sex of the discordant animals (i.e. XX males, XY females) was reconfirmed at 1 year of age by gonadal histology for five randomly selected samples.
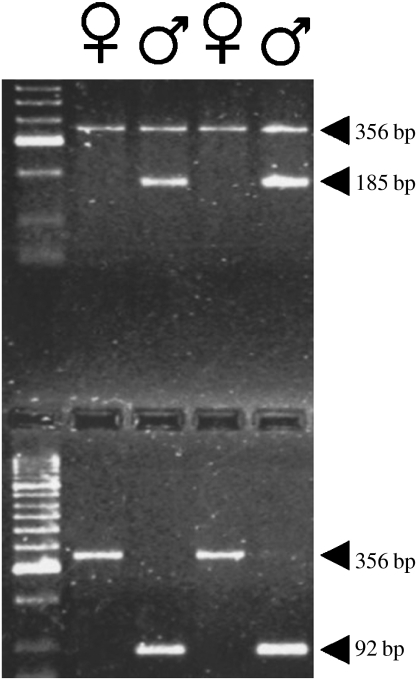
Figure 1.
Agarose gel showing identification of chromosomal sex for two females and two males. Upper half of gel: duplex PCR amplification of 356 bp C-mos fragment (males and females) and 185 bp Y-chromosome fragment (males only) from genomic DNA extracted by high-salt method. Lower half of gel: duplex PCR amplification of 356 bp C-mos fragment (females only) and 92 bp Y-chromosome fragment (males only) from genomic DNA extracted by Chelex method. The Y-chromosome fragment is amplified preferentially over the positive-control C-mos fragment for the Chelex-extracted DNA. Lane 1 shows molecular weight marker.
3. Results
(a) Thermal effects on offspring sex
Hatching success was 90% overall (87.5% of 40 hot-incubated eggs, 92.5% of 40 cold-incubated eggs) and unaffected by treatment (Χ12=0.14, p=0.71). Consistent with the earlier report by Shine et al. (2002), hot incubation produced a balanced sex ratio (54% male, n=19 males, 16 females) whereas cool incubation produced an excess of sons (70% male; 26 males, 11 females, Χ12=5.30, p<0.05). Our genotyping tests unambiguously confirmed sex reversal, with 15% of phenotypic discordant males (XX; 4 out of 26) but no discordant (XY) females. The genotypic sex ratio (22 XY and 15 XX) was not significantly different from parity (Χ12=0.97, p=0.32). No discordant males or females were observed from hot incubation (n=35, lizards screened).
(b) Hormonal effects on offspring sex
Hatching success was not reduced by hormone application to newly laid eggs (79% of 112 controls, 95% of 112 oestradiol-treated eggs). However, sex ratios of the progeny were massively shifted. Control eggs produced 56% males (26/46) from hot incubation and 69% males (29/42) from cold incubation. These control eggs again showed significant excess of males from cold incubation (Χ12=6.76, p<0.01) but not from hot incubation (Χ12=2.7, p=0.10). In contrast, oestradiol-treated eggs produced mostly daughters from both hot (50/52, 97% female) and cold-incubated eggs (52/55, 95% female). Thus, oestradiol application modified sex ratios at both cool (Χ12=40.77, p<0.0001) and hot (Χ12=34.08, p<0.0001) incubation. As expected, approximately half of these phenotypic females were genotypically male (n=11 XY females out of 20 random samples) at both incubation temperatures.
4. Discussion
By implementing a newly developed test for genotypic sex (Y chromosome presence), our study confirms and extends Shine et al.'s (2002) report of multiple sex-determining systems within the scincid lizard B. duperreyi. The previous report relied upon: (i) the occurrence of heteromorphic sex chromosomes in a population of this species from a different site than that used for the incubation studies and (ii) thermally induced shifts in the relative number of sons and daughters, with the classification of offspring sex based on external genitalia at hatching. These aspects raise ambiguity; for example, both karyotypic features and sex-determining systems can vary among conspecific populations (e.g. Conover 1984; Greer 1989; Ezaz et al. 2005). In addition, shifts in offspring sex ratio can be generated by differential mortality rather than disruption of sex-determining systems (Burger & Zappalorti 1988). Given that female B. duperreyi are disadvantaged by cool temperature incubation (Shine et al. 1995, 1997; Elphick & Shine 1998), differential mortality offers a plausible mechanism to explain male-biased offspring sex ratios from cold incubation in this species also. Finally, external morphological traits may not provide unambiguous evidence on hatchling sex (Harlow 1996).
Our study overcomes these problems by demonstrating: (i) sex chromosome heteromorphism in the study population used for the incubation experiments, (ii) congruence between phenotypic sex classification based on hemipenis presence and that based on gonadal histology, (iii) persistence of gonadal phenotypic sex from hatching through to 1 year of age, even in individuals whose genotypic sex was discordant with their phenotypic sex, and (iv) most importantly, showing that incubation temperatures do indeed override genetic factors to determine the phenotypic sex of hatchlings. Our work also demonstrates that offspring sex in B. duperreyi can be modified by yolk hormone levels as well as by incubation temperature, and that the facility to change sex is bidirectional. That is, genotypically male embryos can be transformed (by oestradiol application) into phenotypic females, as well as genotypically female embryos being transformed (by incubation temperature) into phenotypic males.
Many of the correlates of offspring sex ratios reported in vertebrates may reflect indirect effects mediated via maternal physiology (Clutton-Brock 1986; Clutton-Brock & Iason 1986). In contrast, we now have unambiguous evidence that both genes and environmental factors play a causal role in determining offspring sex in B. duperreyi. First, incubation at high temperatures generates a clear match between phenotypic and genotypic sex (XY males and XX females). Second, incubation at low temperatures can override the chromosomal mechanism to produce XX as well as XY males. Finally, offspring sex can be manipulated by adding hormones to the newly laid egg.
The only other reptile known to exhibit a similar sex-determining system is the agamid lizard P. vitticeps (Quinn et al. 2007). This species differs from B. duperreyi not only in habitat type (alpine versus desert) but also in the chromosomal sex-determining system (XY versus ZW), the temperature extreme which overrides genotypic sex (cold versus hot), and which sex of offspring is overproduced under those extreme conditions (sons versus daughters: see Quinn et al. 2007). Clearly, a sex-determining system that incorporates simultaneous genotypic sex determination and temperature-dependent sex determination either is basal to lizard phylogeny (these two lineages probably separated ca 180 Myr ago: Vitt et al. 2003), or has evolved at least twice. Regardless, current paradigms underestimate the complexity of vertebrate sex-determining systems; offspring sex in many squamate reptiles may be the end result of multiple factors operating simultaneously (and interactively) within single populations (as they do in many invertebrates: Bull 1983; Kozielska et al. 2006).
Acknowledgments
All procedures were approved by the University of Sydney Animal Care and Ethics Committee.
The authors would like to thank Melanie Elphick and Jacqui Richardson for their laboratory assistance and the Australian Research Council (FF0561365, DP0345624 and DP0449935) for funding.
References
- Bull J.J. Benjamin/Cummings Publishing Co; Menlo Park, CA: 1983. Evolution of sex determining mechanisms. [Google Scholar]
- Bull J.J. Perspective on sex determination. Past and future. In: Valenzuela N, Lance V.A, editors. Temperature-dependent sex determination in vertebrates. Smithsonian Books; Washington, DC: 2004. pp. 5–8. [Google Scholar]
- Burger J, Zappalorti R.T. Effects of incubation temperatures on sex ratios in pine snakes: differential vulnerability of males and females. Am. Nat. 1988;132:492–505. doi:10.1086/284867 [Google Scholar]
- Clutton-Brock T.H. Sex ratio variation in birds. Ibis. 1986;128:329. doi: 10.1086/415033. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Iason G.R. Sex ratio variation in mammals. Q. Rev. Biol. 1986;61:339–374. doi: 10.1086/415033. doi:10.1086/415033 [DOI] [PubMed] [Google Scholar]
- Conover D.O. Adaptive significance of temperature-dependent sex determination in a fish. Am. Nat. 1984;123:297–313. doi:10.1086/284205 [Google Scholar]
- Deeming D.C. Prevalence of TSD in crocodilians. In: Valenzuela N, Lance V.A, editors. Temperature-dependent sex determination in vertebrates. Smithsonian Books; Washington, DC: 2004. pp. 33–41. [Google Scholar]
- Elphick M.J, Shine R. Longterm effects of incubation temperatures on the morphology and locomotor performance of hatchling lizards (Bassiana duperreyi, Scincidae) Biol. J. Linn. Soc. 1998;63:429–447. doi:10.1006/bijl.1997.0198 [Google Scholar]
- Ewert M.A, Etchberger C.R, Nelson C.E. Turtle sex-determining modes and TSD patterns, and some TSD pattern correlates. In: Valenzuela N, Lance V.A, editors. Temperature-dependent sex determination in vertebrates. Smithsonian Books; Washington, DC: 2004. pp. 21–32. [Google Scholar]
- Ezaz T, Quinn A.E, Miura I, Sarre S.D, Georges A, Graves J.A.M. The dragon lizard Pogona vitticeps has ZZ/ZW micro-sex chromosomes. Chromosome Res. 2005;13:763–776. doi: 10.1007/s10577-005-1010-9. doi:10.1007/s10577-005-1010-9 [DOI] [PubMed] [Google Scholar]
- Greer A.E. Surrey Beatty and Sons; Sydney, Australia: 1989. The biology and evolution of Australian lizards. [Google Scholar]
- Harlow P.S. A harmless technique for sexing hatchling lizards. Herpetol. Rev. 1996;27:71–72. [Google Scholar]
- Kozielska M, Pen I, Beukeboom L.W, Weissing F.J. Sex ratio selection and multi-factorial sex determination in the housefly: a dynamic model. J. Evol. Biol. 2006;19:879–888. doi: 10.1111/j.1420-9101.2005.01040.x. doi:10.1111/j.1420-9101.2005.01040.x [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Three thousand years of questioning sex determination. Cytogenet. Cell Genet. 2000;91:186–191. doi: 10.1159/000056842. doi:10.1159/000056842 [DOI] [PubMed] [Google Scholar]
- Quinn A.E, Georges A, Sarre S.D, Guarino F, Ezaz T, Graves J.A.M. Temperature sex reversal implies sex gene dosage in a reptile. Science. 2007;316:411. doi: 10.1126/science.1135925. doi:10.1126/science.1135925 [DOI] [PubMed] [Google Scholar]
- Shine R, Elphick M.J, Harlow P.S. Sisters like it hot. Nature. 1995;378:451–452. doi:10.1038/378451a0 [Google Scholar]
- Shine R, Elphick M.J, Harlow P.S. The influence of natural incubation environments on the phenotypic traits of hatchling lizards. Ecology. 1997;78:2559–2568. [Google Scholar]
- Shine R, Elphick M, Donnellan S. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecol. Lett. 2002;5:486–489. doi:10.1046/j.1461-0248.2002.00351.x [Google Scholar]
- Valenzuela N. Introduction. In: Valenzuela N, Lance V.A, editors. Temperature-dependent sex determination in vertebrates. Smithsonian Books; Washington, DC: 2004. pp. 1–4. [Google Scholar]
- Vitt L.J, Pianka E.R, Cooper W.E, Jr, Schwenk K. History and the global ecology of squamate reptiles. Am. Nat. 2003;162:44–60. doi: 10.1086/375172. doi:10.1086/375172 [DOI] [PubMed] [Google Scholar]