Abstract
Filial cannibalism (the consumption of one's own offspring) is thought to represent an adaptive strategy in many animals. However, little is known about the details of which offspring are consumed when a parent cannibalizes. Here, we examined patterns of within-brood filial cannibalism in the sand goby (Pomatoschistus minutus). Males spawned sequentially with two females, and we asked whether males cannibalized selectively with regard to egg size or the order in which eggs were received. Males preferentially consumed the larger eggs of the second female they spawned with. Because larger eggs took longer to hatch, and because female 2's eggs were up to 1 day behind those of female 1, such preferential cannibalism might allow males to decrease the time spent caring for the current brood and re-enter the mating pool sooner. More work is needed to understand the fitness consequences of such selective cannibalism.
Keywords: parental care, infanticide, parent–offspring conflict
1. Introduction
It is difficult to imagine how regularly consuming one's own young represents an adaptive strategy, yet filial cannibalism is prevalent in a range of animals (Manica 2002; Klug & Bonsall 2007). Typically, filial cannibalism is viewed as an adaptive trade-off in which energy gained from eggs is used to increase future reproduction or better care for remaining offspring (Manica 2002). Whole-clutch cannibalism represents a termination of care. Any benefits of whole-clutch cannibalism can only be invested in future reproduction, whereas benefits of partial-clutch cannibalism can be used in both current and future reproduction. Because energy is such an obvious benefit of filial cannibalism, much of the work aimed at understanding the adaptive significance of filial cannibalism has focused on energetic benefits (reviewed in Manica 2002). However, some have suggested that energetic benefits alone are unlikely to explain the prevalence of filial cannibalism in natural systems (Smith 1992; Payne et al. 2002; Klug et al. 2006).
The ability to weed out inferior offspring is thought to play a major role in explaining the termination of parental investment in other contexts (e.g. selective abortion in humans and plants: Forbes 1997, Burd 1998; brood reduction: Forbes & Mock 1998), but is rarely considered in relation to filial cannibalism. Previous studies have highlighted the potential importance of selective filial cannibalism (i.e. in relation to the consumption of unfertilized or diseased eggs: Mrowka 1987, Kraak 1996; cannibalism of non-kin after cuckolding: Neff 2003), and recent theoretical work suggests that the ability to cannibalize offspring selectively in relation to offspring phenotype (e.g. expected survivorship, maturation rate) can directly favour the evolution of filial cannibalism (Klug & Bonsall 2007). However, the relationship between offspring phenotype and filial cannibalism remains unknown (but see Salfert & Moodie 1985 regarding preferential consumption of younger eggs). In general, little is known about the details of which eggs are consumed when a parent does decide to cannibalize. Thus, the question remains: if a parent is going to consume their offspring, which offspring should they eat?
To begin to understand the importance of selective filial cannibalism, we evaluated patterns of within-brood cannibalism in the sand goby, Pomatoschistus minutus, a fish in which males alone provide parental care and practise filial cannibalism of eggs. Our primary goal was to determine whether males cannibalize selectively (i.e. exhibit non-random consumption of eggs with regard to some aspect of egg phenotype). Males spawned sequentially with two females, and we asked whether males (i) preferentially consumed eggs of the first or the second female that they spawned with and (ii) exhibited preference with regard to egg size, which has been correlated with post-hatching survival in a range of fishes (Kamler 2005). Additionally, we examined the relationship between egg size and the frequency of whole-clutch cannibalism.
2. Material and methods
Sand gobies were collected near Tvärminne Zoological Station in southern Finland and fed ad libitum frozen Chironomidae larvae daily. Each replicate (n=11) began by placing a male and a female (female 1) in an aquarium equipped with continuous flow-through seawater and a half-flowerpot (8 cm in diameter) as the nesting site. The ceiling of each nest was fitted with a transparent piece of plastic onto which females spawn their eggs. After the male–female pair spawned, the plastic film with eggs was removed, digitally photographed and a subset containing approximately 5% of the eggs (i.e. 20–30 eggs) was cut from the transparency. The subsets of eggs were reared in the absence of the male (described below) to assess any size-specific patterns of egg mortality. We then returned the eggs to the male and placed a second female in the tank. After spawning, we again removed and photographed the eggs, removed a subset of female 2's eggs and returned the eggs to the male. To minimize differences in egg age between the clutches, only cases in which the second female spawned within 24 hours of the first spawning were used. Eggs were followed until just prior to hatching or until all eggs were consumed. Nests were visually inspected daily by shining a light into the nest, and just prior to hatching, (i.e. when eye shine was visible in the embryos), the nest was removed and the eggs were photographed.
The subsets of removed eggs were reared in individual plastic containers equipped with airstones. Water temperature was maintained at approximately 12°C. When eye shine was visible in the majority of eggs, a photograph was taken and egg survivorship was quantified.
For each male's eggs, we superimposed the images immediately following female 1's and female 2's spawning. All eggs were identified as belonging to either female 1 or 2. The image following female 2's spawning (in which all eggs have now been identified) was then superimposed with the final image taken just before hatching, and we determined the specific eggs that had been consumed. Using SigmaScan Pro v. 5.0 (SPSS, Chicago, USA) and the image containing the spawn of both females, we quantified the initial diameter of (i) a random subset of female 1's and female 2's eggs (range 25–75 eggs per female) and (ii) a subset of the specific eggs consumed (range 5–45 eggs per female). These data allowed us to quantify the initial size distributions of (i) all eggs in a nest and (ii) the eggs that were consumed. There was no relationship between position in the nest (i.e. distance from the centre and direction relative to the centre) and egg size (ANOVA, p>0.2 in both cases).
To assess whether males consume eggs in some non-random way with regard to egg size or the order in which eggs are received (i.e. from female 1 versus 2), we used the preference measure α (Manly et al. 1972; Chesson 1983), which accounts for the initial abundance of eggs of varying sizes and the depletion of eggs due to cannibalism. Preference for each egg type (i) was calculated as
where ni0 is the number of eggs of type i present initially; ri is the number of eggs of type i consumed by the male; and m is the total number of different egg types present (modified from Manly et al. 1972 and Chesson 1983). For this estimate, zero indicates no preference (i.e. random consumption of eggs), a positive value indicates a preference for a particular egg type and a negative value suggests that consumption is less than what would be expected from random.
Based on the observed range in egg size, we had sufficient resolution to identify and calculate four size classes (small, small–medium, medium–large, large) for each brood (i.e. each male's eggs). For each nest, we divided the range in egg size (including the eggs of both females) by four to calculate four equal size classes. Thus, for a given brood/nest/male, both females had identically defined size categories (i.e. small size classes for females 1 and 2 were identical within a nest). We then estimated preference for four size classes. Thus, there were a total of eight egg types for each male (two females and four size classes).
The preference data were analysed using a Friedman ANOVA. Linear regressions were used to examine the relationship between mean egg diameter and egg survival, and mean egg diameter and egg development time among the subsets of eggs reared in the absence of males. Logistic regression was used to evaluate the relationship between the within-brood egg diameter (i.e. the mean egg diameter for each nest, including the eggs of both females) and whole-clutch cannibalism.
3. Results
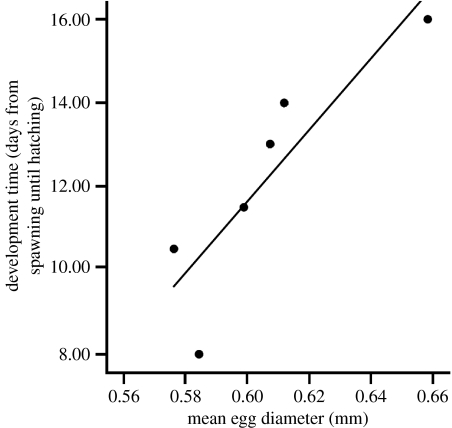
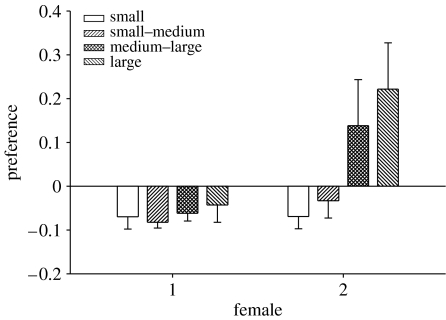
There was no relationship between mean egg diameter and mean egg survivorship among the clutches reared in the absence of males (F1,8=0.14, p=0.72). However, in the absence of males, mean egg size was positively correlated with mean egg development time, suggesting that larger eggs take longer to develop (F1,5=13.85, p=0.02; figure 1). When eggs were with males, four males consumed their entire clutch and seven males exhibited partial-clutch cannibalism. There was no relationship between mean egg diameter and whole-clutch cannibalism (Χ12=0.44, p=0.51). For cases of partial-clutch cannibalism, males consumed 36.2±0.079% of their eggs (i.e. 380.3±65.8 eggs; mean±s.e.). While there was no preference for the eggs of female 1 or the smaller eggs of female 2, males exhibited significant preference for the larger eggs of female 2 (Χ72=15.13, p=0.034; figure 2).
Figure 1.
The relationship between mean initial egg size and mean development time among clutches reared in the absence of males.
Figure 2.
Preference (mean α±s.e.) in egg consumption by parental males that spawned sequentially with two females.
4. Discussion
Male sand gobies preferentially consumed the larger eggs from the second female they spawned with. Larger eggs probably provide a male with more energy (Kamler 2005). However, if males were attempting to maximize their per-offspring energetic gain, we would have expected them to consume larger eggs in all cases. This was not the case. Males exhibited no size-based preference for female 1's eggs in this experiment, and males exhibit no size-based preferences in a single-male/single-female context (Klug 2007). Thus, while important, energetics alone cannot explain the observed patterns of cannibalism.
Preference for the larger eggs of female 2 is potentially associated with decreasing the duration of parental care. Larger eggs took longer to develop and the eggs of female 2 were already slightly behind those of female 1. Thus, the larger eggs of female 2 would probably hatch later and require a longer duration of care than female 1's eggs and the smaller eggs of female 2. Consuming the larger eggs of female 2 potentially allows a male to decrease time spent caring for the current brood, thereby allowing the male to re-enter the mating pool sooner. In this system, nest sites are limited, and simply abandoning the current brood is unlikely to allow a male to re-enter the mating pool quickly. The hypothesis that selective cannibalism reduces the duration of care seems particularly relevant for species like the sand goby, which have multiple brood cycles over a single breeding season. A sand goby male, during a given brood cycle, receives eggs for a few days and then enters a ‘care-only’ phase during which he does not receive additional eggs until the current brood hatches (typically 7–15 days from spawning). It is easy to imagine how even a small reduction (e.g. 1–2 days) in the duration of care over multiple brood cycles might allow a male an additional brood cycle, which in turn might increase the total number of eggs he receives over the breeding season. While more work is needed to quantify the fitness consequences of such selective cannibalism, this hypothesis is consistent with previous theory suggesting that parental fitness is highly sensitive to the maturation rate of eggs (Klug & Bonsall 2007), and also parallels the work on infanticide of non-kin that allows faster sexual access to females (van Schaik & Janson 2000).
It is important to note that the above hypothesis is not mutually exclusive with other hypotheses of filial cannibalism. Indeed, previous work suggests that a range of factors are likely to affect and favour the evolution of filial cannibalism (Manica 2002; Payne et al. 2002; Neff 2003; Klug & Bonsall 2007). For example, both parental condition and density-dependent egg survival affect filial cannibalism in the sand goby (Klug et al. 2006). Additionally, we found no relationship between egg size and whole-clutch cannibalism. Whole-clutch and partial-clutch cannibalism are thought to represent distinct biological phenomena (Manica 2002), and our work further supports this idea.
Acknowledgments
Animal work was approved by the University of Florida IACUC (E504).
We thank C. W. Osenberg for his advice regarding preference estimates, and H. J. Brockmann, R. Kimball, S. Phelps and C. Wynne, and three referees for their feedback. Tvärminne Zoological Station provided logistical support. H.K. was funded by an NSF Graduate Research Fellowship and Doctoral Dissertation grant, and K.L. by the Academy of Finland.
References
- Burd M. “Excess” flower production and selective fruit abortion: a model of potential benefits. Ecology. 1998;79:2123–2132. [Google Scholar]
- Chesson J. The estimation and analysis of preference and its relationship to foraging models. Ecology. 1983;64:1297–1304. doi:10.2307/1937838 [Google Scholar]
- Forbes L.S. The evolutionary biology of spontaneous abortion in humans. Trends Ecol. Evol. 1997;12:446–450. doi: 10.1016/s0169-5347(97)01179-8. doi:10.1016/S0169-5347(97)01179-8 [DOI] [PubMed] [Google Scholar]
- Forbes L.S, Mock D.W. Parental optimism and progeny choice: when is screening for offspring quality affordable? J. Theor. Biol. 1998;192:3–14. doi: 10.1006/jtbi.1997.0596. doi:10.1006/jtbi.1997.0596 [DOI] [PubMed] [Google Scholar]
- Kamler E. Parent–egg–progeny relationships in teleost fishes: an energetics perspective. Rev. Fish Biol. Fish. 2005;15:399–421. doi:10.1007/s11160-006-0002-y [Google Scholar]
- Klug, H. 2007 Evolutionary significance of filial cannibalism in fishes with parental care. PhD thesis, University of Florida, Gainesville, pp. 67–76.
- Klug H, Bonsall M.B. When to care for, abandon, or eat your offspring: the evolution of parental care and filial cannibalism. Am. Nat. 2007;170:886–901. doi: 10.1086/522936. doi:10.1086/522936 [DOI] [PubMed] [Google Scholar]
- Klug H, Lindström K, St Mary C.M. Parents benefit from eating offspring: density-dependent egg survivorship compensates for filial cannibalsim. Evolution. 2006;60:2087–2095. doi: 10.1554/05-283.1. [DOI] [PubMed] [Google Scholar]
- Kraak S.B.M. Female preference and filial cannibalism Aidablennius sphynx (Teleostei, Blenniidae): a combined field and laboratory study. Behav. Process. 1996;36:85–97. doi: 10.1016/0376-6357(95)00019-4. doi:10.1016/0376-6357(95)00019-4 [DOI] [PubMed] [Google Scholar]
- Manica A. Filial cannibalism in teleost fish. Biol. Rev. 2002;77:261–277. doi: 10.1017/s1464793101005905. doi:10.1017/S1464793101005905 [DOI] [PubMed] [Google Scholar]
- Manly B.F.J, Miller P, Cook L.M. Analysis of a selective predation experiment. Am. Nat. 1972;106:719–736. doi:10.1086/282808 [Google Scholar]
- Mrowka W. Filial cannibalism and reproductive success in the maternal mouthbrooding cichlid fish Pseudocrenilabrus multicolor. Behav. Ecol. Sociobiol. 1987;21:257–265. doi:10.1007/BF00292507 [Google Scholar]
- Neff B.D. Paternity and condition affect cannibalistic behavior in nest-tending bluegill sunfish. Behav. Ecol. Sociobiol. 2003;54:377–384. doi:10.1007/s00265-003-0645-9 [Google Scholar]
- Payne A.G, Smith C, Campbell A. Filial cannibalism improves survival and development of beaugregory damselfish embryos. Proc. R. Soc. B. 2002;269:2095–2102. doi: 10.1098/rspb.2002.2144. doi:10.1098/rspb.2002.2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salfert I.G, Moodie G.E.E. Filial egg cannibalism in the brook stickleback, Culae inconstans (Kirtland) Behaviour. 1985;93:82–100. [Google Scholar]
- Smith C. Filial cannibalism as a reproductive strategy in care-giving teleosts. Neth. J. Zool. 1992;42:607–613. [Google Scholar]
- van Schaik C.P, Janson C.H, editors. Infanticide by males and its implications. Cambridge University Press; Cambridge, UK: 2000. [Google Scholar]