Abstract
To test whether male body size affects female reproductive investment in the polygamous crayfish Procambarus clarkii, we described mating behaviour of virgin females paired with either small or large males, and analysed the number, size and weight of both eggs and juveniles sired by either types of male. Along with confirming the overt selection by females of larger mates, we found that the size and weight of both the eggs and the juveniles were higher when sired by larger fathers. This suggests that P. clarkii females exert a form of cryptic choice for large males, seemingly adjusting the quantity of egg deutoplasm in function of the mate body size. The question of why females spend time and energy to brood low-fitness offspring is finally raised.
Keywords: Procambarus clarkii, reproductive investment, egg size, cryptic choice
1. Introduction
In polygamous species, the reproductive interest of the two sexes usually diverge (reviewed in Andersson 1994) leading to a differential resource allocation: males supply sperm in function of the expected reproductive returns and mating opportunities (Dewsbury 1982) and females modulate their primary (egg number/size) and secondary (parental care) reproductive investments according to the mate's attractiveness. Notwithstanding that crayfish are the ideal organisms to investigate this issue, little is known in this taxon except for Austropotamobius italicus. In this species, males produce bigger spermatophores when mating with larger, more fecund females (Rubolini et al. 2006), whereas females lay fewer but larger eggs when their mates are small and large-clawed males (Galeotti et al. 2006).
A recent laboratory study showed that females of the crayfish Procambarus clarkii select large-sized rather than large-clawed mates (Aquiloni & Gherardi 2008a). This suggests that body dimension is taken as an index of male quality in this species. To answer the question of whether females invest more when pairing with large males, we investigate here the effects of male size on P. clarkii's mating behaviour and the female primary reproductive investment.
2. Material and methods
(a) The study species
Procambarus clarkii is an r-selected species with an extended maternal care. It is promiscuous with a relatively long reproductive season (June–October; Scalici & Gherardi 2006). Mates are not limited in the dense populations invading European water bodies (Gherardi 2006). The spermatophores are deposited into a seminal receptacle (annulus ventralis) at the posterior end of the female's seventh thoracic sternite. This structure and its content are lost when females moult at the end of the reproductive season, thus impeding spermatophores to be stored across years. A month after copulation, spawning occurs and the spermatozoa are released by the dissolving spermatophores from the annulus ventralis to fertilize the eggs externally. Juveniles (larval development is direct) hatch approximately 40 days later and remain attached to the female pleopods for three months (Gherardi 2002). Females hold eggs and juveniles secluded in a burrow but the extent of male participation in the construction and defence of the incubation burrow still remains unexplored.
(b) Experimental animals
A total of 200 crayfish was collected with baited traps from Massaciuccoli Lake (Italy) in May 2006, before the start of the reproductive season, which assured that the used females were virgin and males were not sperm depleted. From these, we selected 20 males and 20 females, all sexually responsive and intact. To reset their previous social experience, these were isolated in individual aquaria (25×20×20 cm) for two weeks prior to the observations. Then, pairs were formed by assigning males to one of the two types of pair: males with a cephalothorax length, CL (from the tip of the rostrum to the posterior edge of the carapace) below average (small, S, males; CL: 41.7±0.4 mm) were assigned to S pairs and those with a CL above average (large, L, males; CL: 49.8±0.6 mm) to L pairs (S versus L: t38=0.966, p=0). To eliminate the possible effect of mate size on male behaviour, we used females with the same mean CL (in S pairs: CL, 43.2±0.5; in L pairs: CL, 43.6±0.5; t38=0.470, p=0.672).
(c) Mating behaviour
Observations were conducted in August 2006 from 14.00 to 18.00 hours following random sequences. Mating behaviour was recorded in 20 S and 20 L pairs kept in plastic aquaria (40×25×25 cm) filled with 12 L of still tap water and visually isolated from each other and from possible sources of disturbance. Each aquarium was initially divided into two halves by a removable opaque plastic wall. A male (either S or L) and a female were inserted each into a half of the aquarium and left for 10 min to acclimatize. Each observation, lasting a maximum of an hour, started with the removal of the wall and ended immediately after copulation, if it occurred. The behaviour of each pair was recorded using a digital camera; a number code was given to each videotape for the subsequent blind reading. For each pair, we analysed the following:
Latency until the first mating attempt (MA; in s), i.e. the time elapsed between wall removal and the first MA. MA started when the male tried to turn the female over and ended when the female escaped without ejaculation.
Latency until copulation (CO; in min; as above but until the start of CO). CO is defined as MA except that the female received ejaculation. Ejaculation was denoted by sequential abdominal thrusts by the male.
Number and mean duration (in s) of MA.
Duration (in s) of CO.
Number of approaches to and retreats from the partner (to a distance above 5 cm) by either sex.
For seven S and seven L pairs that failed to copulate during the observation hour, the procedure was repeated for a maximum of 10 days until CO occurred.
(d) Female investment
After copulation, females were inserted into individual incubation aquaria (20×25×25 cm) each containing a shelter. Each female was daily checked to record the time (in days) required for eggs to spawn and for juveniles to hatch, the number of the eggs spawned and aborted, and the number of juveniles hatched. Two days after spawning, 10 eggs from each clutch, randomly selected, were gently detached from the pleopods with forceps. They were wiped with paper towel and weighed three times on an electronic scale. Egg diameter was measured using a micrometric lens mounted on a microscope. The same procedure was followed for the juveniles (10 per brood).
(e) Statistical analyses
The data that met the assumption of normality and homogeneity of variance were compared using two-tailed Student's t-tests (t). Otherwise, we used non-parametric techniques: Mann–Whitney U-test (U), Wilcoxon test (W) and G-tests (G).
3. Results
(a) Mating behaviour
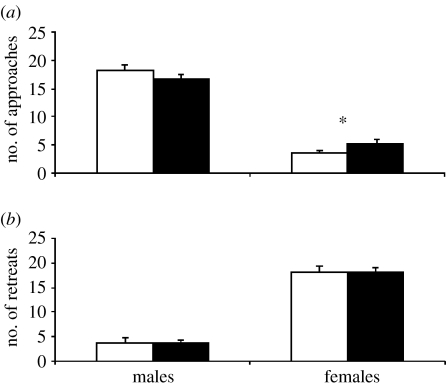
Copulation occurred in 18 S and 18 L pairs (90%). No difference between S and L males was found in: latency until MA; latency until CO; the number of MA; the mean duration of MA; and the duration of CO (table 1). Similarly, both types of male approached (U18=161.5, p=0.295) and retreated from females (U18=195.0, p=0.892) the same number of times (figure 1a). Conversely, females approached more often L rather than S males (U18=112.5, p=0.017; figure 1a), but their retreats were independent of the male size (U18=191.5, p=0.818; figure 1b). Overall males started (W36=3.928, p=0) and females interrupted MA (W36=−5.493, p=0) more often than the other sex.
Table 1.
Behavioural parameters (mean±s.e.) compared between pairs where females were offered a small or a large mate after Mann–Whitney test (U). (MA, mating attempts; CO, copulation.)
| small mates (n=18) | large mates (n=18) | U | p | |
|---|---|---|---|---|
| latency MA (s) | 694±147 | 691±190 | 146.5 | 0.624 |
| latency CO (min) | 83±10 | 100±19 | 157.5 | 0.857 |
| MA (n) | 4±0.74 | 3.3±0.6 | 148.0 | 0.673 |
| duration MA (s) | 94±16 | 95±21 | 143.5 | 0.568 |
| duration CO (s) | 3381±554 | 2334±438 | 109.0 | 0.094 |
Figure 1.
Number (±s.e.) of (a) approaches to and (b) retreats from the partner by the two sexes in pairs with small (n=18; open bars) or large (n=18; filled bars) males. The asterisk in (a) denotes significant differences after Mann–Whitney tests.
(b) Female investment
Out of the 18 that had mated with S (83.3%) and L (77.7%) males, 15 and 14 females, respectively, extruded eggs without any difference between types of mate (G1=0.034, p>0.05). Similarly, no difference was found in the time elapsed until spawning and in the number of the eggs extruded and aborted. Conversely, eggs were significantly heavier and larger when the clutches had been sired by L males (table 2).
Table 2.
Indices of female primary reproductive investment (mean±s.e.) compared between (a) clutches and (b) broods sired by small and large fathers after Mann–Whitney tests (U) and Student's t-tests (t). (Significant values in italics.)
| (a) clutches | small fathers (n=15) | large fathers (n=14) | U/t | p |
|---|---|---|---|---|
| time until spawning (days) | 30±8 | 31±8 | U=97 | 0.726 |
| eggs extruded (n) | 189.9±29.1 | 233.2±36.8 | t=1.046 | 0.350 |
| eggs aborted (n) | 86.5±18.5 | 102.3±21.2 | t=0.566 | 0.576 |
| egg weight (mg) | 3.1±0.1 | 3.5±0.1 | t=2.473 | 0.020 |
| egg diameter (mm) | 3.6±0.1 | 3.8±0.0 | t=2.355 | 0.026 |
| (b) broods | small fathers (n=11) | large fathers (n=12) | U/t | p |
|---|---|---|---|---|
| time until hatching (days) | 42±13 | 41±12 | U=58 | 0.622 |
| juveniles (n) | 141±22.4 | 152.7±30.5 | t=0.299 | 0.768 |
| juvenile weight (mg) | 9.3±0.5 | 10.8±0.5 | t=2.154 | 0.043 |
| juvenile size (mm) | 6.9±0.3 | 7.8±0.2 | t=2.704 | 0.013 |
The number of broods (11 versus 12; G1=0.043, p>0.05), the time until larval hatching and the number of juveniles per brood did not differ between the types of father. However, the juveniles sired by L males were significantly heavier and larger than those of the same age sired by S males (table 2).
4. Discussion
This study confirms previous results (Aquiloni & Gherardi 2008a,b) that P. clarkii females overtly select mates on the basis of their size (they more frequently approach larger males). It also shows that they are apparently able to keep their primary reproductive investment low after having copulated with small males. Such a capability might result from a form of cryptic mate choice made by females who ‘prefer’ to invest more in the brood sired by higher quality males, i.e. the larger ones. However, our results here and the existing knowledge on this species' reproductive biology seem to suggest that females cannot annul the time and energy expended to take care of seemingly low-fitness clutches.
A similar ability to vary the size of the extruded eggs with mate body dimension was previously found by Galeotti et al. (2006) in the crayfish A. italicus: after having mated with small, large-clawed males, females laid fewer but larger eggs than those who had mated with large, small-clawed males. Contrary to A. italicus, however, the preference of P. clarkii females is directed to large males, independently of their claw size (Aquiloni & Gherardi 2008a), and the number of the extruded eggs is kept constant. This might imply that females of this species cannot vary clutch size (which is, however, size dependent; L. Aquiloni 2005, personal observation), but are apparently free to modulate egg size and weight (both of which are size independent; L. Aquiloni 2005, personal observation). After having mated with a low-quality male, they seem to store less deutoplasm in the oocytes before spawning or reabsorb part of it, the deutoplasm thus saved being possibly used in future broods. Evidence of such ability in decapods comes from studies on Homarus americanus, where females reabsorb deutoplasm under unfavourable environmental conditions (Aiken & Waddy 1980). As a consequence, in P. clarkii the eggs sired by large males, richer in deutoplasm, produce heavier and larger juveniles (but this can also be ascribed to the inheritance of ‘large-body’ genes from the father) with higher chances of survival, as suggested for other taxa (Heath & Blouw 1998).
Why should females vary their primary investment with the size of their current mate and favour the larger one? Firstly, by mating with large males, a female can reduce the risk of taking care of unfertilized eggs (Williams 1992) because, as shown in other arthropods (e.g. Andersson 1994), sperm production is often positively correlated with gonad and body sizes. Secondly, large males, which more probably win intrasexual fights (Gherardi 2002), may secure an incubation burrow to the brooding female. Finally, size might be the expression of good genes, and females mating with large males may transmit high quality to their offspring (Hunt et al. 2005). Alternatively, a slight ancestral preference for large males might have led females to select and mate with them; by doing so, females obtain a high fitness due to their ‘sexy sons’ (Weatherhead & Robertson 1979).
Males are not a limited resource in the dense populations of P. clarkii. Thus, particularly at the beginning of the reproductive season, females of this species are expected to abandon (or to cannibalize) the clutch sired by low-quality males, which might allow them to save time and energy for a more advantageous copulation. Conversely, the care they offer to the eggs/juveniles (Gherardi 2002) seems to be independent of their mate's size. Yet, maternal care lasts about four months, which prevents the females, at least in southern Europe, from producing a second clutch in the same year (conversely, reproduction may occur twice per year in the native range; Niquette & D'Abramo 1991).
In essence, by showing a form of mate cryptic choice in P. clarkii, this study has raised the question of why, notwithstanding the ability to recognize male quality and to save deutoplasm when forced to copulate with a small male, females spend time and energy to brood low-fitness offspring. Future studies are expected to solve this issue.
Policy on animal testing. This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research (published on the Animal Behaviour), the legal requirements of Italy, the country in which the work was carried out, and all institutional guidelines.
References
- Aiken, D. E. & Waddy, S. L. 1980 Reproductive biology. In The biology and management of lobsters, vol. 2 (eds J. S. Cobb & B. F. Philips). Ecology and management, ch. 4, pp. 215–276. New York, NY: Academic Press.
- Andersson M.B. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Aquiloni L, Gherardi F. Mutual mate choice in crayfish: large body size is selected by both sexes, virginity by males only. J. Zool. 2008a;274:171–179. doi:10.1111/j.1469-7998.2007.00370.x [Google Scholar]
- Aquiloni L, Gherardi F. Assessing mate size in the red swamp crayfish Procambarus clarkii: effects of visual versus chemical stimuli. Freshwater Biol. 2008b;53:461–469. doi:10.1111/j.1365-2427.2007.01911.x [Google Scholar]
- Dewsbury D.A. Ejaculate cost and male choice. Am. Nat. 1982;119:601–610. doi:10.1086/283938 [Google Scholar]
- Galeotti P, Rubolini D, Fea G, Ghia D, Nardi P.A, Gherardi F, Fasola M. Female freshwater crayfish ad just egg and clutch size in relation to multiple male traits. Proc. R. Soc. B. 2006;273:1105–1110. doi: 10.1098/rspb.2005.3345. doi:10.1098/rspb.2005.3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi F. Behaviour. In: Holdich D.M, editor. Biology of freshwater crayfish. Blackwell Science; Oxford, UK: 2002. pp. 258–290. [Google Scholar]
- Gherardi F. Crayfish invading Europe: the case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2006;39:175–191. doi:10.1080/10236240600869702 [Google Scholar]
- Heath D.D, Blouw D.M. Are maternal effects in fish adaptive or merely physiological side effects? In: Mousseau T.A, Fox C.W, editors. Maternal effects as adaptations. Oxford University Press; New York, NY: 1998. pp. 178–201. [Google Scholar]
- Hunt J, Brooks R, Jennions M.D. Female mate choice as a condition-dependent life-history trait. Am. Nat. 2005;166:79–92. doi: 10.1086/430672. doi:10.1086/430672 [DOI] [PubMed] [Google Scholar]
- Niquette D.J, D'Abramo R. Population dynamics of red swamp crayfish, Procambarus clarkii (Girard, 1852) and white river crayfish, P. acutus acutus (Girard, 1852), cultured in earthen ponds. J. Shellfish Res. 1991;10:179–186. [Google Scholar]
- Rubolini D, Galeotti P, Ferrari G, Spairani M, Bernini F, Fasola M. Sperm allocation in relation to male traits, female size, and copulation behaviour in a freshwater crayfish species. Behav. Ecol. Sociobiol. 2006;60:212–219. doi:10.1007/s00265-005-0158-9 [Google Scholar]
- Scalici M, Gherardi F. Structure and dynamics of an invasive population of the red swamp crayfish (Procambarus clarkii) in a Mediterranean wetland. Hydrobiologia. 2007;583:309–319. doi:10.1007/s10750-007-0615-8 [Google Scholar]
- Weatherhead P.J, Robertson R.J. Offspring quality and the polygyny threshold: “the sexy son hypothesis”. Am. Nat. 1979;113:201–208. doi:10.1086/283379 [Google Scholar]
- Williams G.C. Oxford University Press; Oxford, UK: 1992. Natural selection: domains, levels, and challenges. [Google Scholar]