Abstract
Persistent courtship by male Trinidadian guppies (Poecilia reticulata) is costly for conspecific females. Since male guppies are known to attempt matings with other poeciliid females, we asked whether persistent courtship is also directed towards morphologically similar but phylogenetically distant females encountered following invasion. Skiffia bilineata is one of several endangered viviparous goodeids from Central México, whose remaining habitats are increasingly shared with invasive guppies. Experiments in which guppy sex ratios were manipulated to vary the proportion of heterospecific to conspecific females showed that male guppies courted and attempted forced copulations with S. bilineata females even when females of their own species were in excess. This behaviour places an additional, and previously unrecognized, burden on a group of endemic Mexican fishes already in risk of extinction.
Keywords: sexual harassment, intersexual conflict, invasive species, courtship interference, heterospecific interactions, endangered fishes
1. Introduction
Sexual conflict over mating can result from male–female asymmetries in potential remating rates (Sutherland & de Jong 1991) and may lead to sexual coercion. For instance, in Trinidadian guppies (Poecilia reticulata) where there is more variation in reproductive output among males than females (Becher & Magurran 2004), females are choosy (Houde 1997) and invest heavily in each brood whereas males court females persistently and attempt to forcefully inseminate them by introducing a hooked gonopodium into their cloacae. Persistent courtship leads to loss of feeding opportunities (Magurran & Seghers 1994), increases predation risk (Pocklington & Dill 1995) and may increase energy expenditure. Sexual harassment may be responsible for sexual segregation by causing females to occupy deeper (Croft et al. 2006) and/or faster (Magellan & Magurran 2006) water than males. The intensity of sexual harassment is related to sex ratio, as sneaky mating is more frequent in male-biased populations (e.g. high predation localities; Magurran 1998) where male–male competition is intense (Matthews 1998).
Guppies have been introduced to many tropical and sub-tropical countries, frequently with adverse consequences for native fishes (Man & Hodgkiss 1981; Juliano et al. 1989; Allen 1991). This is usually attributed to competition for habitat and/or food. However, harassment of females by exotic males could place an additional burden on endangered heterospecific populations. Harassment between sister species in seed-eating true bugs (Neacoryphus spp.) leads to a reduction in fecundity of females (McLain & Pratt 1999). Male guppies also harass females of Poecilia picta (Magurran & Ramnarine 2004). Guppies are now found in multiple sites in Mexico, including the last few remaining localities of some endangered goodeids in the Ameca, Balsas, Santiago and Lerma Basins. The Mexican Goodeidae (Goodeinae) are a clade of small viviparous, matrotrophic fishes that resemble poeciliids in their size and habitat use. In the Goodeinae, sperm is shot into the vent during a copulatory embrace, and males lack a gonopodium (Nelson 1975).
Here we quantify the mating behaviour of male guppies towards newly encountered morphologically similar (but phylogenetically distant) goodeine females. Since courtship activity in guppies depends on the availability of conspecific females (Jirotkul 1999), we tested the hypothesis that heterospecific courtship will decrease as the guppy sex ratio becomes more female biased. We used the endangered goodeid Skiffia bilineata (NOM-ECOL-059-1994) from a guppy-free population.
2. Material and methods
Thirty female S. bilineata were collected in November 2005 from a long-known healthy population at Felipe Carrillo, Michoacán, where they co-occur with other goodeids, and with the introduced Poecilia sphenops, Xiphophorus helleri and Heterandria bimaculata. Guppies (P. reticulata) were collected the same month from a spring in Jalisco where they co-occur with the goodeid Zoogoneticus tequila. Although fishes used here had not interacted with the other species, they had encountered heterospecific fishes before capture, thus our test of heterospecific courtship interference is conservative, as a history of interactions may have reduced their responsiveness. A S. bilineata population known to coexist with P. reticulata is presently under threat and we choose not to affect it further; (cf. De la Vega-Salazar et al. 2003). In the laboratory, fishes were kept in 15 visually isolated 40 l home tanks at 24°C with aeration, aquatic moss and filters, treated with protective Stress Coat and kept under a 12 L : 12 D photoperiod. Commercial food flakes were provided twice daily, and the excess were removed after 5 min.
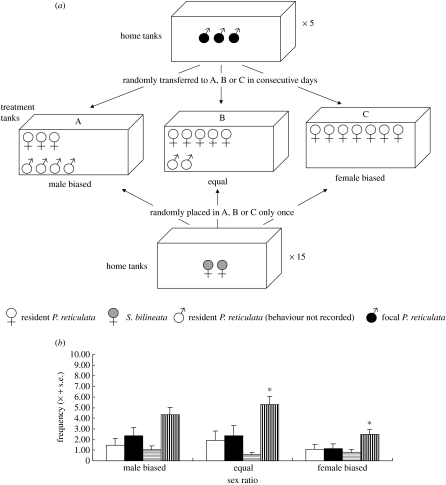
Trios of male guppies (focal males) were placed (and observed) consecutively in treatment tanks (40 l) that contained guppies in the following conditions: male biased (four resident males and three females), equal (two resident males and five females), and female biased (seven females). We quantified attempts by focal males to mate with two S. bilineata females which were introduced together with the focal males into the treatment tanks (figure 1a). Fish density was kept constant, and the design was replicated five times using different focal guppies. We used 15 pairs of S. bilineata females (one pair per trial to avoid possible familiarity; Kelley et al. 1999), and kept them in different home tanks before trials. Thus, neither focal males nor pairs of S. bilineata females were reused.
Figure 1.
(a) The experimental design used in this study; see text for a description. (b) Mean frequency of mating behaviour of focal males towards heterospecific females per 15 min; sigmoid displays (white bars), gonopodial thrusts (black bars), copulation attempts (horizontally stippled bars), ‘following’ (vertically stippled bars), at three sex ratios. (*α<0.05).
Trials (between 10.00 and 16.00) began with the simultaneous introduction of the focal males and the S. bilineata females into a treatment tank. After 1 min, the behaviour of one (haphazardly selected) focal male (identified from sketches) was recorded for 15 min. When focal males had been observed they were returned to their home tank where they remained until the next trial (equal to day). Female S. bilineata were also returned to their home tank. We arbitrarily assigned each group of focal males to a treatment tank and to a time of day for each of three consecutive days. We recorded the frequency of sigmoid displays (Liley 1966) and gonopodial thrusts (in close proximity of a female, the male's gonopodium is swung towards the female's cloaca without necessarily making contact with it), as well as the number of copulation attempts (the male ‘jerked’ backwards while in contact with the female) by focal males over each 15 min period, and the sex and species of the fishes they were directed at. The frequency of ‘following’ of S. bilineata females by focal males and of aggressive interactions (species involved and the direction of the interaction) was also recorded. We measured standard length (SL) and maximum body depth of all females (±0.005 mm) from digital images using Image Tool v. 3.00, and compared the index of maximum body depth (MaxD/SL) of P. reticulata and S. bilineata females. Additionally, we quantified courtship rates of male P. reticulata and S. bilineata (n=30 each) to S. bilineata females over a 5 min period, and simultaneously recorded the frequency of vibration, the typical response of goodeine females to approaching males, involving a costly energetic shaking of the body (Valero et al. 2005).
Non-normal data were transformed (square root) before analysis that was carried out in SPSS using a mixed linear model. To evaluate the effect of sex ratio, we defined treatment as the repeated measure. We nested male within replicate and used treatment as the fixed factor. When comparing body depth, we used species as the fixed factor. When analysing courtship and vibration rates, we defined treatment (male species) as the fixed factor. We report mean±s.e.
3. Results
The frequencies of sigmoid displays and copulation attempts were similar to other reports for P. reticulata in captivity (e.g. Evans & Magurran 1999). Each male directed 5.46±0.84 sigmoid displays and 4.57±0.66 gonopodial thrusts at conspecific females. Aggressive interactions were few (with other males, 0.28±0.13 and with females, 0.28±0.17) and some courtship was targeted at other males (0.06±0.03).
All focal males displayed at, or attempted copulation with, heterospecific females in at least one of the three trials. At least one of the two S. bilineata females received an average of one sigmoid display, gonopodial thrust or copulation attempt by a focal male (table 1). The frequency of these behaviours was unaffected by guppy sex ratio (sigmoid displays: F2,16.3=0.35, p=0.7; gonopodial thrusts: F2,26.7=1.34, p=0.27 and copulation attempts: F2,32.4=0.23, p=0.79; figure 1b), but males performed more ‘following’ at even than at female-biased sex ratios (F2,18.8=4.52, p=0.02; figure 1b). Males also performed more ‘following’ under a male-biased sex ratio (4.33±0.69) but the difference was not significant.
Table 1.
Average number (±s.e.) of sexual behaviours towards female S. bilineata by individual male P. reticulata (n=15) at different guppy sex ratios per 15 min trial.
| male biased | equal | female biased | p | |
|---|---|---|---|---|
| sigmoid | 1.47±0.64 | 1.93±0.87 | 1.07±0.48 | 0.70 |
| gonopodial thrust | 2.33±0.77 | 2.33±0.95 | 1.13±0.49 | 0.27 |
| copulation attempt | 1.00±0.37 | 0.6±0.16 | 0.73±0.33 | 0.79 |
Female S. bilineata had significantly deeper bodies (0.29±0.004) than guppy females (0.26±0.002; F1,61=43.29, p<0.0001). Courtship rates to S. bilineata females by male guppies were significantly higher than by S. bilineata males: display F2,38.89=12.68, p<0.001; copulation attempts F2,40.25=10.51, p<0.001, and courtship by males of both species evoked similar rates of vibration (F2,29.75=0.09, p=0.91).
4. Discussion
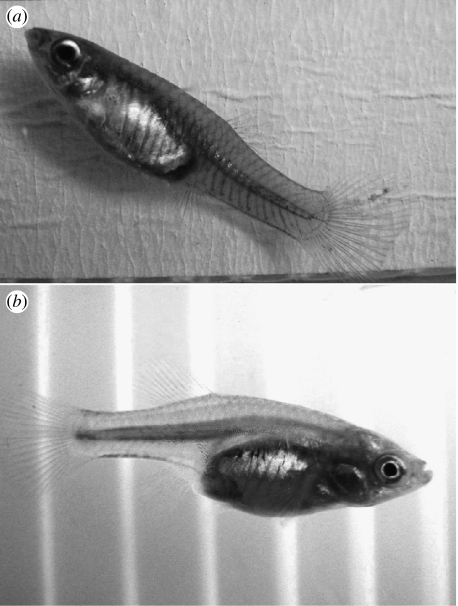
We found that not only male P. reticulata persistently court heterospecific females but also with the exception of ‘following’, this activity is independent of the guppy sex ratio. Other studies have also found no effect of sex ratio on sexual harassment (e.g. Head & Brooks 2006). Here, guppy males devoted 25% of their courtship to heterospecifics. Thus, a typical S. bilineata female received 2.23 sigmoid displays, 2.89 gonopodial thrusts and 1.16 copulation attempts from each male (n=3) over a 15 min trial. Since the species are in different families and cannot hybridize, male P. reticulata are unlikely to gain any benefit from courting/copulating with heterospecific females. They are known to be attracted to large females (Herdman et al. 2004), and we suggest that the attention paid to S. bilineata females may be a non-adaptive consequence of heightened responsiveness to ripe (equal to deep bodied) female guppies, as S. bilineata females have deeper bodies than female guppies and may thus represent a supranormal stimulus to males (figure 2). This may explain why, even in the presence of an excess of guppy females, the males persistently attempt to inseminate S. bilineata females.
Figure 2.
In contrast to (a) female P. reticulata, (b) female S. bilineata have slender caudal peduncles which make them look gravid most of the time.
Persistent courtship by male guppies is likely to impose significant costs on goodeid females, particularly since it occurs at a higher rate than courtship from their own species. For example, goodeid females vibrate vigorously when approached by courting males or aggressive females, which increases oxygen consumption at such times (Valero et al. 2005). Here, S. bilineata females vibrated in response to guppy courtship at the same rate as to conspecific males, thus we expect an increased oxygen consumption in females exposed to heterospecific harassment too. Interspecific courtship may also attract predators (Dill et al. 1999) and reduce feeding rate (Magurran & Seghers 1994). Additionally, the guppy's hooked gonopodium can cause injury to conspecific females during mating (Constantz 1984). It is probable that goodeid females, lacking defences against hooked gonopodia, will suffer cloacal damage during mating attempts.
Guppies have invaded multiple sites in Mexico, including the last few remaining localities of some endangered goodeids (De la Vega-Salazar et al. 2003). Of the approximately 36 species of Mexican goodeids, three are already extinct (or extinct in the wild) with many others threatened. Skiffia bilineata has suffered local extinctions in more than 50% of sites where it was previously present. Small populations, already at risk through stochastic processes, may be most susceptible to the adverse effects of heterospecific courtship.
Acknowledgments
This research adhered to the legal requirements of Mexico and all institutional guidelines.
We thank E. Ávila Luna for helping in collecting and keeping the fishes, which were collected under licence SGPA/DGVS/09253, from SEMARNAT. This project was funded by Leverhulme grant F/00 268/Y.
References
- Allen G.R. Christensen Research Institute; Madang, Papua New Guinea: 1991. Field guide to the freshwater fishes of New Guinea. [Google Scholar]
- Becher S.A, Magurran A.E. Multiple mating and reproductive skew in Trinidadian guppies. Proc. R. Soc. B. 2004;271:1009–1014. doi: 10.1098/rspb.2004.2701. doi:10.1098/rspb.2004.2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantz G.D. Sperm competition in poeciliid fishes. In: Smith R, editor. Sperm competition and the evolution of animal mating systems. Academic Press; Orlando, FL: 1984. pp. 465–485. [Google Scholar]
- Croft D.P, Morrell L.J, Wade A.S, Piyapong C, Ioannou C.C, Dyer J.R.G, Chapman B.B, Wong Y, Krause J. Predation risk as a driving force for sexual segregation: a cross-population comparison. Am. Nat. 2006;167:867–878. doi: 10.1086/504853. doi:10.1086/504853 [DOI] [PubMed] [Google Scholar]
- De la Vega-Salazar M.Y, Avila-Luna E, Macías-Garcia C. Ecological evaluation of local extinction: the case of two genera of endemic Mexican fish. Zoogoneticus and Skiffia. Biodiv. Conserv. 2003;12:2043–2056. doi:10.1023/A:1024155731112 [Google Scholar]
- Dill L.M, Hedrick A.V, Fraser A. Male mating strategies under predation risk: do females call the shots? Behav. Ecol. 1999;10:452–461. doi:10.1093/beheco/10.4.452 [Google Scholar]
- Evans J.P, Magurran A.E. Male mating behaviour and sperm production characteristics under varying sperm competition risk in guppies. Anim. Behav. 1999;58:1001–1006. doi: 10.1006/anbe.1999.1212. doi:10.1006/anbe.1999.1212 [DOI] [PubMed] [Google Scholar]
- Head M.L, Brooks R. Sexual coercion and the opportunity for sexual selection in guppies. Anim. Behav. 2006;71:515–522. doi:10.1016/j.anbehav.2005.04.017 [Google Scholar]
- Herdman E.J.E, Kelly C.D, Godin J.-G.J. Male mate choice in the guppy (Poecilia reticulata): do males prefer larger females as mates? Ethology. 2004;110:97–111. doi:10.1111/j.1439-0310.2003.00960.x [Google Scholar]
- Houde A.E. Princeton University Press; Princeton, NJ: 1997. Sex, color, and mate choice in guppies. [Google Scholar]
- Jirotkul M. Operational sex ratio influences female preference and male–male competition in guppies. Anim. Behav. 1999;58:287–294. doi: 10.1006/anbe.1999.1149. doi:10.1006/anbe.1999.1149 [DOI] [PubMed] [Google Scholar]
- Juliano R.O, Guerrero R, Ronquillo I. The introduction of exotic aquatic species in The Phillipines. In: De Dilva S.S, editor. Exotic aquatic organisms in Asia. Asian Fisheries Society Special Publication 3. Asian Fisheries Society; Manila, The Philippines: 1989. pp. 83–90. [Google Scholar]
- Kelley J.L, Graves J.A, Magurran A.E. Familiarity breeds contempt in guppies. Nature. 1999;401:661–662. doi: 10.1038/44314. doi:10.1038/44314 [DOI] [PubMed] [Google Scholar]
- Liley N.R. Ethological isolating mechanisms in four sympatric species of poeciliid fishes. Behav. Suppl. 1966;13:197. [Google Scholar]
- Magellan K, Magurran A.E. Habitat use mediates the conflict of interest between the sexes. Anim. Behav. 2006;72:75–81. doi:10.1016/j.anbehav.2005.09.022 [Google Scholar]
- Magurran A.E. Population differentiation without speciation. Phil. Trans. R. Soc. B. 1998;353:275–286. doi:10.1098/rstb.1998.0209 [Google Scholar]
- Magurran A.E, Ramnarine I.W. Learned mate recognition and reproductive isolation in guppies. Anim. Behav. 2004;67:1077–1082. doi:10.1016/j.anbehav.2003.10.010 [Google Scholar]
- Magurran A.E, Seghers B.H. A cost of sexual harassment in the guppy, Poecilia reticulata. Proc. R. Soc. B. 1994;258:89–92. doi:10.1098/rspb.1994.0147 [Google Scholar]
- Man S.H, Hodgkiss I.J. Wishing Printing Company; Hong Kong, People's Republic of China: 1981. Hong Kong freshwater fishes. [Google Scholar]
- Matthews, I. M. 1998 Mating behaviour and reproductive biology of the guppy, Poecilia reticulata PhD dissertation, University of St Andrews.
- McLain D.K, Pratt A.E. The cost of sexual coercion and heterospecific sexual harassment on the fecundity of a host-specific, seed-eating insect (Neacoryphus bicrucis) Behav. Ecol. Sociobiol. 1999;46:164–170. doi:10.1007/s002650050606 [Google Scholar]
- Nelson G.C. Anatomy of the male urogenital organs of Goodea atripinnis and Characodon lateralis (Atheriniformes: Cyprinodontoidei), and G. atripinnis courtship. Copeia. 1975;1975:475–482. doi:10.2307/1443645 [Google Scholar]
- Pocklington R, Dill L. Predation on females or males: who pays for bright male traits. Anim. Behav. 1995;49:1122–1124. doi:10.1006/anbe.1995.0141 [Google Scholar]
- Sutherland W.J, de Jong M.C.M. The evolutionarily stable strategy for secondary sexual characters. Behav. Ecol. 1991;2:16–20. doi:10.1093/beheco/2.1.16 [Google Scholar]
- Valero A, Hudson R, Luna E, Macías Garcia C. A cost worth paying: energetically expensive interactions with males protect females from intrasexual aggression. Behav. Ecol. Sociobiol. 2005;59:262–269. doi:10.1007/s00265-005-0033-8 [Google Scholar]