Abstract
Supplementary food given to birds can have contemporary effects by reducing the risk of starvation, increasing survival and altering movements and reproductive performance. There is, however, a widely held perception that birds benefit from extra food over winter, but that it is better that they ‘look after themselves’ during breeding. Here we describe a landscape-scale experiment showing for the first time that the effects of increasing food availability only during the winter can be carried over to the subsequent breeding season. Even though food supplementation stopped six weeks prior to breeding, birds living on sites provisioned over winter had advanced laying dates and increased fledging success compared with birds living on unprovisioned sites. Thus, supplemental feeding of wild birds during winter, in a manner mimicking householders provisioning in gardens and backyards, has the potential to alter bird population dynamics by altering future reproductive performance. With levels of bird feeding by the public continuing to increase, the impacts of this additional food supply on wild bird populations may be considerable.
Keywords: supplementary feeding, foraging, avian reproduction, urbanization
1. Introduction
There is a widespread view that wild birds should be ‘looked after’ in winter; however, as natural food supplies become more readily available in the spring (Newton 1980) human feeding often stops as people feel the birds are able to ‘look after themselves’ (Bromley & Geis 1998). Current estimates suggest that households in the USA and UK together provide in excess of 500 000 tonnes of food for garden birds (O'Leary & Jones 2006). In the UK, sufficient commercial wild bird foods are sold to support over 30 million great tits (Parus major) subsisting entirely on this resource (table 1 in the electronic supplementary material). Given that few individuals rely solely on supplementary food, the numbers of subsidized birds may be even higher. Despite the current level of provisioning, our understanding of the full impacts of such an enormous subsidy is limited.
One way in which food can influence bird populations is via its impact on the timing of breeding (Newton 1998). Previous investigators, who supplemented food supplies immediately prior to and during breeding, have identified advancement in laying dates (Boutin 1990). Two main hypotheses have been proposed to explain this. They are (i) the constraint hypothesis: food availability constrains the female's ability to produce eggs (Perrins 1965) and (ii) the anticipation hypothesis: females use food availability as a cue for the onset of breeding (Lack 1954). The majority of wild bird feeding in temperate regions takes place over winter (Bromley & Geis 1998). Thus, to have an impact on breeding biology, the effects of such feeding would have to be ‘carried over’ into the subsequent breeding season. It is becoming clear that carry-over effects, where past events can influence current fitness, are particularly marked in migratory birds (Marra et al. 1998) but are only rarely reported in resident populations (Wernham & Bryant 1998).
Additional food has been provided well into the breeding period in previous studies of winter food supplementation, making it impossible to distinguish carry-over effects from short-term resource acquisition. Moreover, other studies tend to focus on the period when provisioning is taking place, and are usually conducted at a small scale, using one or two sites, directly feeding birds close to the nest, rather than at a community or landscape scale (Boutin 1990). There is no reason to expect that the directed feeding of individual birds will have the same effects as large-scale diffuse feeding of populations. For example, the diet of the lesser black-backed gull (Larus fuscus) is supplemented through trawler discards, which does not appear to influence clutch size, even though it has been implicated in population increases (Oro 1996). Yet, when provisioned directly at the nest, this same species produces larger clutches (Bolton et al. 1992). Here we report on our results from the first study to examine the potential impacts of supplementation solely during the non-breeding winter period on subsequent breeding success. In addition, it is one of the few provisioning studies using a treatment-control experiment conducted at a landscape scale, more accurately reflecting the diffuse nature of bird feeding by the general public.
2. Material and methods
Ten deciduous woodland sites in County Down, Northern Ireland (figure 1 in the electronic supplementary material) were paired according to a number of ecological and landscape features such as woodland composition and understorey vegetation (for details see the electronic supplementary material) and randomly assigned to fed or unfed treatments. All sites were in isolated woodland blocks in an area dominated by agricultural land. Sites chosen were 12–14 ha in size, dominated by the tree species sycamore (Acer pseudoplatanus), beech (Fagus sylvatica) and oak (Quercus robur) and were at least 3.5 km apart. Wire mesh peanut feeders (1 kg) with effective squirrel guards were positioned hanging from trees 100 m apart, at a density of one per hectare. Supplementary food, peanuts (commonly used by the public to feed birds), were provided ad libitum at fed sites from 1 November 2005 until 8 March 2006, with an average of 66 kg consumed per site. Feeding stopped over six weeks before the first recorded laying date (19 April). Feeder observations and marking of birds caught at feeders during winter (November–February) showed that individuals (including the target species blue tits (Cyanistes caeruleus)) were using the food throughout the winter period and that some individuals were remaining in the area to breed the following spring. Nest-boxes were provided at all sites, at a density of three per hectare and were in position one year prior to starting the experiment. The majority of nest-boxes (85%) were occupied by blue tits and further analyses of breeding parameters are based on this species. Laying date, clutch size and other breeding parameters were determined by a regular inspection of the nest-boxes every 2–3 days from April to June 2006. Individual biometric measurements of all chicks were also recorded. To determine the relative magnitude of treatment effects compared with between site variation and to examine the possibility of an extended carry-over period from feeding in winter 2005–2006 to breeding in 2007 laying date only was determined in 2007.
Variation in the first laying date of each brood was analysed using general linear mixed models (GLMMs) with feeding treatment as a fixed factor and site pair as a random factor. The variation in clutch and brood and chick sizes was analysed by fitting GLMMs, with feeding treatment as a fixed factor, laying date as a covariate and site pair as a random factor. To account for individual variation in chick size within broods, all chicks within a brood were measured and an average for the nest was used. Prior to analysis, fledging success data were ranked and were included as the dependent variable with laying date and feeding treatment in an ordinal logistic regression.
3. Results
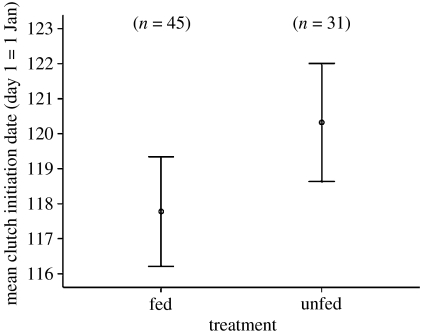
Birds at supplemented sites (2006) laid an average of 2.5 days earlier than birds at control sites (GLMM: F1,70=4.80, p=0.03; figure 1). This compares to the following year (2007) in which no sites received any feeding treatment and where no significant differences were found between the laying dates of previously fed and unfed sites (F1,114=0.09, p=0.763). For full results see tables 2 and 3 in the electronic supplementary material.
Figure 1.
Impact of over-winter provisioning on the laying dates of blue tits. Mean laying date (2006) at fed sites was 2.5 days earlier than at unfed sites. Error bars show 95% CIs. Sample sizes are shown in brackets.
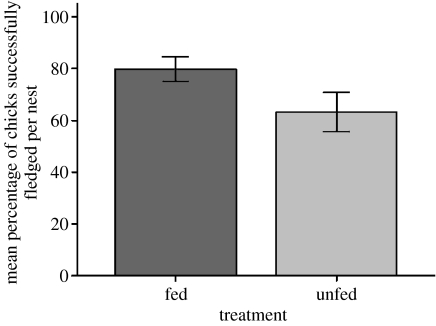
Controlling for the potential effect of an earlier average laying date, feeding also significantly affected the number of chicks that fledged, with almost one extra chick per nest fledging at supplemented sites compared with controls (ordinal logistic regression: feeding treatment Χ2=4.945, p=0.026; figure 2). The effect on fledging success was particularly remarkable since neither clutch size (F1,69=0.001, p=0.976) nor brood size (F1,69=0.026, p=0.872) differed significantly between treatments. There was no significant difference in the body condition of chicks (based on weight/(tarsus)3 ratio) between the two treatments (GLMM: F1,62=0.091, p=0.764).
Figure 2.
The mean percentage of chicks successfully fledged per nest at fed versus unfed sites (2006; based on combined data from the ordinal regression). Standard errors (±1 s.e.) are also given. Owing to the unusual distribution of the data, the difference in fledging success between feeding treatments was tested using ordinal regression (Χ2=4.945, p=0.026), using five ranks based on the percentage of chicks that fledged the nest.
4. Discussion
Our experiment demonstrates two important ways in which the effects of winter feeding can be carried over to influence breeding success. First, over winter feeding allows birds to breed earlier, suggesting that food limitation in the months prior to breeding may be a significant factor regulating the initiation of laying. Second, supplementary feeding enables birds to enhance their productivity by increasing the number of chicks that fledge successfully.
Although blue tits are income breeders, so that they do not rely heavily on endogenous resources for egg formation, there is likely to be a condition threshold that an individual needs to reach before it can begin breeding (Svensson & Nilsson 1995). Since we stopped feeding birds over six weeks prior to the first clutch being initiated, our results indicate that supplementation during winter might enable females to enter the breeding season in better body condition, supporting the ‘constraint hypothesis’ (Perrins 1965). Previous studies have found that birds with access to supplementary food, predominantly peanuts, have higher levels of plasma protein, an indication of improved body condition, and were able to accumulate endogenous resources (Schoech & Bowman 2003).
Benefits of earlier laying have been found in a number of species (Naef-Daenzer et al. 2001). If eggs are laid later, chicks may hatch at a time when natural food availability is declining or may suffer from competition with older conspecifics. Chicks from earlier broods are more likely to be recruited into the breeding population and gain a territory the following year (Arcese & Smith 1985). However, there can be costs associated with breeding too early. Blue tits time their reproduction so that peak food demand for nestlings correlates with peak natural food abundance (Dias & Blondel 1996). If food supplementation causes blue tits to lay early, young may be in the nest before the period of maximum natural food availability. Incubation and brood rearing during periods of lower food availability can cause decreased adult and nestling survival (Nilsson 1994). In this way feeders may act as ecological traps, overriding natural food cues and causing birds to make suboptimal decisions regarding breeding habitat and the timing of breeding.
In our study, differences in fledging success were due to higher rates of chick survival at fed sites compared with that of unfed sites. This is unlikely to be an effect of laying date, as there was no correlation between laying date and fledging success. It could be a consequence of improved parental condition enabling the birds to care better for chicks (Goodburn 1991). This seems probable as brood sizes were comparable across site pairs, hence the number of chicks that parents were provisioning remained constant between treatments.
The carry-over effects of winter feeding could also be driven via the provisioning of important micronutrients that can be stored in the liver. In addition to providing carbohydrate, fat and protein, common feeds such as peanuts and sunflower seeds are excellent sources of other micronutrients (e.g. vitamin E). Birds fed over winter may therefore have benefited from an increased uptake of these specific nutrients and these could be passed on to the chicks via maternal inputs to the eggs. Chicks hatched from eggs with high levels of antioxidants have been shown to have lower mortality rates as antioxidants reduce oxidative stress in the days following hatching (Royle et al. 2001).
It is possible that the improved breeding success in fed woodlands was a consequence of the supplement encouraging higher quality, more competitive birds to settle and breed. This seems improbable as there is currently no evidence that only high-quality birds are attracted to feeders and food was always available ad libitum. In fact all woodlands contained some feeders that were used infrequently, suggesting less competitive birds were not being excluded. Feeders did attract higher numbers of birds and thus our breeders may have included a greater number of higher quality individuals. However, as with the food supply the provision of nest-boxes ensured that nesting sites were not limited. Moreover, higher densities of birds at fed sites would have led to increased competition during the breeding season after supplementary feeding had stopped, making our results conservative.
Thus, there is potential for positive synergistic effects of supplementary feeding, the first being mediated via an increase in over-winter survival (Brittingham & Temple 1998) and the second via an increase in the number of young that are successfully fledged. Considering the high levels of over-winter provisioning and the marked effects on the populations of birds that commonly use feeders (e.g. tits and finches), the impacts on those species that live in close association with human populations may be substantial. In addition, such an extensive over-winter feeding may also influence predictions of the effect of environmental change on the breeding phenology of some species (Both et al. 2006). This may also have wider ecological implications for returning summer migrants that face increased competition from a subsidized resident population of higher population density which is able to breed earlier and produce more offspring (Mönkkönen et al. 2004).
Acknowledgments
We thank Kerry Crawford, Sharon Doake and David Kelly for their assistance with fieldwork. G.R. was funded by a Department for Employment and Learning studentship. Food and nest-boxes were provided by Gardman Ltd.
Supplementary Material
Additional material and methods section; a table with energetic calculation; two tables with results from individual sites and full model outputs of statistical tests
Location of field sites within County Down, Northern Ireland
References
- Arcese P, Smith J.N.M. Phenotypic correlates and ecological consequences of dominance in song sparrows. J. Anim. Ecol. 1985;54:817–830. doi:10.2307/4380 [Google Scholar]
- Bolton M, Houston D, Monaghan P. Nutritional constraints on egg formation in the lesser black-backed gull—an experimental study. J. Anim. Ecol. 1992;61:521–532. doi:10.2307/5607 [Google Scholar]
- Both C, Bouwhuis S, Lessells C.M, Visser M.E. Climate change and population declines in a long distance migratory bird. Nature. 2006;441:81–83. doi: 10.1038/nature04539. doi:10.1038/nature04539 [DOI] [PubMed] [Google Scholar]
- Boutin S. Food supplementation experiments with terrestrial vertebrates—patterns, problems, and the future. Can. J. Zool. 1990;68:203–220. [Google Scholar]
- Brittingham M.C, Temple S.A. Impacts of supplemental feeding on survival rates of black-capped chickadees. Ecology. 1998;69:581–589. doi:10.2307/1941007 [Google Scholar]
- Bromley, P. T. & Geis, A. D. 1998 Feeding wild birds. See http://www.ext.vt.edu/pubs/wildlife/420-006/420-006.html
- Dias P.C, Blondel J. Breeding time, food supply and fitness components of blue tits Parus caeruleus in the Mediterranean region. Ibis. 1996;138:644–649. [Google Scholar]
- Goodburn S.F. Territory quality or bird quality? Factors determining breeding success in the magpie Pica pica. Ibis. 1991;133:85–90. [Google Scholar]
- Lack D. Oxford University Press; London, UK: 1954. The natural regulation of animal numbers. [Google Scholar]
- Marra P.P, Hobson K.A, Holmes R. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science. 1998;282:1884–1886. doi: 10.1126/science.282.5395.1884. doi:10.1126/science.282.5395.1884 [DOI] [PubMed] [Google Scholar]
- Mönkkönen M, Forsman J.T, Thomson R.L. Qualitative geographical variation in interspecific interactions. Ecography. 2004;27:112–118. doi:10.1111/j.0906-7590.2004.03705.x [Google Scholar]
- Naef-Daenzer L, Widmer F, Nuber M. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J. Anim. Ecol. 2001;70:730–738. doi:10.0000/096031097333727 [Google Scholar]
- Newton I. The role of food in limiting bird numbers. Ardea. 1980;68:11–30. [Google Scholar]
- Newton I. Academic Press; London, UK: 1998. Population limitation in birds. [Google Scholar]
- Nilsson J.Å. Energetic bottle-necks during breeding and the reproductive cost of being too early. J. Anim. Ecol. 1994;63:200–208. doi:10.2307/5595 [Google Scholar]
- O'Leary R, Jones D.N. The use of supplementary foods by Australian magpies Gymnorhina tibicen: implications for wildlife feeding in suburban environments. Austral Ecol. 2006;31:208–216. doi:10.1111/j.1442-9993.2006.01583.x [Google Scholar]
- Oro D. Effects of trawler discard availability on egg laying and breeding success in the lesser black-backed gull Larus fuscus in the western Mediterranean. Mar. Ecol. Prog. Ser. 1996;132:43–46. doi:10.3354/meps132043 [Google Scholar]
- Perrins C.M. Population fluctuations and clutch-size in the great tit, Parus major L. J. Anim. Ecol. 1965;34:601–647. doi:10.2307/2453 [Google Scholar]
- Royle N.J, Surai P.F, Hartley I.R. Maternally derived androgens and antioxidants in bird eggs: complementary but opposing effects? Behav. Ecol. 2001;12:381–385. doi:10.1093/beheco/12.4.381 [Google Scholar]
- Schoech S.J, Bowman R. Does differential access to protein influence differences in timing of breeding of Florida scrub-jays (Aphelocoma coerulescens) in suburban and wildland habitats? Auk. 2003;120:1114–1127. doi:10.1642/0004-8038(2003)120[1114:DDATPI]2.0.CO;2 [Google Scholar]
- Svensson E, Nilsson J.Å. Food supply, territory quality and reproductive timing in the blue tit (Parus caeruleus) Ecology. 1995;76:1804–1812. doi:10.2307/1940712 [Google Scholar]
- Wernham C.V, Bryant D.M. An experimental study of reduced parental effort and future reproductive success in the puffin, Fratercula arctica. J. Anim. Ecol. 1998;67:25–40. doi:10.1046/j.1365-2656.1998.00166 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional material and methods section; a table with energetic calculation; two tables with results from individual sites and full model outputs of statistical tests
Location of field sites within County Down, Northern Ireland