Abstract
Understanding life-history evolution requires knowledge about genetic interactions, physiological mechanisms and the nature of selection. For platyfish, Xiphophorus maculatus, extensive information is available about genetic and physiological mechanisms influencing life-history traits. In particular, alleles at the pituitary locus have large and antagonistic effects on age and size at sexual maturation. To examine how predation affects the evolution of these antagonistic traits, I examined pituitary allele evolution in experimental populations differing in predation risk. A smaller size, earlier maturation allele increased in frequency when predators were absent, while a larger size, later maturation allele increased in frequency when predators were present. Thus, predation favours alleles for larger size, at the cost of later maturation and reproduction. These findings are interesting for several reasons. First, predation is often predicted to favour early reproduction at smaller sizes. Second, few studies have shown how selection acts on alleles that affect age and size at sexual maturation. Finally, many studies assume that trade-offs between these life-history traits result from antagonistic pleiotropy, with alleles that positively affect one trait negatively affecting another, yet this is rarely known. This study unequivocally demonstrates that genetically based trade-offs affect life-history evolution in platyfish.
Keywords: maturation, age, size, life history, antagonistic pleiotropy, predation
1. Introduction
Variation in age and size at sexual maturation is common and can have important fitness consequences. Fitness benefits of earlier maturation at smaller sizes include a higher probability of surviving to reproduce and a shorter generation time (Fisher 1930; Cole 1954; Roff 2002). Yet, early reproduction can result in lower initial fecundity and reduced offspring survivorship. Delayed maturation at larger sizes can thus have meaningful fitness benefits. Despite a considerable body of work, few studies have addressed why selection maintains genetic variation for the timing of maturation in vertebrates, and for none of the previous studies has the genetic basis of sexual maturation been well characterized.
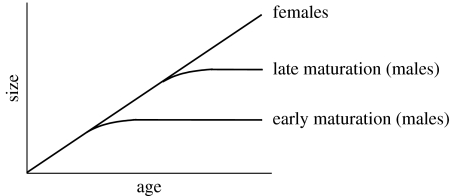
In many systems, indirect evidence indicates that there is likely to be genetic variation in age and size at maturation. An important question in evolutionary biology is: why is such genetic variation maintained, given the profound fitness consequences of maturing earlier versus later? Addressing this question requires information on how selection acts not only on phenotypic traits but also on the genetic, physiological and phenotypic trade-offs that constrain how traits evolve (Stearns 1989; Harshman & Zera 2006). For example, trade-offs between traits like age and size at maturation are often assumed to result from antagonistic pleiotropy, with alleles at a given locus affecting how energy is allocated between different alternatives. The genetic bases of these traits, however, are rarely understood, and even less is known about physiological mechanisms that cause different alleles to produce different life-history phenotypes. One notable exception is the platyfish, Xiphophorus maculatus, for which variation at the polymorphic, sex-linked pituitary locus (P-locus) controls the age at which the gonadotropic zone of the pituitary gland differentiates and becomes physiologically active, triggering gonadal maturation, and thus sexual maturation (Schreibman & Kallman 1977). Nine P-alleles have been identified in platyfish and, depending on the P-genotype, the age at maturation ranges from two months to over a year (Kallman 1989). Individuals with early P-genotypes mature at small sizes while individuals with late P-genotypes mature at larger sizes (figure 1). While age and size of platyfish at sexual maturation can be affected by environmental conditions, P-allele genotypes respond to environmental conditions as distinct classes (McKenzie et al. 1983; Stearns & Koella 1986). The ultimate effect of P-alleles differs for the sexes: females continue growth after maturation, but male post-maturation growth decreases precipitously. P-alleles thus affect lifetime male size. Given that selection often reduces the variation for life-history traits, what maintains the genetic polymorphism at the pleiotropic P-locus?
Figure 1.
Antagonistic pleiotropy results in trade-offs between age and size at sexual maturation in platyfish, with alternate P-alleles affecting life histories differently.
Predation is an important source of selection affecting fitness in many organisms, but its effect depends on how prey size affects an individual's risk of being captured and eaten. If the risk is greater for larger individuals, then selection should favour early maturation and reproduction at smaller sizes, but if the risk is greater for smaller individuals, selection should favour delayed maturation and reproduction at larger sizes (Charlesworth 1994). Studies have suggested that predation often favours smaller size (Reznick et al. 1990; Winemiller et al. 1990; Lafferty 1993), yet other studies suggest that larger size may reduce predation risk (Werner et al. 1983; Basolo & Wagner 2004). Few studies, however, have experimentally addressed how size-related variation in adult predation risk directly affects alleles for age and size at maturation.
Wild platyfish populations differ in the level of risk from piscivorous fishes: some populations co-occur with predatory Cichlasoma spp. while others lack these fishes. I sampled wild populations and found that adult size was greater in populations sympatric with predators than in populations that are not (A. L. Basolo, unpublished data). This indirect evidence suggests that predation could favour P-alleles for later maturation at larger sizes. I tested this hypothesis directly with a laboratory evolution study.
2. Material and methods
P-allele evolution was tested by establishing populations of platyfish with known P-allele genotypes. Genetically linked pigmentary alleles allowed the phenotypic identification of P-genotypes (table 1). Pigmentary alleles do not appear to affect endocrine events (Kallman & Schreibman 1973), and thus do not influence the effects of P-alleles on age and size at maturation. (Similar lines with linked pigmentary alleles were previously used to track allelic change during sex ratio evolution; Basolo 1994.)
Table 1.
Platyfish P-genotypes.
| male | female | ||
|---|---|---|---|
| XP1YP2 | XP1YP6 | XP1XP1 | |
| age at sexual maturation | earlier | later | earlier |
| size at sexual maturation | smaller | larger | smaller |
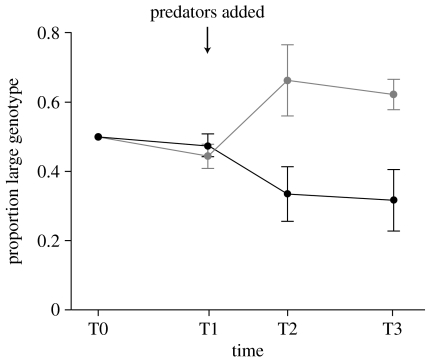
Genetic lines of the following P-genotypes were bred and divided (section C in the electronic supplementary material) across eight tanks: (i) early maturation, smaller sized female (P1P1); (ii) early maturation (11 weeks, on average), smaller sized male (P1P2); and (iii) late maturation (16 weeks, on average), larger sized male (P1P6). An equilibration period allowed the establishment of population age structure (figure 2: T0–T1). After 38 weeks, the P-allele frequencies in each population were quantified at T1 using the pigmentary alleles, and then a cichlid, Cichlasoma octofasciatum, originating from a platyfish field location, was randomly placed in four populations, producing four predator-present and four predator-absent replicates. The genotype frequencies of mature males were quantified twice over the subsequent 33 weeks (T2, 15 weeks; T3, 18 weeks). In addition, size was measured at T2. To control for density effects, after each scoring, the platyfish in the predator-present replicates were returned to a tank, but the platyfish in predator-absent replicates were reduced to the mean number in the predator-present replicates, retaining the replicate-specific P-genotype frequencies. (Also, refer to electronic supplementary material.)
Figure 2.
Evolutionary change in the frequency of the large male genotype (±s.e.) in predator-present (grey line) and predator-absent (black line) populations. T0, population establishment; T1, genotype frequencies quantified, followed by the introduction of predators to four populations. Changes tracked from T1–T3 (T1, n=361; T2, n=271; T3, n=506).
3. Results
The frequency of the later maturation, larger size P1P6-genotype was significantly greater in predator-present when compared with predator-absent replicates at T2 and T3 (T2, t6=2.47, p=0.048; T3, t6=2.73, p=0.034; figure 2). In addition, at T2, males (predator-present, male mass=1.18 mg±0.062 s.e.; predator-absent, male mass=0.81 mg±0.11 s.e.; t6=2.86, p=0.029) and females (predator-present, female mass =1.81 mg ±0.03 s.e.; predator-absent, female mass =1.60 mg ±0.06 s.e.; t6=3.16, p=0.020) were significantly heavier in the predator-present than in the predator-absent replicates. These results are consistent with the hypothesis that predation favours P-alleles for larger size and later maturation, and that this source of selection is sufficiently strong to counter the generation advantage of earlier maturation.
Within the predator-present replicates, the P1P6-genotype increased in frequency, with a concomitant decrease in the P1P2-genotype frequency (T1 versus T3: paired t-test, t3=6.32, p<0.008; figure 2). Although the P1P6-genotype frequency decreased in the predator-absent replicates, with a concomitant increase in the P1P2-genotype frequency, this change was not significant (T1 versus T3: paired t-test, t3=1.57, p=0.216), suggesting that additional sources of selection could be operating to maintain the larger, later maturation P1P6-genotype.
4. Discussion
This study unequivocally demonstrates that, despite the generally accepted advantage of faster generation time, predation in platyfish favours later maturation and larger size alleles. The P-alleles thus have antagonistic pleiotropic effects (Williams 1957) resulting in a trade-off between age and size at sexual maturation. This study also suggests that body size differences in wild platyfish populations (A. L. Basolo, unpublished data), and in the congener Xiphophorus helleri (Basolo & Wagner 2004), are at least partly due to the differences in predation risk. Finally, the combined evidence supports the conclusion that opposing sources of selection, including generation time and predation, act on variation at the P-locus and probably contribute to the maintenance of the P-locus polymorphism.
A primary explanation for larger size in platyfish populations with predators is that there are structural constraints such that once prey reach a critical size beyond the gape width of predators (Werner et al. 1983), platyfish are released from a high predation risk. Thus, by achieving larger sizes that make capture or consumption difficult or costly, predation risk is lowered. While this study demonstrates that natural selection via predation can favour alleles for larger size, this is probably not the whole story. Field studies with many systems indicate that multiple sources of selection simultaneously affect size evolution (Houde 1997; Quinn & Kinnison 1999; Hamon & Foote 2005). For example, in many vertebrates, including Xiphophorus with P-alleles (Basolo 1998; A. L. Basolo & M. A. Nootz, unpublished data), intra- and intersexual selection favour larger size; thus, sexual selection also probably plays a role in P-allele maintenance. Because changes in P-allele frequencies can be detected over relatively short periods, platyfish could prove useful for testing additional factors that might affect the evolution of age and size at maturation.
Despite numerous studies, there is no general rule governing how predation affects age and size at maturation. Even when the effects of closely related predators on closely related prey are investigated, opposite results are found. In the cichlid–guppy predator–prey systems (Crenicichla alta–Poecilia reticulata; Crenicichla saxatilis–P. reticulata), for example, predation favours smaller prey size (Reznick et al. 1990; Winemiller et al. 1990), while in the cichlid–platyfish (this study) and cichlid–swordtail (Basolo & Wagner 2004) systems, predation favours larger size. This divergence between related taxa in the evolutionary response to similar sources of selection is enigmatic; comparative studies could prove useful for understanding factors that allow some species to escape predation by growing larger, and prevent other species from doing the same.
In many systems, there is variation in life-history traits, yet the proximate basis is poorly understood. For platyfish, more than 70 years of research has yielded a body of knowledge about phenotypic effects of many life-history and coloration loci. As a result, both genetic variation at the P-locus and physiological consequences of P-alleles have been characterized (Bao & Kallman 1982). For platyfish, the opposing effects of a generation time advantage and a reduced predation-risk advantage probably contribute to maintaining the P-locus polymorphism. There is thus a trade-off between age and size at maturation resulting from antagonistic pleiotropy. As this study demonstrates, systems like the southern platyfish can provide a deeper understanding of the evolution of traits that are thought to be well understood. In addition, experiments using such systems with nearly Mendelian life-history traits are particularly promising because they allow the rapid detection of responses to selection, and thus could potentially improve our knowledge of how multiple factors interact to influence life-history evolution. Finally, these results may have implications for applied fields, such as fisheries.
Acknowledgments
Fish were collected and returned to the laboratory in accordance with procedures approved by the Ecosystems Management Unit, Belize Fisheries Department.
I thank W. Wagner and K. Kallman for their field assistance, permit agencies in Belize and J. Endler, E. Hebets, K. Fowler, K. Kallman, J. Vavra, W. Wagner and T. Zera for their insightful input. UNL–IACUC approved protocols: 00-10-051 and 04-04-023. Research funding: NSF-IBN-01112656.
Supplementary Material
Tank conditions and maintenance of X. maculatus for laboratory evolution study; experimental X. maculatus P-genotypes for laboratory evolution study; replicate experimental populations for the laboratory evolution study
References
- Bao I.Y, Kallman K.D. Genetic control of the hypothalamic–pituitary axis and the effect of hybridization on sexual maturation. J. Exp. Zool. 1982;220:297–309. doi:10.1002/jez.1402200305 [Google Scholar]
- Basolo A.L. The dynamics of Fisherian sex ratio evolution: theoretical and experimental investigations. Am. Nat. 1994;144:471–487. doi:10.1086/285687 [Google Scholar]
- Basolo A.L. Shift of investment in sexually selected traits: tarnishing of the silver spoon. Anim. Behav. 1998;55:665–671. doi: 10.1006/anbe.1997.0634. doi:10.1006/anbe.1997.0634 [DOI] [PubMed] [Google Scholar]
- Basolo A.L, Wagner W.E. Covariation between predation risk, body size and fin elaboration in the green swordtail. Biol. J. Linn. Soc. 2004;83:87–100. doi:10.1111/j.1095-8312.2004.00369.x [Google Scholar]
- Charlesworth B. Cambridge University Press; Cambridge, UK: 1994. Evolution in age-structured populations. [Google Scholar]
- Cole L.C. The population consequences of life history phenomena. Q. Rev. Biol. 1954;29:103–137. doi: 10.1086/400074. doi:10.1086/400074 [DOI] [PubMed] [Google Scholar]
- Fisher R.A. Clarendon; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Hamon T.R, Foote C.J. Concurrent natural and sexual selection in wild male sockeye salmon, Oncorhynchus nerka. Evolution. 2005;59:1104–1118. [PubMed] [Google Scholar]
- Harshman L.G, Zera A.J. The cost of reproduction: the devil in the details. Trends Ecol. Evol. 2006;22:80–86. doi: 10.1016/j.tree.2006.10.008. doi:10.1016/j.tree.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Houde A.E. Princeton University Press; Princeton, NJ: 1997. Sex, color and mate choice in guppies. [Google Scholar]
- Kallman K.D. Genetic control of size at maturity in Xiphophorus. In: Meffe G.K, Snelson F.F, editors. Ecology and evolution of livebearing fishes. Prentice Hall; Upper Saddle River, NJ: 1989. pp. 163–184. [Google Scholar]
- Kallman K.D, Schreibman M.P. A sex-linked gene controlling gonadotrop differentiation and its significance in determining the age of sexual maturation and size of the platyfish, Xiphophorus maculatus. Gen. Comp. Endocrin. 1973;21:287–304. doi: 10.1016/0016-6480(73)90061-0. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D. The marine snail, Cerithidea californica, matures at smaller sizes where parasitism is high. Oikos. 1993;68:3–11. doi:10.2307/3545303 [Google Scholar]
- McKenzie Q.D, Jr, Crews D, Kallman K.D, Policansky D, Sohn J.J. Age, weight and the genetics of the platyfish, X. maculatus. Copeia. 1983;1983:770–774. doi:10.2307/1444344 [Google Scholar]
- Quinn T.P, Kinnison M.T. Size-selective and sex-selective predation by brown bears on sockeye salmon. Oecologia. 1999;121:273–282. doi: 10.1007/s004420050929. doi:10.1007/s004420050929 [DOI] [PubMed] [Google Scholar]
- Reznick D.A, Bryga H, Endler J.A. Experimentally induced life-history evolution in a natural population. Nature. 1990;346:357–359. doi:10.1038/346357a0 [Google Scholar]
- Roff A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Schreibman M.P, Kallman K.D. The genetic control of the pituitary–gonadal axis in the platyfish, Xiphophorus maculatus. J. Exp. Zool. 1977;200:277–294. doi: 10.1002/jez.1402000209. doi:10.1002/jez.1402000209 [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Trade-offs in life history evolution. Funct. Ecol. 1989;3:259–268. doi:10.2307/2389364 [Google Scholar]
- Stearns S.C, Koella J.C. The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution. 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. doi:10.2307/2408752 [DOI] [PubMed] [Google Scholar]
- Werner E.E, Gilliam J.F, Hall D.J, Mittelbach G.G. An experimental test of the effects of predation risk on habitat use in fish. Ecology. 1983;64:1540–1548. doi:10.2307/1937508 [Google Scholar]
- Williams G.C. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. doi:10.2307/2406060 [Google Scholar]
- Winemiller K.O, Leslie M.A, Roche R. Phenotypic variation in male guppies from natural inland populations: corroboration of Haskin's sexual selection/predation hypothesis. Environ. Biol. Fishes. 1990;29:179–191. doi:10.1007/BF00002218 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tank conditions and maintenance of X. maculatus for laboratory evolution study; experimental X. maculatus P-genotypes for laboratory evolution study; replicate experimental populations for the laboratory evolution study