Abstract
The strength of selection to increase the span of a life stage is dependent upon individuals at that stage being able to contribute towards individual fitness and the probability of their surviving to that stage. Complete reproductive cessation and a long post-reproductive female lifespan as found in humans are also found in killer whale (Orcinus orca) and short-finned pilot whale (Globicephala macrorhynchus), but not in the long-finned pilot whale (Globicephala melaena). Each species forms kin-based, stable matrilineal groups and exhibits kin-directed behaviours that could increase inclusive fitness. Here, the initial mortality rate and mortality rate-doubling time of females of these three closely related whale species are compared. The initial mortality rate shows little variation among pilot whale species; however mortality rate accelerates almost twice as fast in the long-finned pilot whale as it does in killer whale and short-finned pilot whale. Selection for a long post-reproductive female lifespan in matrilineal whales may therefore be determined by the proportion of females surviving past the point of reproductive cessation.
Keywords: senescence, whale, menopause, life history
1. Introduction
Life history traits are shaped by natural selection to maximize individual fitness through the resolution of trade-offs between somatic maintenance and reproductive output (e.g. Hendry et al. 2004; Reznick et al. 2006). In species with maternally-dependent offspring, females can have a post-reproductive lifespan equivalent to the length of the time taken to raise the last born offspring to independence (Packer et al. 1998). However a long post-reproductive female lifespan such as the human menopause extends beyond independence from maternal care of all offspring and is hypothesized to allow multigenerational transfers of fitness to kin through provisioning and care of grandchildren by menopausal females (Hawkes et al. 1998; Lee 2003). The most compelling evidence in support of this hypothesis comes from demographic studies in humans showing that the presence of menopausal females were associated with an increase in their daughter's reproductive success and grandchildren's survival (Sear et al. 2000; Lahdenperä et al. 2004; Shanley et al. 2007). However a number of species that have extended social networks and exhibit kin-directed behaviour that would permit multigenerational transfers have little or no post-reproductive female lifespan (Packer et al. 1998). Therefore, it appears that the potential for a female to increase inclusive fitness is not the sole requirement for the selection of a long post-reproductive lifespan.
Classical life history theories predict that the age-dependent selection gradient for somatic repair and against the accumulation of deleterious mutations and antagonistic pleiotropy will depend upon the rate of acceleration of extrinsic mortality (Hamilton 1966; Kirkwood 1977; Charlesworth 1994). Empirical support for these hypotheses comes from studies showing guppies suffering lower predation rates to be exhibiting delayed somatic senescence and having an increased lifespan (Reznick et al. 1997, 2006). However, the selection for somatic maintenance should be confined to life stages that contribute towards individual fitness (Reznick et al. 2006).
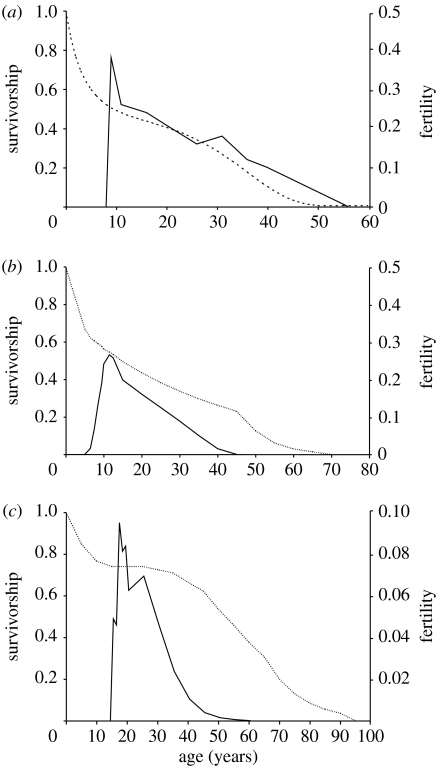
A long post-reproductive female lifespan has been reported in two closely related whale species: killer whale, Orcinus orca (Olesiuk et al. 1990) and short-finned pilot whale, Globicephala macrorhynchus (Kasuya & Marsh 1984; Marsh & Kasuya 1984). Killer whale and short-finned pilot whale females undergo reproductive senescence on a trajectory independent of somatic aging and cease reproducing at just over halfway through their maximum lifespan of 90 and 65 years, respectively (Kasuya & Marsh 1984; Marsh & Kasuya 1984; Olesiuk et al. 1990; figure 1). No female older than 48 years had given birth during multi-decadal field studies on wild populations of killer whales (Olesiuk et al. 1990) and no female over 36 years was pregnant in corpses inspected from a Japanese drive fishery for short-finned pilot whales (Kasuya & Marsh 1984; Marsh & Kasuya 1984). Long-finned pilot whale, Globicephala melaena, females have a maximum lifespan of 59 years (Bloch et al. 1993; figure 1) and females as old as 55 years have been found to be pregnant (Martin & Rothery 1993). Although female long-finned pilot whales undergo reproductive senescence in that reproductive output declines on a trajectory in line with somatic ageing, less than 4% of mature females inspected had ceased to ovulate (Martin & Rothery 1993), compared with 25% of mature female short-finned pilot whales (Kasuya & Marsh 1984; Marsh & Kasuya 1984). All the three species live in highly stable, multigenerational matrilineal social groups (Bigg et al. 1990; Amos et al. 1993; Heimlich-Boran 1993) and exhibit kin-directed behaviours such as food provisioning (Hoelzel 1991; Marsh & Kasuya 1991; Ford & Ellis 2006). The difference in life history suggests that multigenerational transfers are not the sole determinant of the extent of a post-reproductive lifespan. Here I review the published life history data on these species and compare the acceleration of mortality rate (figure 2).
Figure 1.
Age-dependent survivorship and fertility of (a) the long-finned pilot whale; the dotted line indicates the proportion of females surviving (Bloch et al. 1993) and the solid line indicates the proportion of females that were pregnant (Martin & Rothery 1993); (b) the short-finned pilot whale; the dotted line indicates the proportion of females surviving and the solid line indicates the proportion of females that were pregnant (Kasuya & Marsh 1984); (c) killer whale; the dotted line indicates the proportion of females surviving and the solid line indicates the number of viable calves produced per female (Olesiuk et al. 1990).
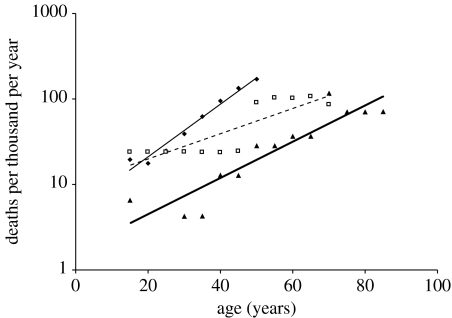
Figure 2.
Female mortality rates as a function of the age of three matrilineal whale species, killer whale (triangles), long-finned pilot whale (diamonds) and short-finned pilot whale (squares), based on life tables from Kasuya & Marsh (1984), Olesiuk et al. (1990) and Bloch et al. (1993).
2. Material and methods
Data were taken from published life tables (Kasuya & Marsh 1984; Marsh & Kasuya 1984; Olesiuk et al. 1990; Bloch et al. 1993). The killer whale demographic data were collected between 1973 and 1987 from two annually censused killer whale populations in the north-eastern Pacific: the northern residents (n=140–171) and southern residents (n=71–84), which used natural markings to identify individuals (see Bigg et al. 1990; Olesiuk et al. 1990). Age estimates (see Olesiuk et al. (1990) for methodology) are considered accurate to be within ±0.5 years for offspring born within the study period; ±3 years for females that were juveniles at the start of the study; ±8 years for females that were adults at the start of the study; and ±10 years for females that were post-reproductive at the start of the study (Bigg et al. 1990; Olesiuk et al. 1990). The long-finned and short-finned pilot whale data were obtained by physiological inspection of lethal drive fishery catch in the Faeroes (n=1482 females; Bloch et al. 1993; Martin & Rothery 1993) and Japan (n=373 females; Kasuya & Marsh 1984; Marsh & Kasuya 1984), respectively. Age was determined by counting dentinal and cemental growth layers of teeth (Kasuya & Marsh 1984; Marsh & Kasuya 1984; Bloch et al. 1993; Martin & Rothery 1993). These drive fisheries are thought to result in the complete capture of entire family groups (Amos et al. 1993) and there should therefore be no bias in the age distribution or reproductive status of sampled individuals.
Using the Gompertz function (Gompertz 1825), m(t)=Aeαt, where m is the mortality rate at age t and α is the rate constant for age-related increases of mortality, I calculated the initial mortality rate (IMR) with the start of adult life approximated to be age 15 years and the subsequent mortality rate-doubling time (MRDT=ln 2/α).
3. Results and discussion
The data are consistent with life-history theory in that the species with a higher proportion of individuals surviving to old age (e.g. lower MRDT) have a longer maximum lifespan. In killer whale (MRDT=14.20 years) and short-finned pilot whale females (MRDT=20.21 years), the rate of aging is approximately half that of female long-finned pilot whales (MRDT=9.76 years). Selection for somatic repair and against deleterious mutation accumulation or pleiotropic genes with deleterious late-life effects are therefore expected to be stronger until later in life in killer whales and short-finned pilot whales than in long-finned pilot whales (Hamilton 1966; Kirkwood 1977). Changes to life history traits can occur rapidly (<15 generations) and vary intra-specifically in response to changes in the level of age-dependent extrinsic mortality (Reznick et al. 1997). Variation in mortality rate acceleration could therefore have led to the differences in life histories between the short- and long-finned pilot whales in the time since they diverged.
Selection for the extension of lifespan should be limited only to life-stages that make a direct contribution to fitness (e.g. Hendry et al. 2004; Reznick et al. 2006). The benefits from a living post-reproductive female in stable social groups could directly impact the fitness of multi-generations through interactions such as cooperative foraging and food provisioning (e.g. Marsh & Kasuya 1984; Hoelzel 1991; Ford & Ellis 2006). A post-reproductive lifespan may also occur in other whale species with stable matrilineal social groups such as false killer whale (Pseudorca crassidens) or sperm whale (Physeter macrocephalus; Marsh & Kasuya 1986; McAuliffe & Whitehead 2005). This life history trait has so far not been found in odontocete species that live in fluid social groups that offer less opportunity for multi-generational transfers (Marsh & Kasuya 1986; Chivers & Danil 2007).
The IMR was similar for the long-finned pilot whale (0.015) and short-finned pilot whale (0.017) and may reflect the level of intrinsic mortality, while the MRDT may reflect variation between the two species in susceptibility to age-dependent sources of extrinsic mortality such as disease, starvation or predation. However the cause of the observed differences in mortality rate acceleration between the two pilot whale species is unknown. Both the studied populations are subject to exploitation by drive fisheries, and the level of exploitation by the Faroese long-finned pilot whale fishery is historically higher than that by the Japanese short-finned pilot whale fishery (Bloch et al. 1993). However, drive fisheries result in the removal of entire family groups (Amos et al. 1993) and remove all age classes equally; therefore the level of exploitation should not change the age distribution or the age-dependent selection gradient.
The selection of a long post-reproductive female lifespan such as that found in natural populations of humans, killer whales and short-finned pilot whales appears to be dependent upon a combination of factors. Firstly, a post-reproductive female's potential to contribute towards individual fitness. Secondly, the probability that a high proportion of females survive until this late life stage. The latter appears to be the principal determinant, possibly explaining why a long post-reproductive lifespan is found in so few species in the wild.
Acknowledgments
A.D.F. is supported by a Sixth Century Scholarship from the University of Aberdeen and would like to thank Claire Embling and Jane Reid for their comments on an earlier draft of this manuscript.
References
- Amos B, Schlötterer C, Tautz D. Social structure of pilot whales revealed by analytical DNA profiling. Science. 1993;260:670–672. doi: 10.1126/science.8480176. doi:10.1126/science.8480176 [DOI] [PubMed] [Google Scholar]
- Bigg M.A, Olesiuk P.F, Ellis G.M, Ford J.K.B, Balcomb K.C., III Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State. Rep. Int. Whaling Comm. 1990;12:383–405. Special Issue. [Google Scholar]
- Bloch D, Lockyer C.H, Zachariassen M. Age and growth parameters of the long-finned pilot whale off the Faroe Islands. Rep. Int. Whaling Comm. 1993;14:163–207. Special Issue. [Google Scholar]
- Charlesworth B. 2nd edn. Cambridge, UK; Cambridge University Press: 1994. Evolution in age-structured populations. [Google Scholar]
- Chivers S.J, Danil K. Growth and reproduction of female short-beaked common dolphins, Delphinus delphis, in the eastern tropical Pacific. Can. J. Zool. 2007;85:108–121. doi:10.1139/Z07-059 [Google Scholar]
- Ford J.K.B, Ellis G.M. Selective foraging by fish-eating killer whales Orcinus orca in British Columbia. Mar. Ecol. Prog. Ser. 2006;316:185–199. doi:10.3354/meps316185 [Google Scholar]
- Gompertz B. On the nature and function expressive of the law of human mortality, and on a new mode of determining life contingencies. Phil. Trans. R. Soc. Lond. 1825;115:513–583. doi: 10.1098/rstb.2014.0379. doi:10.1098/rstl.1825.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.D. The moulding of senescence by natural selection. J. Theor. Biol. 1966;12:12–15. doi: 10.1016/0022-5193(66)90184-6. doi:10.1016/0022-5193(66)90184-6 [DOI] [PubMed] [Google Scholar]
- Hawkes K, O'Connell J.F, Blurton Jones N.G, Alvarez H, Charnov E.L. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. doi:10.1073/pnas.95.3.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimlich-Boran, J. R. 1993 Social organization of the short-finned pilot whale, Globicephala macrorhynchus, with special reference to the social ecology of delphinids. PhD thesis, Cambridge University, Cambridge.
- Hendry A.P, Morbey Y.E, Berg O.K, Wenburg J.K. Adaptive variation in senescence: reproductive lifespan in a wild salmon population. Proc. R. Soc. B. 2004;271:259–266. doi: 10.1098/rspb.2003.2600. doi:10.1098/rspb.2003.2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzel A.R. Killer whale predation on marine mammals at Punta Norte, Argentina; food sharing, provisioning and foraging strategy. Behav. Ecol. Sociobiol. 1991;29:197–204. doi:10.1007/BF00166401 [Google Scholar]
- Kasuya T, Marsh H. Life history and reproductive biology of the short-finned pilot whale, Globicephala macrorhynchus, off the Pacific Coast Japan. Rep. Int. Whaling Comm. 1984;6:259–310. Special Issue. [Google Scholar]
- Kirkwood T.B.L. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. doi:10.1038/270301a0 [DOI] [PubMed] [Google Scholar]
- Lahdenperä M, Lummaa V, Helle S, Tremblay M, Russell A.F. Fitness benefits of prolonged post-reproductive lifespan in women. Nature. 2004;428:178–181. doi: 10.1038/nature02367. doi:10.1038/nature02367 [DOI] [PubMed] [Google Scholar]
- Lee R.D. Rethinking the evolutionary theory of aging: transfers, not births, shape senescence in social species. Proc. Natl Acad. Sci. USA. 2003;100:9637–9642. doi: 10.1073/pnas.1530303100. doi:10.1073/pnas.1530303100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H, Kasuya T. Ovarian changes in the short-finned pilot whale, Globicephala macrorhynchus. Rep. Int. Whaling Comm. 1984;6:311–335. Special Issue. [Google Scholar]
- Marsh H, Kasuya T. Evidence for reproductive senescence in female cetaceans. Rep. Int. Whaling Comm. 1986;8:57–74. Special Issue. [Google Scholar]
- Marsh H, Kasuya T. Changes in the role of a female pilot whale with age. In: Pryor K, Norris K.S, editors. Dolphin societies. University of California Press; Berkeley, CA: 1991. pp. 281–286. [Google Scholar]
- Martin A.R, Rothery P. Reproductive parameters of female long-finned pilot whales (Globicephala melas) around the Faroe Islands. Rep. Int. Whaling Comm. 1993;14:263–304. Special Issue. [Google Scholar]
- McAuliffe K, Whitehead H. Eusociality, menopause and information in matrilineal whales. Trends Ecol. Evol. 2005;20:650. doi: 10.1016/j.tree.2005.09.003. doi:10.1016/j.tree.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Olesiuk P.F, Bigg M.A, Ellis G.M. Life history and population dynamics of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State. Rep. Int. Whaling Comm. 1990;12:209–243. Special Issue. [Google Scholar]
- Packer C, Tatar M, Collins A. Reproductive cessation in female mammals. Nature. 1998;392:807–811. doi: 10.1038/33910. doi:10.1038/33910 [DOI] [PubMed] [Google Scholar]
- Reznick D, Bryant M, Holmes D. The evolution of senescence and post-reproductive lifespan in guppies (Poecilia reticulata) PLoS Biol. 2006;4:136–143. doi: 10.1371/journal.pbio.0040007. doi:10.1371/journal.pbio.0040007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick D.N, Shaw F.H, Rodd F.H, Shaw R.G. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata) Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. doi:10.1126/science.275.5308.1934 [DOI] [PubMed] [Google Scholar]
- Sear R, Mace R, McGregor I.A. Maternal grandmothers improve nutritional status and survival of children in rural Gambia. Proc. R. Soc. B. 2000;267:1641–1647. doi: 10.1098/rspb.2000.1190. doi:10.1098/rspb.2000.1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley D.P, Sear R, Mace R, Kirkwood T.B.L. Testing evolutionary theories of menopause. Proc. R. Soc. B. 2007;274:2943–2949. doi: 10.1098/rspb.2007.1028. doi:10.1098/rspb.2007.1028 [DOI] [PMC free article] [PubMed] [Google Scholar]