Abstract
Background
Several candidate genes on the short arm of chromosome 6 including the HLA locus, TNF, LTA and AGER could be associated with late diabetic complications. The aim of our study was therefore to explore whether polymorphisms (TNF -308 G→A, LTA T60N C→A and AGER -374 T→A) in these genes alone or together (as haplotypes) increased the risk for diabetic complications.
Methodology/Principal Findings
The studied polymorphisms were genotyped in 742 type 1 and 2957 type 2 diabetic patients as well as in 206 non-diabetic control subjects. The Haploview program was used to analyze putative linkage disequilibrium between studied polymorphisms. The TNF, LTA and AGER polymorphisms were associated with the HLA-DQB1 risk genotypes. The AGER -374 A allele was more common in type 1 diabetic patients with than without diabetic nephropathy (31.2 vs. 28.4%, p = 0.007). In a logistic regression analysis, the LTA but not the AGER polymorphism was associated with diabetic nephropathy (OR 2.55[1.11–5.86], p = 0.03). The AGER -374 A allele was associated with increased risk of sight threatening retinopathy in type 2 diabetic patients (1.65[1.11–2.45], p = 0.01) and also with increased risk for macrovascular disease in type 1 diabetic patients (OR 2.05[1.19–3.54], p = 0.01), but with decreased risk for macrovascular disease in type 2 diabetic patients (OR 0.66[0.49–0.90], p = 0.009). The TNF A allele was associated with increased risk for macrovascular complications in type 2 (OR 1.53 [1.04–2.25], p = 0.03, but not in type 1 diabetic patients.
Conclusions/Significance
The association between diabetic complications and LTA, TNF and AGER polymorphisms is complex, with partly different alleles conferring susceptibility in type 1 and type 2 diabetic patients. We can not exclude the possibility that the genes are part of a large haplotype block that also includes HLA-DQB1 risk genotypes.
Introduction
The etiology of diabetic complications is complex, and inflammation may play a role [1]. The mRNA expression for pro-inflammatory cytokines such as IL-1 and Tumor Necrosis Factor Alpha (TNF-α) is increased in the retina and animal studies suggest that inhibition of TNF-α has beneficial effects in prevention of diabetic retinopathy [2], [3]. Recently, we have shown that type 1 diabetic patients with proliferative retinopathy have increased levels of TNF- α [4]. Similarly, inflammatory markers are elevated in diabetic nephropathy [5] and inflammation is associated with development of macrovascular complications such as myocardial infarction [6]. TNF-α and lymphotoxin-α (LT-α, also known as TNF-β) belong to the same TNF family and are encoded by the same gene cluster. TNF-α is mainly produced by activated macrophages and LT-α by T, B and natural killer cell lymphocytes [7]. Promoter variants -308A→G and -238G→A in the gene coding for TNF-α (TNF) affect transcriptional regulation of the gene coding for LT-α (LTA) [8]. The receptor for advanced glycation end products (RAGE) is also mainly considered as an intracellular signal and transducer or proinflammatory peptide [9].
LTA, TNF and the gene encoding for RAGE (AGER) are all located within the MHC complex on the short arm of chromosome 6. The HLA locus is among the most polymorphic in the human genome. Some studies have suggested a direct role of HLA in development of diabetic nephropathy [10], retinopathy [11] and macrovascular disease [12]. Since this region also harbours a variety of other genes involved in inflammation, the results could also reflect variation in other genes than HLA. We have recently shown AGER -374T→A polymorphism to be associated with diabetic nephropathy and possibly with retinopathy in type 1, but not in type 2 diabetic patients [13]. The results concerning the risk allele (A) in the AGER gene were in conflict with a previous study [14], and a possible explanation could be that the AGER gene is in linkage disequilibrium with other genes, such as TNF and LTA.
Variation in TNF and LTA genes has been associated with diabetic nephropathy [15], retinopathy [16] as well as with cardiovascular and cerebrovascular disease [17], [18]. A large genome-scan in Japanese patients identified a susceptibility locus for myocardial infarction on chromosome 6p21 [19], especially the 256A→G and T60N (also referred to as T26N in some studies) variants in the LTA gene, were associated with myocardial infarction. However, a recent, rather large study from USA could neither confirm the association with myocardial infarction, nor the association with inflammatory biomarkers [20].
The TNF/LTA locus is in linkage disequilibrium with HLA-DQB1 [21] and we have previously shown that the AGER -374T→A polymorphism is associated with the HLA-DQB1 risk genotypes [13]. In a recent study the HLA 8.1 ancestral haplotype was shown to be strongly linked to the C allele of the AGER -429T→C promoter polymorphism [22]. Our aim was to study whether variants in these genes form a putative haplotype associated with increased risk of diabetic nephropathy, retinopathy and macrovascular disease.
Results
Type 2 diabetic patients were older and had higher BMI than type 1 diabetic patients or non-diabetic controls (Table 1). Genotype distributions of LTA, TNF and AGER polymorphisms are shown in Table 2. All three variants deviated from the Hardy–Weinberg equilibrium in type 1, but neither in type 2 diabetic patients nor in non-diabetic controls. In type 1 diabetic patients there was an excess of heterozygous patients with LTA (p<0.0002), TNF (p = 0.02) and AGER (p = 0.008). The genotype frequencies of the LTA, TNF and AGER polymorphisms were different between type 1 diabetic patients and controls and also between type 1 and type 2 diabetic patients. The LTA, TNF and AGER polymorphisms were associated with the HLA-DQB1 genotypes (Table 3). The minor allele (A) of the LTA polymorphism was less common in patients with than without HLA-DQB1 genotypes risk genotypes (37.2 vs. 43.9%, p = 0.0001). No difference in allele frequencies of the TNF polymorphism was seen between patients with or without HLA-DQB1 risk genotypes, however the minor allele (A) of the AGER polymorphism was more common in patients with than without HLA-DQB1 risk alleles (34.5 vs. 22.9%), p<0.000001). Because of lack of Hardy–Weinberg equilibrium in type 1 diabetic patients, we could not confirm whether the LTA, TNF and AGER polymorphisms were in LD in type 1 diabetic patients. In type 2 diabetic patients and controls the LTA and TNF polymorphisms were in LD (D′ = 1.00 [0.99–1.00] , r2 = 0.4). The AGER polymorphism was neither in LD with LTA (D′ 0.13 [0.06–0.20], r2 = 0.004) nor with TNF (D′ 0.36 [0.23–0.43], r2 = 0.009) in type 2 diabetic patients.
Table 1. Clinical characteristics of the patients and non-diabetic control subjects.
| Controls | Type 1 | Type 2 | |
| N (M/F) | 107/99 | 458/375 | 2240/1616 |
| Age (yrs.) | 59.7±12.6a | 38.2±13.6 | 61.2±11.7b |
| Age at diagnosis (yrs.) | - | 18.2±9.1 | 55.6±12.2 |
| Diabetes duration (yrs.) | - | 18.1[8.5–29.6] | 2.58[0.09–9.54] |
| BMI (kg/m2) | 25.8±3.8a | 23.8±3.1 | 29.7±5.6a |
| HbA1c (%) | - | 7.2±1.3 | 6.6±1.3 |
| Systolic BP (mmHg) | - | 130.6±18.9 | 144.8±21.8 |
| Diastolic BP (mmHg) | - | 74.2±9.6 | 80.9±10.9 |
| Smokers (current or previous) | - | 51.5% | 61.1% |
p<0.000001.
p = 0.01. Type 1 and type 2 diabetic patients vs. controls.
Table 2. Genotype frequencies (%) of LTA T60N (C→A), TNF -308 G→A and AGER -374 T→A polymorphism in nondiabetic controls and in type 1 and type 2 diabetic patients.
| Controls | Type 1 | p | Corrected p | Type 2 | Corrected p | ||
| LTA T60N (CC/CA/AA) | 77/88/35 (38.5/44.0/17.5) | 186/409/131 (25.6/56.3/18.0) | 0.0003 | 0.05 | 1089/1395/436 (37.3/47.8/14.9) | 0.73 | 1.00 |
| TNF -308 (GG/GA/AA) | 133/66/6 (64.9/32.2/2.9) | 350/329/50 (48.0/45.1/6.9) | 0.00002 | 0.00009 | 1908/906/113 (65.2/31.0/3.9) | 0.93 | 1.00 |
| AGER -374 (TT/TA/AA) | 127/67/11 (62.0/32.7/5.4) | 350/335/48 (47.7/45.7/6.5) | 0.0003 | 0.007 | 1624/1108/198 (55.4/37.8/6.8) | 0.07 | 0.31 |
The uncorrected p-value refers to differences in genotype frequencies (CC vs CA/AA, GG vs. GA/AA and TT vs. TA/AA). Corrected p-value refers to differences in allele frequencies after 100 000 permutations (haploview).
Table 3. Minor allele frequencies according to HLA-DQB1 genotype in type 1 diabetic patients.
| LTA (A allele)a | TNF (A allele)b | AGER (A allele)c | |
| 02/0301 | 26 (59.1) | 17 (38.6) | 5 (11.9) |
| 02/0302 | 214 (46.7) | 174 (38.2) | 131 (28.0) |
| 02/0602d | 2 (100.0) | 1 (50.0) | 1 (50.0) |
| 02/0603d | 6 (50.0) | 5 (41.7) | 2 (16.7) |
| 02/0604 | 26 (68.4) | 13 (34.2) | 2 (5.0) |
| 02/X | 114 (67.1) | 101 (58.7) | 20 (11.6) |
| 0301/0302 | 29 (45.3) | 13 (20.3) | 28 (43.8) |
| 0301/0602d | 1 (50.0) | 0 (0.0) | 2 (100.0) |
| 0301/0603d | 5 (83.3) | 0 (0.0) | 1 (16.7) |
| 0301/0604d | 3 (50.0) | 0 (0.0) | 1 (16.7) |
| 0301/X | 9 (40.9) | 0 (0.0) | 9 (40.9) |
| 0302/0602d | 1 (16.7) | 0 (0.0) | 3 (37.5) |
| 0302/0603 | 33 (43.4) | 6 (20.0) | 14 (46.7) |
| 0302/0604 | 33 (43.4) | 4 (5.1) | 20 (26.3) |
| 0302/X | 54 (22.7) | 18 (7.5) | 116 (48.3) |
| 0602/03/04/Xd | 8 (42.9) | 12 (14.3) | 11 (21.4) |
| 0604/X | 10 (50.0) | 1 (5.0) | 5 (25.0) |
| X/X | 9 (45.0) | 2 (10.0) | 8 (40.0) |
Numbers are number of alleles N (%).
P<0.000001, Chi-Square = 94.2 df = 11.
P<0.000001, Chi-Square = 196.3,df = 11.
P<0.000001, Chi-Square = 98.8,df = 11.
Expected value <5 in type 1 diabetes. These genotypes were pooled in the statistical analysis. X could mean either a homozygous allele or any allele other than 02, 0301, 0302, 0602 or 0604.
Diabetic Nephropathy
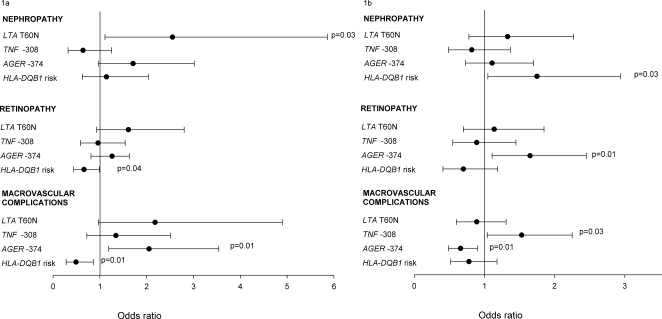
Type 1 diabetic patients with diabetic nephropathy had a higher frequency of the RAGE -374 A allele (31.2%) compared to those who maintained normoalbuminuria ≥10 years (28.4%) (P = 0.007) (Table 4) and the difference remained even after correction for multiple comparisons (p = 0.02). The allele frequencies of TNF -308 G→A and LTA T60N polymorphisms were similar in type 1 diabetic patients with and without diabetic nephropathy. No differences in allele or haplotype frequencies of the studied polymorphisms were observed between type 2 diabetic patients with and without diabetic nephropathy (Table 4). In a logistic regression analysis with age, duration, BMI, HbA1c, systolic and diastolic blood pressure, sex, previous or current smoking and studied polymorphisms and HLA-DQB1 risk genotypes as independent variables, having the LTA T60N A allele in type 1 diabetic patients was associated with increased risk diabetic nephropathy (OR 2.55[1.11–5.86],p = 0.03), (Figure 1a). In type 2 diabetic patients, the HLA-DQB1 risk allele but not LTA, TNF and AGER polymorphisms was associated with increased risk for diabetic nephropathy (1.75 [1.05–2.94], p = 0.03). LTA, TNF and AGER polymorphisms were not associated with diabetic nephropathy in type 2 diabetic patients (Figure 1b).
Table 4. Allelic association of LTA T60N (C→A), TNF -308G→A and AGER -374 T→A polymorphism with diabetic nephropathy, retinopathy and macrovascular complications.
| Type 1 | Type 2 | ||||||||
| NEPHROPATHY | |||||||||
| N | p | corrected p | N | p | corrected p | ||||
| LTA T60N C→A | Controls | 340 | 27.9/53.2/18.9 | 0.317 | 0.918 | 442 | 33.3/47.3/19.5 | 0.205 | 0.592 |
| Cases | 113 | 18.6/63.7/17.7 | 314 | 37.2/45.9/16.9 | |||||
| TNF -308 G→A | Controls | 342 | 47.7/46.5/5.8 | 0.503 | 0.983 | 438 | 63.9/30.4/5.7 | 0.559 | 0.970 |
| Cases | 113 | 44.2/48.7/7.1 | 314 | 65.0/30.9/4.1 | |||||
| AGER -374 T→A | Controls | 345 | 52.2/41.2/6.7 | 0.007 | 0.023 | 439 | 54.2/37.6/8.2 | 0.119 | 0.509 |
| Cases | 114 | 34.2/57.9/7.9 | 315 | 58.1/37.1/4.8 | |||||
| RETINOPATHY | |||||||||
| LTA T60N C→A | Controls | 310 | 29.4/53.5/17.1 | 0.154 | 0.461 | 584 | 34.1/49.8/16.1 | 0.608 | 0.986 |
| Cases | 310 | 21.6/60.6/17.7 | 296 | 37.5/45.6/16.9 | |||||
| TNF -308 G→A | Controls | 307 | 50.2/43.6/6.2 | 0.573 | 0.982 | 583 | 65.0/30.7/4.3 | 0.368 | 0.878 |
| Cases | 315 | 46.0/48.9/5.1 | 295 | 67.8/28.8/3.4 | |||||
| AGER -374 T→A | Controls | 313 | 48.9/45.3/5.8 | 0.295 | 0.774 | 583 | 54.9/36.5/8.6 | 0.734 | 0.998 |
| Cases | 315 | 44.8/47.9/7.3 | 298 | 50.3/44.0/5.7 | |||||
| MACROVASCULAR COMPLICATIONS | |||||||||
| LTA T60N C→A | Controls | 609 | 27.6/55.2/17.2 | 0.034 | 0.122 | 1802 | 38.1/47.5/14.4 | 0.102 | 0.456 |
| Cases | 112 | 15.2/64.3/20.5 | 885 | 35.0/48.6/16.4 | |||||
| TNF -308 G→A | Controls | 611 | 49.3/44.0/6.7 | 0.426 | 0.916 | 1804 | 67.3/29.2/3.5 | 0.003 | 0.017 |
| Cases | 113 | 42.5/52.2/5.3 | 886 | 61.4/34.1/4.5 | |||||
| AGER -374 T→A | Controls | 617 | 48.8/44.6/6.6 | 0.299 | 0.797 | 1809 | 54.4/38.1/7.5 | 0.010 | 0.052 |
| Cases | 111 | 41.4/52.3/6.3 | 888 | 58.8/35.9/5.3 | |||||
The uncorrected p-value refers to differences in allele frequencies. Corrected p-value refers to differences in allele frequencies using after 100 000 permutations (Haploview).
Figure 1. Logistic regression analysis in type 1 (1a) and type 2 (1b) diabetic patients with LTA T60N (C→A), TNF -308 G→A, AGER -374 T→A polymorphisms and HLA-DQB1 risk genotypes as independent and diabetic complication as dependent variable.
Age, systolic and diastolic blood pressure, sex, previous/current smoking are included in all models. BMI is included in the models for nephropathy and macrovascular disease, duration in the models for nephropathy and retinopathy and age at diagnosis in the model for macrovascular disease.
Diabetic retinopathy
The allele (or haplotype) frequencies of the studied polymorphisms did not differ between patients with and without sight-threatening retinopathy, neither in type 1 nor in type 2 diabetic patients (Table 4). In a logistic regression analysis with age, duration, HbA1c, systolic and diastolic blood pressure, sex, current/previous smoking and genotypes as independent variables, HLA-DQB1 risk genotype was associated with decreased risk for sight-threatening retinopathy in type 1 diabetic patients (0.66[0.44–0.99], p = 0.04). In contrast LTA, TNF and AGER polymorphisms were not associated with sight-threatening retinopathy in type 1 diabetic patients (Figure 1a). In type 2 diabetic patients the AGER A allele was associated with increased risk for sight-threatening retinopathy (1.65[1.11–2.45], p = 0.01) (Figure 1b).
Macrovascular complications
Type 1 diabetic patients with a history of macrovascular complications had higher frequency of the LTA A allele than patients without macrovascular complications (52.7% vs. 44.8%, p = 0.03) (Table 4). The allele frequencies of the TNF and AGER polymorphisms did not differ between type 1 diabetic patients with and without history of macrovascular disease.
In a logistic regression analysis, the AGER – 374 A allele was associated with increased risk for macrovascular complications (OR 2.05[1.19–3.54], p = 0.01), Figure 1a) in type 1 diabetic patients. The HLA-DQB1 risk genotype was associated with decreased risk for macrovascular disease in type 1 diabetic patients (OR 0.49[0.28–0.86], p = 0.01). The gene-gene interaction was tested in a separate logistic regression model (assuming a dominant model) by adding a term (AGER)×(HLA risk genotype). The gene-gene interaction term was however not significant and was therefore not included in the final model (Figure 1a).
In type 2 diabetic patients the frequency of both TNF -308 A allele and AGER -374 T allele genotype was higher in patients with than without macrovascular complications (21.6% vs. 18.1%, p = 0.003 and 76.7% vs. 73.5%, p = 0.03, respectively) (Table 4), the significance of difference in frequency of AGER -374 polymorphism did not stand multiple comparisons (Table 4). The AA haplotype of TNF and LTA was more common in type 2 diabetic patients with than without macrovascular disease (21.5% vs. 18.1%, p = 0.003). In a logistic regression analysis the TNF -308 A allele (OR 1.53[1.04–2.25], p = 0.03) was associated with increased and the AGER -374 A allele with decreased risk (OR 0.66[0.49–0.90], p = 0.009) for macrovascular disease (Figure 1b). The gene-gene interaction was tested in a separate logistic regression model (assuming a dominant model) by adding a term (AGER)×(TNF). The gene-gene interaction term was however not significant and was therefore not included in the final model (Figure 1b).
Discussion
The key finding of the present study was that the polymorphisms in the TNF, LTA and AGER genes were associated with high risk HLA-DQB1 alleles on chromosome 6p21 and that they influenced the risk for late diabetic complications. The TNF, LTA and AGER alleles were in Hardy-Weinberg equilibrium in as well in non-diabetic controls as in type 2 diabetic patients. In type 1 diabetic patients all of the studied polymorphisms deviated from Hardy-Weinberg equilibrium having excess of heterozygous alleles. This is well in line with previous observations of a synergistic effect of the DR3 and DR4 haplotypes DRB1*0301-DQA1*0501-DQB1*0201 and DRB1*0401-DQA1*0301-DQB1*0302 which are strongly associated with type 1 diabetes thus leading to excess of heterozygous versus homozygous patients [23]. Deviation from Hardy-Weinberg equilibrium will influence the estimated haplotype frequencies especially in a situation with excess of homozygous patients [24]. Therefore, we could not test whether LTA, TNF and AGER polymorphisms are in linkage disequilibrium in type 1 diabetic patients as suggested by Laki et. al. who recently showed that the HLA 8.1 ancestral haplotype (8.1 AH) was strongly linked to the AGER -429T→C polymorphism and the AGER -429 allele should therefore be considered as candidate member of the HLA 8.1 ancestral haplotype [22].
The association patterns between diabetic complications and polymorphisms in LTA, TNF, AGER and HLA was complex and none of the studied polymorphism was associated with all diabetic complications in either type 1 or type 2 diabetic patients. For example, the A allele of the AGER -374 polymorphism was more common in type 1 diabetic patients with than without diabetic nephropathy. In a regression model however, when all of the polymorphisms and HLA-DQB1 risk genotype were included in the model, LTA rather than AGER was a risk factor for diabetic nephropathy in type 1 diabetic patients. Similarly, the AGER A allele was associated with increased risk for sight-threatening retinopathy but decreased risk for macrovascular disease in type 2 diabetic patients which raises a question, whether this could represent a survival bias because of the strong association between TNF and AGER polymorphisms and macrovascular disease.
The lack of association in type 1 diabetic patients could of course be due to small sample size. Another possible source of bias could be population stratification due to ethically diverse samples, which is not very likely given the fact that all patients were Scandinavians. Previous studies on the putative association between polymorphisms in the LTA, TNF and RAGE genes and micro-and macrovascular complications in type 2 diabetes have given conflicting results [14]–[20], [25]–[30]. Differences in study design, insufficient power and inclusion of different ethnic groups might explain some of the observed differences, as would inclusion of type 2 diabetic patients with LADA [30]. To circumvent this problem we excluded adult patients who were GAD antibody positive or required insulin therapy during the first year.
Some of the discrepancy in the published literature could also be due to the complex pattern of LD in the region. The HLA region on the short arm of chromosome 6 contains several genes involved in inflammatory responses. The haplotypic structure is complex and there is also a complex interaction between genes and gene products, as illustrated by the TNF gene polymorphism that can influence transcription of LTA [8], and receptor for advanced glycation end-products (RAGE), which after binding to its ligand can increase production of pro-inflammatory cytokines, among them TNF-α [31].
Taken together the data show that polymorphisms in the LTA, TNF and AGER genes increase risk of diabetic micro- and macroangiopathy either alone or together. Given the strong association with HLA-risk genotypes we can not rule out that they are part of a same haplotype and their risk on disease can therefore only be judged from studies assessing them all.
Materials and Methods
A detailed description of study subjects and analytical methods has been given previously [13]. The study population was mainly the same as in the previous study. However, additional 98 type 1 and 796 type 2 diabetic patients were included and genotyped for the AGER -374T→A polymorphism in this study and all the patients were also genotyped for the TNF -308 G→A and LTA T60N C→A polymorphisms. Patients were classified as having type 1 or type 2 diabetes by the attending physician using the World Health Organization (WHO) guidelines of 1985 [23] or, when diagnosed after January 1, 2001, according to the new WHO guidelines [24]. Type 1 diabetic patients with age at onset >35 years (N = 224) and type 2 diabetic patients positive for GAD antibodies (N = 197) were excluded. In addition, type 2 diabetic patients with an age at diagnosis <35 years and with permanent insulin treatment during the first year from diagnosis were excluded (N = 108). A total of 3699 (742 type 1 and 2957 type 2 diabetes) Scandinavian patients and 206 Scandinavian, non-diabetic control subjects were included in the study. Control subjects were selected among spouses of patients with hypertension; they were not allowed to have any first degree relatives with diabetes. No information on hypertension or myocardial infarction in non-diabetic control subjects was available. The Ethics committee of Malmö/Lund approved the study. Informed consent was obtained from all patients.
Assessment of complications
Diabetic nephropathy
The urinary albumin concentration was determined by immunonephelometry (Beckman Instruments, CA, USA) until 1998 and thereafter by an immunoturbimetric method (Beckman Coulter, Beckman Instruments, CA, USA). Albuminuria was reported either as µg/min (AER), mg/24 hours or as a urinary albumin/creatinine ratio (g/mol). Microalbuminuria was defined as 20–200 µg/min, 30–300 mg/24 hours or 2.0–25 in males and 2.8–25 g/mol in females. For the definition of microalbuminuria we also considered older values given as the urinary albumin concentration measured in a first morning specimen. Values of 30–300 mg/l were considered as microalbuminuria. Values above the upper limit were indicative of macroalbuminuria. Macroalbuminuria was considered present when at least two values above the cut-off limit for macroalbuminuria were recorded. One positive measurement only was considered as macroalbuminuria if the patient thereafter was treated with ACE inhibitors or angiotensin II receptor blockers or if the patient had had persistent microalbuminuria previously. Patients with other kidney diseases were excluded from the analysis. Normoalbuminuria required that all urinary albumin measurements were within the normal range, otherwise the albuminuria status was considered unknown. Duration of albuminuria was calculated from the onset of microalbuminuria when known, or from the latest measurement with no albuminuria. If not known (60% of all the cases with micro- or macroalbuminuria, 39% in type 1 diabetic patients) the duration was calculated from the first registered value of micro- or macroalbuminuria. When calculating the genotype frequencies in patients with normoalbuminuria, only patients with diabetes duration ≥10 years were included.
Diabetic Retinopathy
Information about the retinopathy status was available in 3072 patients. Patients were divided into two groups; subjects with no or non-proliferative retinopathy without macular edema and subjects with sight-threatening retinopathy, which included patients with proliferative retinopathy and/or photocoagulation treatment (panretinal and/or focal/grid for macular edema). The duration of sight-threatening retinopathy was defined from the first information of diagnosis or laser treatment. When calculating the genotype frequencies in patients without sight-threatening retinopathy only those with diabetes duration ≥10 years were included.
Macrovascular disease
Macrovascular disease was defined as previously diagnosed myocardial infarction, angina pectoris, transitory ischemic attack (TIA), stroke and/or peripheral vascular disease. Information on previous macrovascular disease was available in 93% of the patients.
In logistic regression analysis age at diagnosis was used in stead of duration because the macrovascular disease duration was often unknown and sometimes a decade before the onset of diabetes. Lipid levels were not used in the regression analysis, because according to the clinical guidelines patients with previous episode of myocardial infarction or stroke should be treated with statins. Consequently, current cholesterol levels were lower in patients with previous episode of macrovascular disease and we did not have access to historical data.
Genotyping
T60N C→A (rs1041981), TNF -308 G→A (rs1800629), and AGER -374 T→A (rs1800624) polymorphisms were genotyped using the allelic discrimination method on the ABI 7900 instrument (Applied Biosystems, Foster City, CA). A subset of patient (629 type 1 and 1108 type 2 diabetic patients and all control subjects were previously genotyped for HLA-DQB1 [13]. The genotyping success rates were 99.0% for the LTA, 98.7% for the TNF and 98.4% for the AGER polymorphisms. Re-genotyping was performed in a separate analysis in random samples from those which passed. A total of 226 (LTA), 234 (TNF) and 225 (AGER) patients were re-genotyped with a 100% genotyping concordance rate.
Statistical analysis
Data are presented as mean±SD or as median and interquartile range [25th–75th]. Chi-square test was used to test for frequency differences between studied genotypes. To test differences between group means, the Student's two-tailed t-test was used for normally distributed values and Mann-Whitney U-test for non-normally distributed medians. In order to assess factors associated with diabetic nephropathy, retinopathy and macrovascular disease, a multiple logistic regression analysis with forward selection was performed. All data were analysed with a NCSS 2004 (NCSS statistical software, Kaysville, UT, USA). A p-value <0.05 was considered statistically significant. To evaluate putative haplotype blocks, linkage disequilibrium (LD) between the SNPs was analyzed using Haploview 3.32 and D′ values were calculated with 95% confidence intervals (CI) when the genotype frequencies were in Hardy-Weinberg equilibrium [32]. A corrected p-value was obtained after 100,000 permutations of individual SNPs and haplotype blocks including the TNF, LTA and AGER polymorphisms. Power analysis was made using Genetic Power calculator [33]. HW-QuickCheck software [34] was used for testing of putative excess of heterozygous/ homozygous patients.
Power calculations
Power assuming α = 0.05 and relative risk of 1.3 was 11%, 31% and 32% for type 1 diabetic patients and 62%, 81% and 80% for type 2 diabetic patients with or without diabetic nephropathy for the LTA, TNF and AGER polymorphisms. The power for retinopathy was 68%, 86% and 85% in type 1 and 58%, 73% and 73% in type 2 diabetes and for macrovascular disease 20%, 28% and 28% in type 1 and 97%, 99% and 99% in type 2 diabetes.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by Skane County Council Research and Development Foundation and the Medical Research Council of Lundberg.
References
- 2.Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, et al. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol. 2002;160:1683–1693. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. Faseb J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 4.Gustavsson C, Agardh CD, Bengtsson B, Agardh E. TNF-alpha is an independent serum marker for proliferative retinopathy in type 1 diabetic patients. Journal of Diabetes and Its Complications (In Press) 2007 doi: 10.1016/j.jdiacomp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Dalla Vestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(Suppl 1):S78–82. doi: 10.1681/asn.2004110961. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 7.Makhatadze NJ. Tumor necrosis factor locus: genetic organisation and biological implications. Hum Immunol. 1998;59:571–579. doi: 10.1016/s0198-8859(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 8.Knight JC, Keating BJ, Rockett KA, Kwiatkowski DP. In vivo characterization of regulatory polymorphisms by allele-specific quantification of RNA polymerase loading. Nat Genet. 2003;33:469–475. doi: 10.1038/ng1124. [DOI] [PubMed] [Google Scholar]
- 9.Vlassara H, Palace MR. Glycoxidation: the menace of diabetes and aging. Mt Sinai J Med. 2003;70:232–241. [PubMed] [Google Scholar]
- 10.Dyck R, Bohm C, Klomp H. Increased frequency of HLA A2/DR4 and A2/DR8 haplotypes in young saskatchewan aboriginal people with diabetic end-stage renal disease. Am J Nephrol. 2003;23:178–185. doi: 10.1159/000070747. [DOI] [PubMed] [Google Scholar]
- 11.Agardh D, Gaur LK, Agardh E, Landin-Olsson M, Agardh CD, et al. HLA-DQB1*0201/0302 is associated with severe retinopathy in patients with IDDM. Diabetologia. 1996;39:1313–1317. doi: 10.1007/s001250050575. [DOI] [PubMed] [Google Scholar]
- 12.Dahlen GH, Slunga L, Holmlund G, Lango A, Lindblom B. Lp(a) lipoprotein and HLA-DR genotype in early coronary artery disease. Eur J Immunogenet. 1993;20:95–102. doi: 10.1111/j.1744-313x.1993.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 13.Lindholm E, Bakhtadze E, Sjogren M, Cilio CM, Agardh E, et al. The -374 T/A polymorphism in the gene encoding RAGE is associated with diabetic nephropathy and retinopathy in type 1 diabetic patients. Diabetologia. 2006;49:2745–2755. doi: 10.1007/s00125-006-0412-3. [DOI] [PubMed] [Google Scholar]
- 14.Pettersson-Fernholm K, Forsblom C, Hudson BI, Perola M, Grant PJ, et al. The functional -374 T/A RAGE gene polymorphism is associated with proteinuria and cardiovascular disease in type 1 diabetic patients. Diabetes. 2003;52:891–894. doi: 10.2337/diabetes.52.3.891. [DOI] [PubMed] [Google Scholar]
- 15.Manchanda PK, Kumar A, Kaul A, Mittal RD. Correlation between a gene polymorphism of tumor necrosis factor-alpha (G/A) and end-stage renal disease: a pilot study from north India. Clin Chim Acta. 2006;370:152–157. doi: 10.1016/j.cca.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Kumaramanickavel G, Sripriya S, Vellanki RN, Upadyay NK, Badrinath SS, et al. Tumor necrosis factor allelic polymorphism with diabetic retinopathy in India. Diabetes Res Clin Pract. 2001;54:89–94. doi: 10.1016/s0168-8227(01)00269-8. [DOI] [PubMed] [Google Scholar]
- 17.Um JY, An NH, Kim HM. TNF-alpha and TNF-beta gene polymorphisms in cerebral infarction. J Mol Neurosci. 2003;21:167–171. doi: 10.1385/JMN:21:2:167. [DOI] [PubMed] [Google Scholar]
- 18.Bernard V, Pillois X, Dubus I, Benchimol D, Labouyrie JP, et al. The -308 G/A tumor necrosis factor-alpha gene dimorphism: a risk factor for unstable angina. Clin Chem Lab Med. 2003;41:511–516. doi: 10.1515/CCLM.2003.077. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki K, Ohnishi Y, Iida A, Sekine A, Yamada R, et al. Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction. Nat Genet. 2002;32:650–654. doi: 10.1038/ng1047. [DOI] [PubMed] [Google Scholar]
- 20.Asselbergs FW, Pai JK, Rexrode KM, Hunter DJ, Rimm EB. Effects of lymphotoxin-alpha gene and galectin 2 gene polymorphisms on inflammatory biomarkers, cellular adhesion molecules, and risk of coronary heart disease. Clin Sci (Lond) 2006 doi: 10.1042/CS20060200. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Groop L, Nilsson A, Weng J, Tuomi T. A combination of human leukocyte antigen DQB1*02 and the tumor necrosis factor alpha promoter G308A polymorphism predisposes to an insulin-deficient phenotype in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2767–2774. doi: 10.1210/jc.2002-020506. [DOI] [PubMed] [Google Scholar]
- 22.Laki J, Kiszel P, Vatay A, Blasko B, Kovacs M, et al. The HLA 8.1 ancestral haplotype is strongly linked to the C allele of -429T>C promoter polymorphism of receptor of the advanced glycation endproduct (RAGE) gene. Haplotype-independent association of the -429C allele with high hemoglobin(A1C) levels in diabetic patients. Mol Immunol. 2007;44:648–655. doi: 10.1016/j.molimm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Noble JA, Valdes AM, Cook M, Klitz W, Thomson G, et al. The role of HLA class II genes in insulin-dependent diabetes mellitus: molecular analysis of 180 Caucasian, multiplex families. Am J Hum Genet. 1996;59:1134–1148. [PMC free article] [PubMed] [Google Scholar]
- 24.Single RM, Meyer D, Hollenbach JA, Nelson MP, Noble JA, et al. Haplotype frequency estimation in patient populations: the effect of departures from Hardy-Weinberg proportions and collapsing over a locus in the HLA region. Genet Epidemiol. 2002;22:186–195. doi: 10.1002/gepi.0163. [DOI] [PubMed] [Google Scholar]
- 25.Hudson BI, Stickland MH, Futers TS, Grant PJ. Effects of novel polymorphisms in the RAGE gene on transcriptional regulation and their association with diabetic retinopathy. Diabetes. 2001;50:1505–1511. doi: 10.2337/diabetes.50.6.1505. [DOI] [PubMed] [Google Scholar]
- 26.dos Santos KG, Canani LH, Gross JL, Tschiedel B, Pires Souto KE, et al. The -374A allele of the receptor for advanced glycation end products gene is associated with a decreased risk of ischemic heart disease in African-Brazilians with type 2 diabetes. Mol Genet Metab. 2005;85:149–156. doi: 10.1016/j.ymgme.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Falcone C, Campo I, Emanuele E, Buzzi MP, Geroldi D, et al. -374T/A polymorphism of the RAGE gene promoter in relation to severity of coronary atherosclerosis. Clin Chim Acta. 2005;354:111–116. doi: 10.1016/j.cccn.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Padovani JC, Pazin-Filho A, Simoes MV, Marin-Neto JA, Zago MA, et al. Gene polymorphisms in the TNF locus and the risk of myocardial infarction. Thromb Res. 2000;100:263–269. doi: 10.1016/s0049-3848(00)00315-7. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Ng MC, So WY, Ma R, Ko GT, et al. Association between tumour necrosis factor-alpha G-308A polymorphism and risk of nephropathy in obese Chinese type 2 diabetic patients. Nephrol Dial Transplant. 2005;20:2733–2738. doi: 10.1093/ndt/gfi101. [DOI] [PubMed] [Google Scholar]
- 30.Zeggini E, Groves CJ, Parkinson JR, Halford S, Owen KR, et al. Large-scale studies of the association between variation at the TNF/LTA locus and susceptibility to type 2 diabetes. Diabetologia. 2005;48:2013–2017. doi: 10.1007/s00125-005-1902-4. [DOI] [PubMed] [Google Scholar]
- 31.Yeh CH, Sturgis L, Haidacher J, Zhang XN, Sherwood SJ, et al. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495–1504. doi: 10.2337/diabetes.50.6.1495. [DOI] [PubMed] [Google Scholar]
- 32.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 33.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- 34.Kalinowski ST. hw-quickcheck: an easy-to-use computer program for checking genotypes for agreement with Hardy-Weinberg expectations. Molecular Ecology Notes. 2006;6:974–979. [Google Scholar]