Abstract
Aims
The purpose of this study was to evaluate the effects of photoinitiator type and water content on the polymerization rate (Rp) and degree of conversion (DC) of a model BisGMA/HEMA-based resin.
Materials and methods
The comonomer mixture consisted of BisGMA/HEMA (60/40 by weight). Different two- or three-component photoinitiator systems were incorporated. Two-component systems were 0.5% CQ (camphorquinone) and 0.5% DMAEMA (2-(dimethylamino) ethyl methacrylate) or 0.5% CQ and 0.5% 4E (Ethyl 4-dimethylaminobenzoate). The three-component systems were added 1% DPIHP (diphenyliodonium hexafluorophosphate) to the above systems. Each system was tested as made, or after addition of 5, 10, 15wt% water. When cured under a conventional dental light, the Rp and DC of each formulation was determined using time-resolved attenuated total reflection (ATR)-Fourier transform infrared (FTIR) spectroscopy.
Results
For mixtures containing two-component initiator systems, when the hydrophobic initiator CQ was used in combination with hydrophilic DMAEMA, Rps and DCs were dramatically decreased as a function of water content. The Rps and DCs of the hydrophobic CQ/4E system were higher than those of the CQ/DMAEMA system in the presence of water. For three-component initiator systems, incorporation of DPIHP enhanced the polymerization of all mixtures in the presence of water compared to their counterpart two-component initiators. Interestingly, the CQ/DMAEMA caused greater DC and Rp when DPIHP was used.
Significance
The hydrophobicity/hydrophilicity of photoinitiator components significantly affects both the DC as well as Rp when in the presence of water. The results indicate that formulation of photoinitiator components should be based on the effectiveness of the bonding systems under both dry and wet conditions.
Keywords: Dental adhesives, Photopolymerization, FTIR, Photoinitiator, Degree of conversion
1. Introduction
The development of so-called “wet bonding technique” [1–2] leads to higher bond strengths by preventing collapse of demineralized dentin and by enhancing infiltration of adhesive into exposed collagen networks. This technique requires adhesives to be more hydrophilic, in order that they can wet the dentin substrates and tolerate additional moisture [3]. However, water content of demineralized dentin cannot be well-controlled clinically [4–6]. Thus, the behavior of adhesives in the presence of different contents of water is very crucial for the success of a bonded restoration. During the past decade, much research has been performed to evaluate the effect of water on dentin adhesives and composites [7–18].
Water can affect dental adhesives in many aspects before and after photopolymerization. During polymerization, water sorption and diffusion of methacrylated-based adhesive resins were found to be related to the hydrophobicity or hydrophilicity of the adhesive [9]. Water absorption in resins has negative effects on their mechanical properties: the more water the polymers absorb, the mechanical properties decrease [10–13, 16, 17]. Phase separation of BisGMA based adhesives in the presence of water [14] has been shown, and the effect of water content on polymerization has also been studied on a BisGMA/HEMA-based system [17]. The conversion of the resin decreased from ~54% to ~25% when more than 20% water was added [17]. The extent of monomer conversion [19–22] and the presence of phase separations in the presence of water [6, 23–26] during polymerization are very influential on the structure and properties of the resulting polymer.
In dental adhesive formulations, hydrophobic components, such as BisGMA, are usually employed to enhance the mechanical properties and compatibility with restorative resin composites [16]. Hydrophilic components, such as HEMA, are employed to improve water-compatibility and enhance the infiltration of adhesives into wet, demineralized dentin [14]. In the presence of water, phase separation of the uncured hydrophobic/hydrophilic components can easily develop [14]. The presence of water and monomer phase separation affects the polymerization rate and degree of conversion, and induces an inhomogeneous structure of the cured adhesive, which might be a potential mechanism for degradation [27–29]. Therefore, the performance of photoinitiators in the presence of water becomes very critical. However, this aspect seems not to have been investigated thoroughly.
The majority of commercial dentin resins contain camphorquinone (CQ)/amine pairs as photoinitiating systems.[20] The effectiveness of the systems depends on the H-atom donor ability of amines used as coinitiators and the compatibility of initiator components with monomers. CQ is relatively hydrophobic. However, some amines, such as 2-(dimethylamino) ethyl methacrylate (DMAEMA), are hydrophilic and some amines like ethyl 4-dimethylaminobenzoate (4E) are relatively hydrophobic (Table 1). In the presence of water, it is unknown how the hydrophilicity/hydrophobicity of the components in photoinitiator systems would affect polymerization of adhesives. Recently, the third component, for example, the iodonium salt (DPIHP) is applied into the above two-component systems [32]. DPIHP, which is an electron acceptor, has the following double roles [32]: one is to regenerate the dye molecules (CQ) by replacing inactive, terminating radicals with active, phenyl initiating radicals, and another role is to generate additional active phenyl radicals. Due to its unique abilities, the DPIHP is expected to be an efficient, water compatible initiating component.
Table 1.
Comparison of solubility of different initiator components in water and monomer
| Initiator Components | Abbreviation | Solubility in water | Solubility in BisGMA/HEMA (60/40) |
|---|---|---|---|
| Camphorquinone | CQ | ~0.7 mg/g | > 10 mg/g |
| 2-(dimethylamino) ethyl methacrylate | DMAEMA | >10 mg/g | > 10 mg/g |
| Ethyl-4-(dimethylamino) benzoate | 4E | <0.5 mg/g | > 10 mg/g |
| Diphenyliodonium hexafluorophosphate | DPIHP | ~5 mg/g | ~5 mg/g |
The type of coinitiator and water have been found to significantly affect the photoreactivity and polymerization of HEMA/CQ-based mixtures using photo-differential scanning calorimetry method [20]. However, these mixtures have no phase separation in the presence of water and are very different from the current adhesive formulations that contain significant amounts of hydrophobic components such as BisGMA. The purpose of this study was to determine the effect of water content on the polymerization rate and DC of a model BisGMA/HEMA-based resin that contain photoinitiator systems with different hydrophilic/hydrophobic components. It is hypothesized that (1) the hydrophilicity/hydrophobicity of photoinitiator system components would affect polymerization of BisGMA/HEMA based adhesives in the presence of water; the more hydrophobic the photoinitiator components are, the less they will be affected by water. In addition (2), because of its ability to enhance radical efficacy, addition of DPIHP will significantly increase both final conversion and overall conversion rate of all photoinitiator systems that do not contain it.
2. Materials and methods
2.1. Model resin and photoinitiator systems
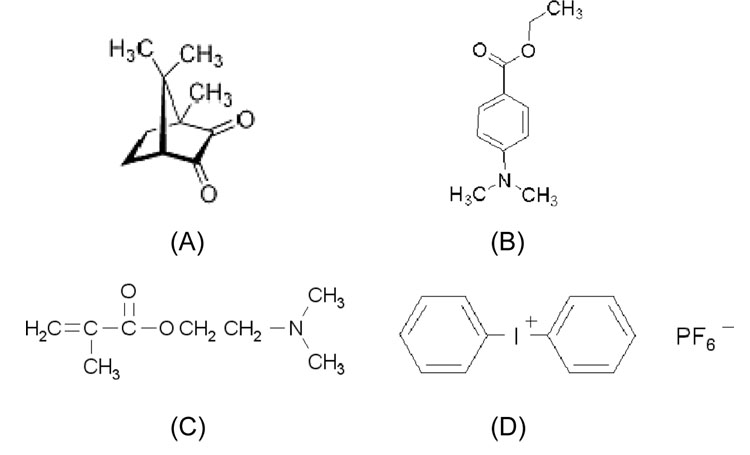
The comonomer mixture consisted of 2, 2-bis[4-(2-hydroxy-3-methacryloxypropoxy) phenyl]-propane (BisGMA) (Polysciences, Washington, PA) and hydroxyethylmethacrylate (HEMA) (Acros Organics, Morris Plains, NJ) used in a mass ratio of 60/40. This composition is similar to that used in some commercial dental adhesive formulations such as Single Bond (3M ESPE, St. Paul, MN).[14] The two-component visible light photoinitiators systems (all from Aldrich, Milwaukee, WI) used either 0.5 % CQ and 0.5 % DMAEMA or 0.5 % CQ and 0.5% 4E. The three-component systems were formed by adding 1% DPIHP (diphenyliodonium hexafluorophosphate) to each of the above two-component groups. The chemical structures of the different photoinitiator components are shown in Fig. 1. The initiator component solubilities in water and BisGMA/HEMA (60/40) mixture were tested and are shown in Table 1. Among these chemicals, CQ and 4E are relatively hydrophobic, DMAEMA and DPIHP are hydrophilic.
Fig. 1.
Chemical structures of components used in the initiator systems. (A) CQ; (B) 4E; (C) DMAEMA; (D) DPIHP.
The monomers/photoinitiator groups were prepared in the absence of visible light, and shaking and sonication were required to yield well-mixed solutions. When water was added to the solutions, heavy water (D2O) (Cambridge Isotope Laboratories, Inc. Andover, MA, USA) was used due to its absence of overlapping water band at 1640 cm−1, where monitoring of the aliphatic C=C bond is essential for determining monomer conversion. D2O was added to make 5, 10 and 15 wt% solutions. All compounds were used as received.
2.2. Obtaining real-time FTIR spectra
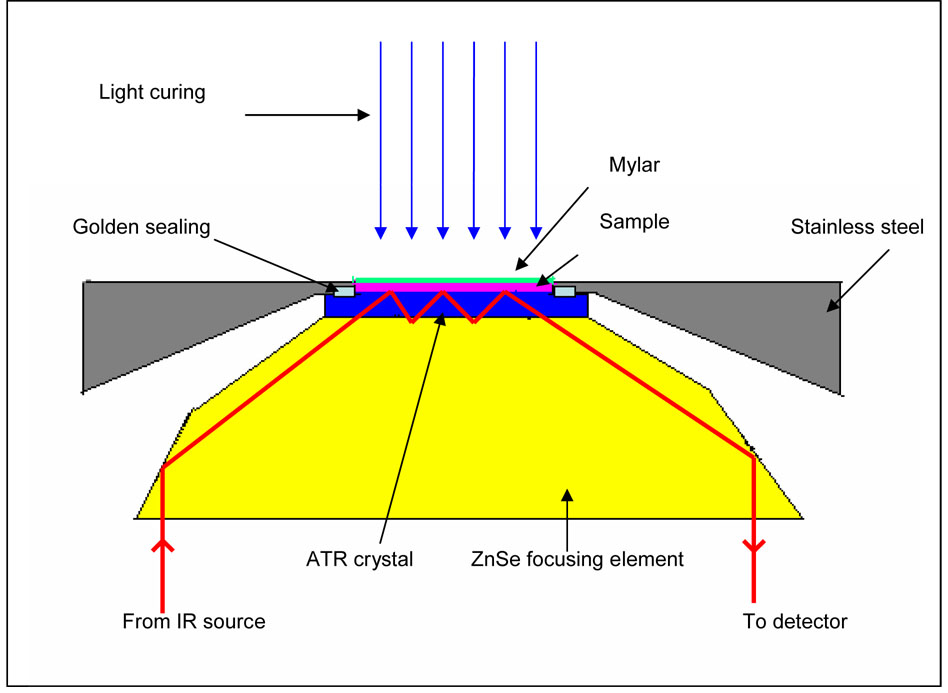
Real-time in-situ monitoring of the photocuring of the different solutions was made using an infrared spectrometer (Spectrum One, Fourier transform infrared spectrophotometer, Perkin-Elmer, Waltham, MA, USA) at a resolution of 4 cm−1 [30, 31]. The adhesive/water mixtures were cast on the diamond crystal top-plate of an attenuated total reflectance (ATR) accessory (Perkin-Elmer, Waltham, MA, USA), covered with a mylar film. A diagrammatic sketch of the sampling accessory is shown in Fig. 2. The ATR crystal was zinc selenide (ZnSe) with a transmission range between 650~4000 cm−1. A time-based spectrum collector (Spectrum TimeBase, Perkin-Elmer) was used for continuous and automatic collection of spectra during polymerization at a rate of one spectrum every 0.4–0.6 s. At this high rate and resolution, the signal-to-noise ratio of spectra is sufficient for spectral analysis. A 40s-exposure from a conventional dental light polymerization unit (Spectrum Light, Dentsply, Milford, DE, USA) emitting 550 mW/cm2 was applied after 50 spectra had been recorded. Real-time IR spectra were continuously recorded for 300 s after light activation began. Three separate replications for each adhesive formulation were obtained. Data on the degree of conversion and polymerization rate were analyzed using analysis of variance (ANOVA), together with the T test.
Fig. 2.
Optical layout of the universal attenuated total reflectance (UATR) sampling accessory. Time-resolved FTIR spectra were collected to monitor the real-time polymerization behavior of dental adhesives under a conventional dental curing light. The distance between the light guide end and the mylar film was less than 5 mm.
2.3. Calculation of monomer conversion and polymerization rate
The polymerization rate and degree of conversion were calculated for each adhesive formulation. The maximal polymerization rate (Rmax) was determined using the maximum slope of linear region of the DC-time plots by using the least square linear fitting. The DC was calculated based on the last 20 spectra of time-resolved spectra. One DC value was an average of 60 points.
3. Results
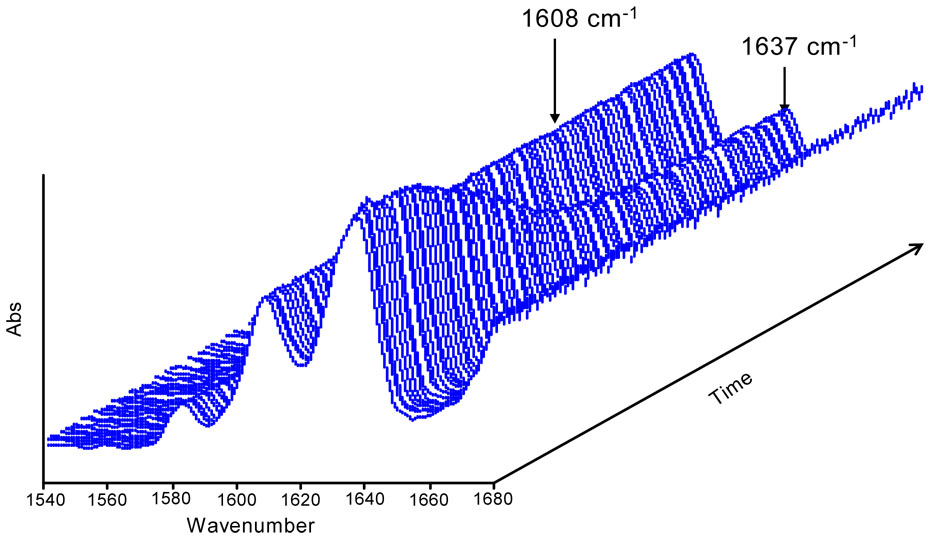
Representative real-time spectra of one model adhesive formulation during photopolymerization are presented in Fig. 3. It can be seen that the absorbance of the 1608 cm−1 band (associated with phenyl groups in BisGMA) remains unchanged and thus can be used as an unchanging, internal standard to which the changing aliphatic C=C group at 1637 cm−1 can be compared. Absorbance decrease at 1637 cm−1 indicates consumption of methacrylate C=C bonds during polymerization. The changes in absorbance ratios of 1637 cm−1(C=C) / 1608 cm−1 (phenyl) were monitored and degree of conversion (DC) was calculated by using the following equation [30]:
| (1) |
Fig. 3.
Representative time-resolved FTIR spectra for the BisGMA/HEMA model adhesive containing the CQ/4E initiator system in the presence of 10 wt% water.
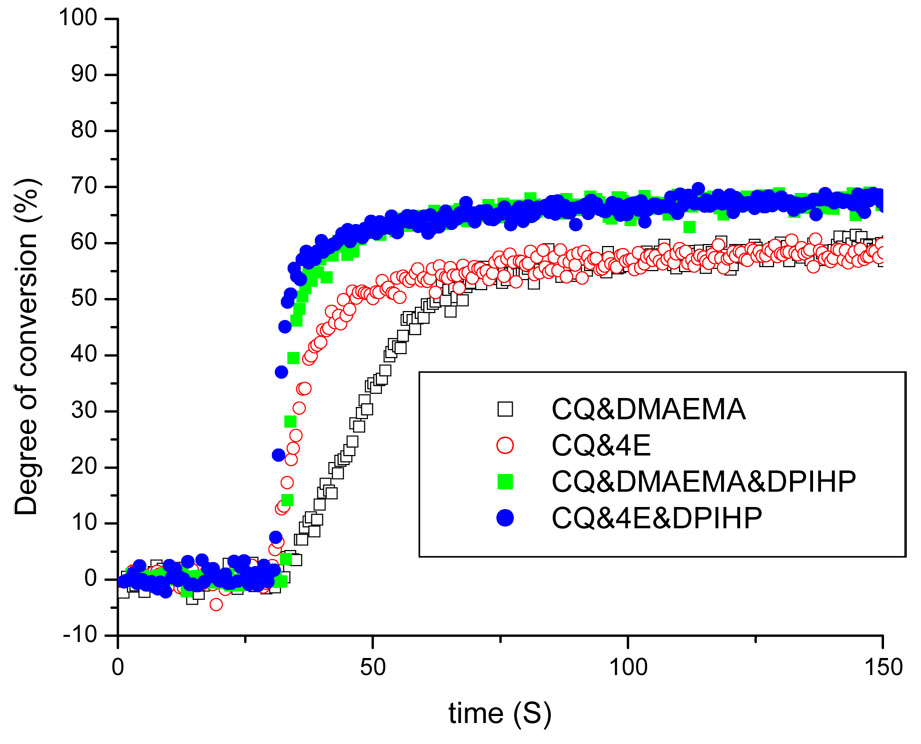
Time-based conversions of systems containing no water, but using the different initiators are shown in Fig. 4. Comparison of the plots in Fig. 4 revealed different photopolymerization kinetics in the presence of the various initiator systems. Conversion (DC) of mixtures containing two-component systems appeared to plateau ~40 s after light initiation. Addition of DPIHP caused conversion to plateau at an earlier time: ~20 s. The DCs of model adhesives containing two-component systems were similar, at approximately 60%. Conversion values (~ 70%) of the three-component systems were significantly higher than those containing only the two-component systems (P < 0.02).
Fig. 4.
Real-time conversion plots of the BisGMA/HEMA model adhesives containing different initiator systems. Curing light exposure duration was 40 s.
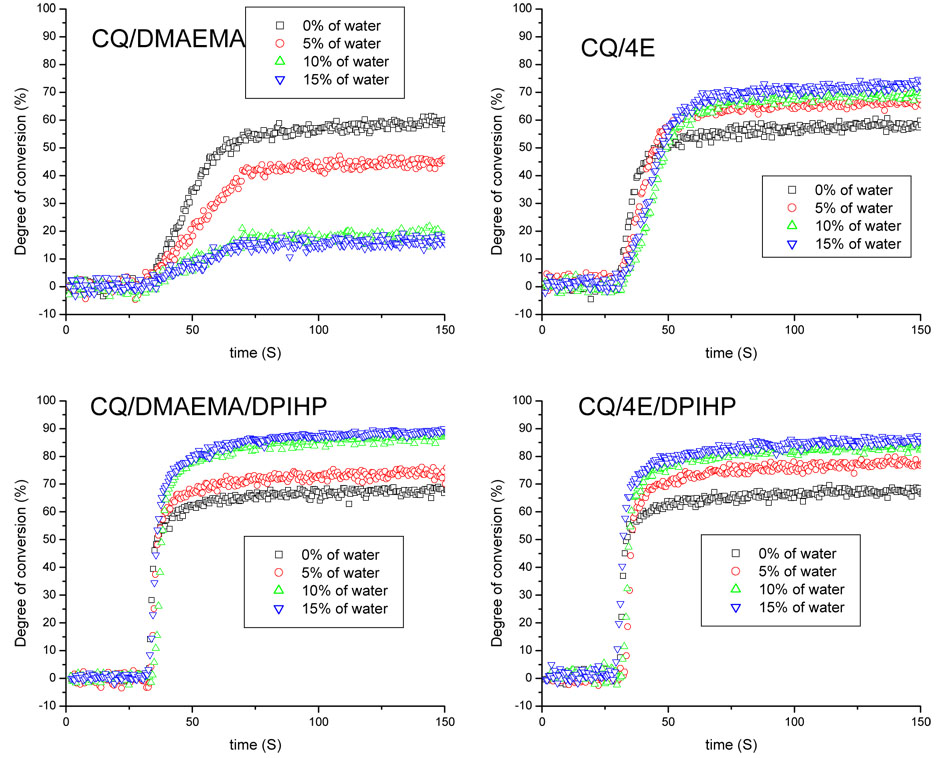
Fig. 5 shows the DC as a function of water content for the four initiator combinations. It can be seen that addition of water has a dramatic effect on both the rate of polymerization and conversion plateau of systems initiated by CQ/DMAEMA: with increasing water content, both the rate and final extent of conversion decreases (Fig. 5A). However, for other three initiator systems, addition of water does not seem to have such a dramatic effect (Figs. 5B–5D). Most of the changes in DC were completed within 60 s after the light activation (Fig. 5). No obvious change in conversion can be observed after 60 s time period.
Fig. 5.
Real-time conversion plots as a function of water content for the BisGMA/HEMA model adhesives containing different initiators. Curing light exposure duration was 40 s.
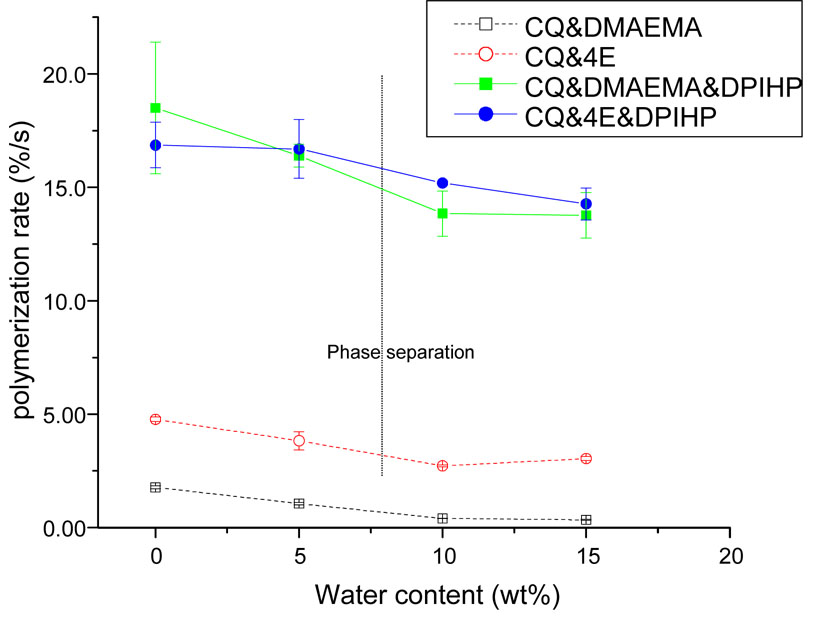
The polymerization rates of formulations using the different photoinitiator systems as a function of water content are shown in Fig. 6. For all the initiator systems, maximal polymerization rates decreased slightly with the increase in water content. The CQ/DMAEMA combination showed the lowest rate with and without the presence of water. The polymerization rate dropped from 1.77±0.07 to 0.34±0.01 %/s when the water content increased from 0 to 15 wt%. At each level of water content, the polymerization rate of CQ/4E was greater that that of CQ/DMAEMA. For example, in the presence of 10 wt% water, the polymerization rate of the CQ/4E system was 2.72±0.05 %/s; however, the value of the CQ/DMAEMA system was 0.40±0.01 %/s. Addition of DPIHP dramatically increased polymerization rates for both of the two-component systems, with or without water present. For example, incorporation of DPIHP into the CQ/DMAEMA system increased the polymerization rate from 1.77±0.07 to 18.5±2.9 %/s without water. In the presence of water (15 wt%), the polymerization rate increased from 0.34±0.01 %/s to 13.8±1.0 %/s. In the CQ/4E system, the rate of polymerization increased from 3.04±0.09 %/s to 14.3±0.7 %/s (in the presence of 15 wt% of water). In mixtures containing only the two-component systems, polymerization rates showed significant difference between CQ/DMAEMA and CQ/4E (p < 0.02) in the presence of different water contents (0 to 15 wt%). For the three-component systems, polymerization rates were not significantly affected by water content (p > 0.1).
Fig. 6.
Polymerization rates of the model adhesives containing different initiator systems and different water content. The black vertical line indicates that the monomers/water phase separation occurs, which is at the presence of ~8 wt% water content. The small vertical bars signify standard deviations (n=3). In mixtures containing only the two-component systems, polymerization rates showed significant difference between CQ/DMAEMA and CQ/4E (p < 0.02) in the presence of different water contents (0 to 15 wt%). For the three-component systems, polymerization rates were not significantly affected by water content (p > 0.1).
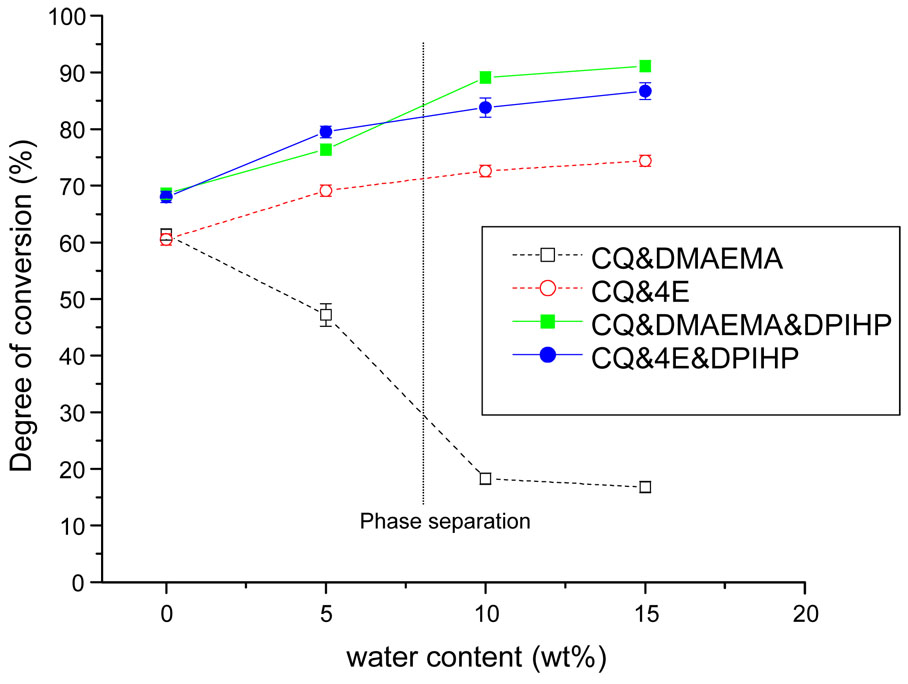
Conversion values observed when using the different photoinitiator systems as a function of water content are shown in Fig. 7. For the two-component systems, conversions of mixtures initiated by CQ/DMAEMA showed a dramatic decrease as a function of water content. For example, the DC dropped from 61.4±1% to 16.8±1% when the water content increased from 0 to 15 wt%. However, when CQ/4E was used, conversion values slightly increased with increasing water content. At each level of water content, the DC of CQ/4E was greater that that of CQ/DMAEMA. For example, in the presence of 10 wt% water, the DC of the CQ/4E system was 72.6±1%; however, the value of the CQ/DMAEMA system was 18.3±1%. Incorporation of DPIHP into the two-component systems dramatically increased conversion as water content increased (p<0.02). The most dramatic improvement in conversion caused by addition of DPIHP was seen in the CQ/DMAEMA system. Mixtures containing only CQ/DMAEMA showed the lowest conversion values in the presence of water. On the contrary, when DPIHP was added to this system, conversion values increased dramatically, and were the highest (91.1±1%) in the presence of 15 wt% water among all the initiator systems.
Fig. 7.
The degree of conversion of the model adhesives containing different initiator systems and different water content. The black vertical line indicates that the monomers/water phase separation occurs, which is at the presence of ~8 wt% water content. The small vertical bars signify standard deviations. Incorporation of DPIHP into the two-component systems dramatically increased conversion as water content increased (p<0.02).
4. Discussion
The results of this study support the two proposed hypotheses that the hydrophilicity/hydrophobicity of photoinitiator system components dramatically affects polymerization of BisGMA/HEMA based resins in the presence of water and addition of DPIHP significantly increase both final conversion and overall conversion rate of all photoinitiator systems.
The results have indicated that the efficacy of the initiator systems is influenced by the compatibility of monomers with initiating components. It was shown that there were dramatic differences in the photopolymerization rate of BisGMA/HEMA mixtures between the two-component CQ/DMAEMA and CQ/4E initiator systems (p<0.02) (Fig. 4,Fig. 6). The solubility in water and BisGMA/HEMA (60/40) mixtures of the components used in the initiator systems are shown in Table 1. Hydrophilic co-initiator DMAEMA appeared not compatible with the relatively hydrophobic BisGMA/HEMA mixture. The polymerization rate of monomers containing hydrophobic 4E co-initiator was higher than that of monomers containing hydrophilic DMAEMA co-initiator. This result is different from that of our previous study on the photopolymerization of HEMA [20]. In that study, it was reported that hydrophilic co-initiators outperformed the hydrophobic co-initiators; and the CQ system containing only hydrophobic coinitiator didn’t even initiate the polymerization of HEMA [20]. These results indicate that the type of initiator components has a big effect on the polymerization of different monomers, and compatibility between monomers and initiators is critical in determining the efficiency of photopolymerization.
The addition of DPIHP to the two-component initiator systems increased the polymerization rate dramatically (Fig. 4, Fig. 6). Although DPIHP is generally hydrophilic due to its ionic nature (Fig. 1), phenyl radical produced by this molecule is relatively hydrophobic. It is expected that with the addition of DPIHP, the compatibility between monomers and initiators will be increased no matter what hydrophobic (i.e., 4E) or hydrophilic (i.e., DMAEMA) co-initiators are used. This is probably the reason why the incorporation of DPIHP into both the two-component systems would increase the rate of polymerization.
It is generally accepted that the quality of adhesives bonding to dentin is closely related to the infiltration and photopolymerization of adhesive monomers. The photopolymerization behaviors (i.e., the reactivity and degree of conversion) of adhesives play a very important role in determining the quality of the interfacial hybrid layer. Water or saliva on the etched dentin surface is omnipresent in the mouth of patients, makes the bonding process very complex and difficult. Especially for adhesives using wet-bonding techniques, the coexistence of adhesive monomers and water is the characteristics during adhesive polymerization process. This requires adhesives should be able to polymerize in the presence of water or saliva. The results of this study indicated that the polymerization rate and DC of adhesives were different in the presence of water when different initiator systems were employed in the formulations.
It was previously reported that the BisGMA/HEMA mixtures underwent micro-phase separation with the increase of water content [14]. The concentration of water required for phase separation of BisGMA/HEMA model adhesives, ranging in composition from 70/30 to 30/70, was investigated [14]. The content of water increased from 5 to 25 wt% as the weight ratio in the BisGMA/HEMA mixtures varied from 30 to 70 wt% HEMA. The model adhesives used in this study consist of 60 wt% of BisGMA and 40 wt% of HEMA. According to the phase diagram [14], when the water content is higher than 8 wt%, there is micro-phase separation in the specimens. Water contents of 5, 10 and 15 wt% were chosen in this study to investigate the effect of water on photopolymerization of model adhesives with and without micro-phase separation.
The type of initiators affects both the polymerization rate and final DC of BisGMA/HEMA mixtures in the presence of water (Fig 5, Fig 6, Fig 7). Among the initiators, the DMAEMA showed the lowest reactivity in the presence of different water contents. The addition of the third component DPIHP to the initiator systems notably increased the efficacy of the photopolymerization at the different water contents. With the presence of water, it was shown that the polymerization rates tended to decrease with the increase of water content (Fig. 6). Generally, the incorporation of water would dilute the concentrations of radicals and monomers, so the polymerization rate would decrease.
The polymerization rate decreasing with the increase of water content does not mean that the DC would also decrease. On the contrary, adhesives containing three initiator systems showed a slightly increased DC while the polymerization rates decreased, except for the CQ/DMAEMA system. The increase of DC in the presence of water may be influenced by other factors. For example, the addition of water may decrease the viscosity of the mixtures, which would provide some benefits to the C=C double-bond conversion.
Among the four initiator systems, the CQ/DMAEMA system showed the lowest DC in the presence of water, and the DC decreased as a function of water content. This may be caused by the difference in hydrophilicity of the two components in the initiator system. Based on the water solubility tests, CQ is relatively hydrophobic and DMAEMA is hydrophilic (Table 1). There are following reasons which might lead to a very lower DC in the presence of water. When BisGMA/HEMA undergoes micro-phase separation, the hydrophilic DMAEMA and hydrophobic CQ tend to exist in the hydrophilic HEMA phase and hydrophobic BisGMA phase, respectively. This decreases the chance for them to contact, so less radicals will be generated. In addition, according to the initiating mechanism [32], the active radicals are on the DMAEMA molecules. These active radicals are hydrophilic, which tend to exist in the hydrophilic HEMA/water phase. Since there were less monomers in the hydrophilic phase during phase separation, this might be the reason why the DC of model adhesives decreased as a function of water content. For the CQ/4E initiator system, both components are hydrophobic. They are present in the BisGMA monomer rich hydrophobic phase, which makes them easier to generate radicals and initiate the polymerization. The increase of the water content will decrease the viscosity which leads to a slight increase of DC.
In the cases of three-component initiator systems, it can be seen that adhesives containing these initiator systems showed the increased DC with the increase of the water content. The remarkable improvement was for the CQ/DMAEMA/DPIHP initiator system. Its counterpart two-component initiator system showed the worst initiating behavior in the presence of water. However, with the addition of DPIHP, it showed the highest DC in the presence of water. In this system, besides the active phenyl hydrophobic radicals in the hydrophobic phase, there still are active hydrophilic DMAEMA radicals in the hydrophilic phase. As a result of the coexistence of both hydrophilic and hydrophobic active radicals, the DC showed a dramatic increase in the presence of water. Same mechanism can be used to explain the high efficiency of CQ/4E/DPIHP initiator system. When the water content was less than 5 wt%, the two CQ containing three-component system showed no significantly different DCs (p>0.1). However, when the water content was higher (10 wt% to 15 wt%), the model adhesives containing CQ/DMAEMA/DPIHP initiator system showed significant higher (p<0.02) DCs than those of model adhesives containing CQ/4E/DPIHP initiator system. One of the reasons was that the CQ/4E/DPIHP initiator system could only generate hydrophobic radicals. When the phase separation occurred, there were less active radicals in the hydrophilic phase.
In summary, the different initiator systems showed quite different initiating behaviors with/without the presence of water. Besides their reactivity, the hydrophilicity/hydrophobicity of the initiator components and water played a critical role in determining the initiating/polymerization behaviors of adhesives. For adhesives containing both hydrophilic HEMA and hydrophobic BisGMA components, where, how many and what kind of radicals could be generated by the initiator system all are very important factors. For adhesive mixtures containing two-component initiator systems, when hydrophobic CQ was used in combination with hydrophilic DMAEMA, the polymerization rates and DCs were dramatically decreased as a function of water content. The polymerization rates and DCs of the hydrophobic CQ/4E combination initiator system were higher than those of the CQ/DMAEMA system in the presence of water. For three-component initiator systems, the incorporation of DPIHP enhanced the polymerization of the mixtures in the presence of water as compared to their counterpart two-component initiators. DPIHP can recycle the CQ, and at the same time, it will generate hydrophobic phenyl radicals, which are very active in initiating the polymerization. In our model adhesives, in the presence of water the monomer rich phase is hydrophobic, so the initiator systems which can generate active hydrophobic radical are more tolerant to water. The optimal initiator system should be able to generate enough both hydrophilic and hydrophobic active radicals in the case of phase separation. Under in-vivo conditions, the photopolymerization is usually performed in the presence of water and/or saliva. Our results indicate that the selection and combination of photoinitiator components should be based on the curing behavior of resin monomers under both dry and wet conditions.
ACKNOWLEDGEMENTS
This work is a contribution from the UMKC Center for Research on Interfacial Structure & Properties (UMKC-CRISP). This investigation was supported by Research Grant: R01DE14392 (PI: Spencer), K25DE015281 (PI: Wang) from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kanca J. Improved bond strength through acid etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123:235–243. doi: 10.14219/jada.archive.1992.0248. [DOI] [PubMed] [Google Scholar]
- 2.Gwinnett AJ. Dentin bond strength after air drying and rewetting. Am J Dent. 1994;7:144–148. [PubMed] [Google Scholar]
- 3.Fusayama T, Nakamura M, Kurosaki N, Iwaku M. Non-presure adhesion of a new adhesive restoration resin. J Dent Res. 2005;58:1364–1370. doi: 10.1177/00220345790580041101. [DOI] [PubMed] [Google Scholar]
- 4.Tay FR, Gwinnett AJ, Wei SHY. An optical, micromorphological study of surface moisture in the acid-conditioned, resin-dentine interface. Am J Dent. 1996;9:43–48. [PubMed] [Google Scholar]
- 5.Tay FR, Gwinnett AJ, Wei SHY. The overwet phenomenon: A scanning electron microscopic study of surface moisture in the acid conditioned, resin-dentine interface. Am J Dent. 1996;9:109–114. [PubMed] [Google Scholar]
- 6.Tay FR, Gwinnett AJ, Wei SHY. The overwet phenomenon: A transmission electron microscopic study of surface moisture in the acid-conditioned, resin-dentine interface. Am J Dent. 1996;9:161–166. [PubMed] [Google Scholar]
- 7.Sauro S, Watson TF, Tay FR, Chersoni S, Breschi L, Bernardi F, Prati C. Water uptake of bonding systems applied on root dentin surfaces: A SEM and confocal microscopic study. Dent Mater. 2006;22:671–680. doi: 10.1016/j.dental.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Tay FR, Pashley DH. Have dentin adhesive become too hydrophilic? J Can Dent Assoc. 2003;69:726–731. [PubMed] [Google Scholar]
- 9.Malacarne J, Carvalho RM, Goes MF, Svizero N, Pashley DH, Tay FR, Yiu CK, Carrilho MRO. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Fong H. effects of water contents and postcuring conditions on BisGMA/TEGDMA dental restorative composite resins. J Appl Polym Sci. 2004;94:492–502. [Google Scholar]
- 11.Ito S, Hasshimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg FA, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 12.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Yiu C, King N, Pashley DH, Suh BI, Carvalho RM, Carrilho M, Tay FR. Effects of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004;25:5789–5796. doi: 10.1016/j.biomaterials.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Spencer P, Wang Y. Adhesive phase separation at dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 15.Miyasaka K, Nakabayashi N. Effect of phenyl-P/HEMA acetone primer on wet bonding to EDTA-conditioned dentin. Dent Mater. 2001;17:499–503. doi: 10.1016/s0109-5641(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 16.Paul SJ, Leach M, Rueggeberg, Pashley DH. Effects of water content on the physical properties of model dentine primer and bonding resins. J Dent. 1999;27:209–214. doi: 10.1016/s0300-5712(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen T, Soderholm KJ. Some effects of water on dentin bonding. Dent Mater. 1995;11:132–136. doi: 10.1016/0109-5641(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Arnold AM, Arnold MA, Williams VD. Measurement of water sorption by resin composite adhesives with near-infrared spectroscopy. J Dent Res. 1992;71:438–442. doi: 10.1177/00220345920710030301. [DOI] [PubMed] [Google Scholar]
- 19.Ogliari FA, de Sordi MLT, Ceschi MA, Petzhold CL, Demarco FF, Piva E. 2,3-epithiopropyl methacrylate as functionalized monomer in a dental adhesive. J Dent. 2006;34:472–477. doi: 10.1016/j.jdent.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Spencer P, Yao X, Ye Q. Effect of coinitiator and water on the photoreactivity and photopolymerization of HEMA/camphoquinone-based reactant mixtures. J Biomed Mater Res A. 2006;78A:721–728. doi: 10.1002/jbm.a.30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanehira M, Finger WJ, Hoffmann M, Endo T, Komatsu M. Relationship between degree of polymerization and enamel bonding strength with self-etching adhesives. J Adhes Dent. 2006;8:211–216. [PubMed] [Google Scholar]
- 22.Dickens SH, Cho BH. Interpretation of bond failure through conversion and residual solvent measurements and Weibull analyses of flexural and microtensile bond strengths of bonding agents. Dent Mater. 2005;21:354–364. doi: 10.1016/j.dental.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Van Landuyt KL, Peumans M, De Munck J, Lambrechts P, Van Meerbeek B. Extension of a one-step self-etch adhesive into a multi-step adhesive. Dent Mater. 2006;22:533–544. doi: 10.1016/j.dental.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, Inoue S, Peumans M, Suzuki K, Lambrechts P, Van Meerbeek B. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005;84:183–188. doi: 10.1177/154405910508400214. [DOI] [PubMed] [Google Scholar]
- 25.Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. Interfacial chemistry of the dentin/adhesive bond. J Dent Res. 2000;79:1458–1463. doi: 10.1177/00220345000790070501. [DOI] [PubMed] [Google Scholar]
- 26.Tay FR, Gwinnett JA, Wei SHY. Micromorphological spectrum from overdrying to overwetting acid-conditioned dentin in water-free, acetone-based, single-bottle primer adhesives. Dent Mater. 1996;12:236–244. doi: 10.1016/s0109-5641(96)80029-7. [DOI] [PubMed] [Google Scholar]
- 27.Tay FR, Pashley DH. Water treeing - a potential mechanism for degradation of dentin adhesive. Am J Dent. 2003;16:6–12. [PubMed] [Google Scholar]
- 28.Hashimoto M, Ito S, Tay FR, Svizero NR, Sano H, Kaga M, Pashley DH. Fluid movement across the resin-dentin interface during and after bonding. J Dent Res. 2004;83:843–848. doi: 10.1177/154405910408301104. [DOI] [PubMed] [Google Scholar]
- 29.Chersoni S, Sippa P, Breschi L, Ferrari, Tay FR, Pashley DH, Prati Carlo. Water movement in the hybrid layer after different dentin treatment. Dent Mater. 2004;20:796–803. doi: 10.1016/j.dental.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Ye Q, Wang Y, Williams K, Spencer P. Characterization of photopolymerization of dentin adhesives as a function of light source and irradiance. J Biomed Mater Res B Appl Biomater. 2007;80:440–446. doi: 10.1002/jbm.b.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties, and structure in model dentin adhesives. J Biomed Mater Res A. 2007;80:342–350. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padon KS, Scranton AB. A mechanistic investigation of a three-component radical photoinitiator system comprising methylene blue, n-methyldiethanolamine, and diphenyliodonium chloride. J Polym Sci A Polym Chem. 2000;38:2057–2066. [Google Scholar]