Abstract
Episodic memory is widely conceived as a fundamentally constructive, rather than reproductive, process that is prone to various kinds of errors and illusions. With a view towards examining the functions served by a constructive episodic memory system, we consider recent neuropsychological and neuroimaging studies indicating that some types of memory distortions reflect the operation of adaptive processes. An important function of a constructive episodic memory is to allow individuals to simulate or imagine future episodes, happenings and scenarios. Since the future is not an exact repetition of the past, simulation of future episodes requires a system that can draw on the past in a manner that flexibly extracts and recombines elements of previous experiences. Consistent with this constructive episodic simulation hypothesis, we consider cognitive, neuropsychological and neuroimaging evidence showing that there is considerable overlap in the psychological and neural processes involved in remembering the past and imagining the future.
Keywords: constructive memory, false recognition, mental simulation, neuroimaging, amnesia, Alzheimer's disease,
1. Introduction
The analysis of human memory comprises a variety of approaches, conceptual frameworks, theoretical ideas and empirical findings. Despite the wealth of contrasting and sometimes conflicting ideas, there are some basic observations on which memory researchers can agree. One of the least controversial—but most important—observations is that memory is not perfect. Instead, memory is prone to various kinds of errors, illusions and distortions. For instance, it has been proposed that memory's imperfections can be classified into seven basic categories or ‘sins’ (Schacter 1999, 2001). Each of the memory sins has important practical implications, ranging from annoying everyday instances of absent-minded forgetting to misattributions and suggestibility that can distort eyewitness identifications. But for memory researchers, such imperfections are most important because they provide critical evidence for the fundamental idea that memory is not a literal reproduction of the past, but rather is a constructive process in which bits and pieces of information from various sources are pulled together; memory errors are thought to reflect the operation of specific components of this constructive process. This characterization of memory dates at least to the pioneering ideas of Bartlett (1932) and has been a major influence in contemporary cognitive psychology for nearly 40 years.
The situation is rather different when we turn to cognitive neuroscience approaches, which attempt to elucidate the neural underpinnings of memory. Here, sustained interest in constructive aspects of memory has developed only more recently. Such interest has been driven mainly by observations concerning the memory distortion known as confabulation, in which patients with damage to various regions within prefrontal cortex and related regions produce vivid but highly inaccurate ‘recollections’ of events that never happened (e.g. Johnson 1991; Moscovitch 1995; Burgess & Shallice 1996; Dalla Barba et al. 1999; Schnider 2003; Moulin et al. 2005). During the past decade, investigations of memory distortions in other patient populations, as well as neuroimaging studies of accurate versus inaccurate remembering in healthy individuals, have contributed to an increase in research on the cognitive neuroscience of constructive memory (for reviews, see Schacter et al. 1998a; Schacter & Slotnick 2004).
In the present paper, we focus on episodic memory, the system that enables people to recollect past experiences (Tulving 1983, 2002). We consider some recent work concerning the neural basis of memory construction with a view to addressing a question concerning its function: why does memory involve a constructive process of piecing together bits and pieces of information, rather than something more akin to a replay of the past? Several researchers have grappled with this issue and proposed various reasons why human memory, in contrast to video recorders or computers, does not store and retrieve exact replicas of experience (e.g. Bjork & Bjork 1988; Anderson & Schooler 1991; Schacter 1999, 2001). We focus on one hypothesis concerning the origins of a constructive episodic memory: that an important function of this type of memory is to allow individuals to simulate or imagine future episodes, happenings and scenarios. As we discuss later, a number of investigators have recently articulated a broad view of memory that not only considers the ability of individuals to re-experience past events, but also focuses on the capacity to imagine, simulate or pre-experience episodes in the future (Tulving 1983, 2002, 2005; Suddendorf & Corballis 1997; Atance & O'Neill 2001, 2005; Klein & Loftus 2002; Suddendorf & Busby 2003, 2005; D'Argembeau & Van der Linden 2004; Dudai & Carruthers 2005; Hancock 2005; Buckner & Carroll 2007; Schacter & Addis 2007). This latter ability has been referred to by such terms as prospection (Gilbert 2006; Buckner & Carroll 2007) and episodic future thinking (Atance & O'Neill 2001, 2005). Since the future is not an exact repetition of the past, simulation of future episodes may require a system that can draw on the past in a manner that flexibly extracts and recombines elements of previous experiences—a constructive rather than a reproductive system. If this idea has merit, then there should be considerable overlap in the psychological and neural processes involved in remembering the past and imagining the future. We consider some recent cognitive, neuropsychological and neuroimaging evidence that is consistent with this hypothesis.
2. Constructive memory: from cognitive psychology to cognitive neuroscience
Any discussion of constructive memory must acknowledge the pioneering ideas of Bartlett (1932), who rejected the notion that memory involves a passive replay of a past experience via the awakening of a literal copy of experience. Although Bartlett did not advocate the extreme position sometimes ascribed to him that memory is always inaccurate (Ost & Costall 2002), he clearly rejected the importance of reproductive memory: ‘the first notion to get rid of is that memory is primarily or literally reduplicative, or reproductive. In a world of constantly changing environment, literal recall is extraordinarily unimportant…if we consider evidence rather than supposition, memory appears to be far more decisively an affair of construction rather than one of mere reproduction’ (Bartlett 1932, pp. 204–205). Bartlett emphasized the dependence of remembering on schemas, which he defined as ‘an active organization of past reactions, or of past experiences’ (p. 201). Though usually adaptive for the organism, the fact that remembering relies heavily on construction via a schema also has a downside: ‘condensation, elaboration and invention are common features or ordinary remembering, and these all very often involve the mingling of materials belonging originally to different ‘schemata’’ (p. 205).
Bartlett's (1932) ideas have influenced countless modern attempts to conceive of memory as a constructive rather than a reproductive process. For example, Schacter et al. (1998a) described a ‘constructive memory framework’ that links ideas about memory construction from cognitive psychology with various brain systems. Schacter et al. noted evidence supporting the idea that representations of new experiences should be conceptualized as patterns of features in which different features represent different facets of encoded experience, including outputs of perceptual systems that analyse specific physical attributes of incoming information and interpretation of these attributes by conceptual or semantic systems analogous to Bartlett's schemas. In this view, constituent features of a memory are distributed widely across different parts of the brain, such that no single location contains a literal trace or engram that corresponds to a specific experience (cf. Squire et al. 2004; Thompson 2005). Retrieval of a past experience involves a process of pattern completion (Marr 1971; McClelland et al. 1995; Norman & O'Reilly 2003), in which the rememberer pieces together some subset of distributed features that comprise a particular past experience, including perceptual and conceptual/interpretive elements.
Since a constructive memory system is prone to error, it must solve many problems to produce sufficiently accurate representations of past experience. For example, the disparate features that constitute an episode must be linked or bound together at encoding; failure to adequately bind together appropriate features can result in the common phenomenon of source memory failure, where people retrieve fragments of an episode but do not recollect, or misrecollect, how or when the fragments were acquired, resulting in various kinds of memory illusions and distortions (e.g. Johnson et al. 1993; Schacter 1999). Furthermore, bound episodes must be kept separate from one another in memory: if episodes overlap extensively with one another, individuals may recall the general similarities or gist (Brainerd & Reyna 2005) common to many episodes, but fail to remember distinctive item-specific information that distinguishes one episode from another, resulting in the kinds of gist-based distortions that Bartlett (1932) and many others have reported. Similarly, retrieval cues can potentially match stored experiences other than the sought-after episode, thus resulting in inaccurate memories that blend elements of different experiences (McClelland 1995), so retrieval often involves a preliminary stage in which the rememberer forms a more refined description of the characteristics of the episode to be retrieved (Burgess & Shallice 1996; Norman & Schacter 1996). Breakdowns in this process of formulating a retrieval description as a result of damage to the frontal cortex and other regions can sometimes produce striking memory errors, including confabulations regarding events that never happened (e.g. Burgess & Shallice 1996; Dab et al. 1999; Ciaramelli et al. 2006; Gilboa et al. 2006).
During the past decade, research in cognitive neuroscience has made use of neuroimaging and neuropsychological approaches to address questions concerning memory errors and distortions that bear on constructive aspects of memory (for a review, see Schacter & Slotnick 2004). We do not attempt an exhaustive review here, but instead focus on two lines of research that are most relevant to our broader claims regarding a possible functional basis for constructive aspects of memory. First, we will consider research concerning false recognition in patients with memory disorders that provides evidence indicating that false recognition – rather than reflecting the operation of a malfunctioning or flawed memory system – is sometimes a marker of a healthy memory system, such that damage to the system can reduce, rather than increase, the incidence of this memory error. Second, we consider neuroimaging studies that provide insight into the extent to which accurate and inaccurate memories depend on the same underlying brain regions. A growing body of evidence indicates that there is indeed extensive overlap in the brain regions that support true and false memories, at least when false memories are based on what we refer to as general similarity or gist information.
3. False recognition in amnesia and dementia
As noted earlier, patients with damage to regions of prefrontal cortex and related brain areas sometimes exhibit the memory distortion known as confabulation. Such patients also sometimes show pathological levels of false recognition, claiming incorrectly that novel information is familiar (e.g. Delbecq-Derouesné et al. 1990; Schacter et al. 1996a; Ward et al. 1999). The fact that brain damage can increase the incidence of memory distortion leads naturally to the view that recollective errors reflect the operation of a diseased or malfunctioning system. By contrast, however, two related lines of research that have emerged during the past decade indicate that some types of memory distortion reflect the adaptive operation of a healthy memory system. These studies of amnesic and demented patients have examined the incidence of robust false recognition effects, in which healthy people exhibit high levels of false alarms after studying a series of semantically or perceptually related words or pictures. For example, in the Deese–Roediger–McDermott (DRM) paradigm (Deese 1959; Roediger & McDermott 1995), participants study lists of words (e.g. tired, bed, awake, rest, dream, night, blanket, doze, slumber, snore, pillow, peace, yawn and drowsy) that are related to a non-presented lure word (e.g. sleep). On a subsequent old–new recognition test containing studied words (e.g. tired and dream), new words that are unrelated to the study list items (e.g. butter) and new words that are related to the study list items (e.g. sleep), participants frequently claim that they previously studied the related lure words. In many instances, false recognition of the related lure words is indistinguishable from the true recognition rate of studied words (for review of numerous DRM studies, see Gallo 2006).
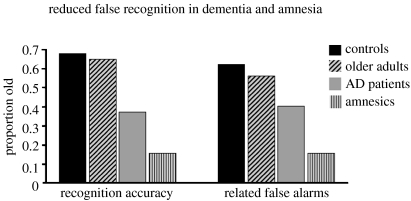
A number of studies have consistently revealed that amnesic patients with damage to the hippocampus and related structures in the medial temporal lobe (MTL) show significantly reduced false recognition of non-studied lure words that are either semantically or perceptually related to previously studied words (figure 1; Schacter et al. 1996c, 1997, 1998b; Melo et al. 1999; Ciaramelli et al. 2006). This false recognition ‘deficit’ roughly parallels patients' true recognition deficit and occurs even though amnesics typically show similar or even increased levels of false recognition to unrelated lure words. Amnesics also show reduced false recognition of non-studied visual shapes that are perceptually similar to previously presented shapes (Koutstaal et al. 1999). Parallel studies have been reported in patients with Alzheimer's disease (AD), who typically have neuropathology that includes, but is not limited to, MTL regions. Like amnesics, AD patients show reduced false recognition of lure items that are either semantically or perceptually related to previously studied items (Balota et al. 1999; Budson et al. 2000, 2001, 2003).
Figure 1.
Performance of patients with amnesia and Alzheimer's disease on the Deese–Roediger–McDermott (DRM) paradigm (Roediger & McDermott 1995). Participants study lists of words (e.g. tired, bed, awake, rest, dream, night, etc.) that are related to a non-presented lure word (e.g. sleep). A subsequent old–new recognition test contains studied words (e.g. tired, dream), new words that are unrelated to the study list items (e.g. butter) and new words that are related to the study list items (e.g. sleep). Both patient groups show significantly reduced recognition accuracy (i.e. hits—false alarms to new unrelated words) and also make fewer related false alarms (i.e. false alarms to new related words—false alarms to new unrelated words) relative to age-matched controls. Note that the ‘controls’ were the age-matched control group for the amnesic patients (data for controls and amnesics are obtained from Schacter et al. 1996c) and the ‘older adults’ were the age-matched control group for Alzheimer's patients (data for older adults and Alzheimer's patients are obtained from Budson et al. 2000). AD, Alzheimer's disease.
One interpretation of this pattern of results is that healthy controls form and retain a well-organized representation of the semantic or perceptual gist of a list of related study items. Related lures that match semantic or perceptual features of this representation are likely to be falsely recognized, while unrelated words that do not match it are likely to be correctly rejected. As a result of MTL damage, amnesic and AD patients may form and retain only a weak or degraded gist representation and thus make fewer false alarms to semantic associates or perceptually similar items than do controls. Support for this interpretation comes from a study that used a modified version of the DRM semantic associates procedure (Verfaellie et al. 2002). Participants were instructed to call ‘old’ any item that is semantically related to the theme or gist of a previously studied list, even if the item itself had not appeared on the list. Evidence from the healthy controls suggests that such a task provides a more direct probe of gist information than a standard old/new recognition task (Brainerd & Reyna 1998; Schacter et al. 2001). Verfaellie et al. (2002) reported that even in this meaning test, amnesic patients provided fewer ‘old’ responses to semantically related lure words than do controls, thereby supporting the idea of a degraded gist representation. Budson et al. (2006) reported similar results in patients with AD, using a paradigm in which participants studied categorized pictures and were given a version of a ‘meaning test’ in which they were instructed to respond ‘yes’, when either a studied or non-studied picture came from a studied category.
In the foregoing studies, involving meaning tests, participants were asked to remember explicitly aspects of previously presented materials; it is well known that both amnesic and AD patients exhibit deficits on explicit memory tasks. Thus, it is conceivable that patients do form and retain a normal gist representation, but do not express this information on explicit tests. Since amnesic patients can show intact priming effects on various implicit or indirect memory tasks (for review, see Schacter et al. 2004), Verfaellie et al. (2005) examined whether use of an implicit task might reveal intact retention of gist information in amnesics. They did so by having patients and controls study lists of semantic associates (e.g. resort, sun, beach, parties, etc.) that were all associated to a non-presented related lure word (e.g. vacation). On the subsequent stem completion test, participants were provided three-letter word beginnings that had multiple possible completions; some could be completed with previously studied words (e.g. bea___) and some with related lures (e.g. vac___). Previous research using a similar paradigm with healthy subjects revealed the existence of a ‘false priming’ effect: compared with a baseline condition, participants were more likely to complete stems of related lures with the lure item following study of a list of semantic associates (not surprisingly, priming was also observed for previously studied words, e.g. McDermott 1997; McKone & Murphy 2000). Verfaellie et al. reported that amnesic patients showed intact priming for previously studied words, replicating earlier results, but showed no priming for related lures. By contrast, controls showed significant priming for both studied words and related lure words.
These results further strengthen the idea that impaired false recognition of similar words and objects in amnesic and AD patients reflects an impoverished or diminished gist representation, while suggesting that the deficit extends beyond the strict confines of episodic memory. They also support the idea that this type of memory error in control populations reflects the normal operation of healthy adaptive memory processes. This latter conclusion is also supported by the results of functional neuroimaging studies.
4. Neuroimaging studies of true and false recognition
In a number of studies using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), subjects studied lists of DRM semantic associates and were later scanned while making judgements about old words, related lures and unrelated lures. Consistent with the results from amnesic and AD patients, these studies have revealed significant and comparable levels of activation in the MTL, including the hippocampus, during both true and false recognition of related lures (e.g. Schacter et al. 1996b; Cabeza et al. 2001; for more detailed review, see Schacter & Slotnick 2004).
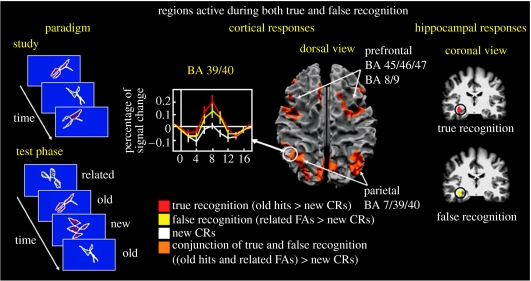
More recent neuroimaging studies of gist-based false recognition using paradigms other than the DRM procedure have replicated and extended these results. Slotnick & Schacter (2004) used a prototype recognition paradigm in which the critical materials were abstract, unfamiliar shapes; all shapes in the study list were visually similar to a non-presented prototype (figure 2). Participants made significantly more ‘old’ responses to studied shapes than to new related shapes and also made significantly more ‘old’ responses to new related shapes (i.e. prototypes) than to new unrelated shapes. This latter result confirms the presence of a false recognition effect that was presumably driven by memory for the ‘perceptual gist’ of the studied exemplars that resembled the prototype. Slotnick & Schacter documented that a number of regions previously implicated in true recognition, including MTL, fusiform cortex, lateral parietal cortex and multiple regions in dorsolateral and inferior prefrontal cortex, showed significant and comparable levels of activity during false recognition of new related shapes and true recognition of studied shapes (figure 2).
Figure 2.
Neural regions engaged during both true and false recognition (adapted from Slotnick & Schacter 2004). A prototype recognition paradigm was employed; all stimuli presented during study were abstract, unfamiliar shapes. During recognition testing, participants made recognition judgements about old studied shapes, new prototypical shapes visually related to studied shapes and new shapes unrelated to studied shapes. A number of regions previously implicated in true recognition, including hippocampus, lateral parietal cortex, and dorsolateral and inferior prefrontal cortex, showed significant and comparable levels of activity during false recognition of new related shapes (i.e. prototypes) and true recognition of studied shapes compared with correct rejections of new unrelated shapes. The percentage of signal changed extracted from the left lateral parietal cortex is also shown. BA, Brodmann area; CR, correct rejection; FA, false alarm.
Garoff-Eaton et al. (2006) also used abstract shapes as target items in a slightly different experimental paradigm that focused on the relationship between processes underlying related and unrelated false recognition. In both types of false recognition, subjects respond ‘old’ to new items. However, in related false recognition, semantic or perceptual overlap between the new item and a previously studied item drives the false recognition response, whereas the basis for ‘old’ response to unrelated items is unclear. Standard signal detection models of memory typically do not distinguish between related and unrelated false alarms: both are seen to result from a single underlying process that supports familiarity or memory strength sufficient to surpass a subject's criterion for saying ‘old’ (e.g. Miller & Wolford 1999; Slotnick & Dodson 2005; but see, Wixted & Stretch 2000). However, data from studies of false recognition in amnesic patients reviewed earlier point towards different mechanisms underlying related and unrelated false recognition, because amnesics typically show reduced related false recognition compared with controls, together with either increased or unchanged unrelated false recognition.
In the experiment by Garoff-Eaton et al. (2006), subjects studied abstract shapes drawn from the same set as those developed by Slotnick & Schacter (2004). On a subsequent recognition test, they were presented either with the same shape from the study list, a related shape that was visually similar to one of the studied shapes or a new unrelated shape. Participants were instructed to respond ‘same’ when a test shape was identical to a previously studied shape, ‘similar’ when a new shape was visually similar to a previously studied one and ‘new’ to unrelated novel shapes. Behavioural data revealed significantly more ‘same’ responses (0.59) to same shapes than to either new related or new unrelated shapes, and significantly more ‘same’ responses to related (0.31) than to unrelated (0.20) shapes. A conjunction analysis of the fMRI data that assessed common neural activity during true recognition (i.e. ‘same’/same) and related false recognition (i.e. ‘same’/related new) compared with unrelated false recognition (i.e. ‘same’/new) indicated significant activity in a network of regions previously associated with episodic remembering, including hippocampus/MTL, several regions within prefrontal cortex, medial and inferior parietal lobes and ventral temporal/occipital regions. In striking contrast, a conjunction analysis that assessed common activity during related and unrelated false recognition, in comparison with true recognition, showed no significant activity in any region. When contrasting unrelated false recognition with true recognition and related false recognition, significant activity was observed in regions of left superior and middle temporal gyri (BA 22/38), regions previously associated with language processing. Unrelated false recognition may have occurred when subjects mistakenly applied a verbal label generated during the study list to a novel shape, whereas related false recognition was driven largely by perceptual similarity between studied shapes and related new shapes.
Overall, these data strengthen the argument that related or gist-based false recognition depends on many of the same neural processes as true recognition and shares relatively little in common with unrelated false recognition. Of course, we do not wish to imply that gist-based false recognition is neurally indistinguishable from true recognition. A number of PET and fMRI studies have provided evidence that brain activity can distinguish between true recognition and related false recognition (for review, see Schacter & Slotnick 2004). Some of these studies have supported what Schacter & Slotnick (2004) termed the sensory reactivation hypothesis, which holds that true memories contain more sensory and perceptual details than do related false memories (e.g. Mather et al. 1997; Norman & Schacter 1997). Slotnick & Schacter (2004; see also Kahn et al. 2004) provided some of the strongest evidence for this hypothesis: they showed increased activation in early visual areas, when subjects made recognition decisions about previously studied shapes compared with related new shapes. Interestingly, this early visual area activity for old shapes occurred equally strongly when subjects responded ‘old’ and when they responded ‘new’ to the studied shapes, suggesting that this putative sensory reactivation effect reflected some type of non-conscious or implicit memory (Slotnick & Schacter 2004; for further evidence, see Slotnick & Schacter 2006).
In summary, both neuropsychological and neuroimaging studies of gist-based false recognition support the idea that this type of memory error reflects, to a very large extent, the healthy operation of constructive processes that support the ability to remember what has actually happened in the past.
5. Remembering the past and imagining the future: what kind of overlap?
The foregoing research provides not only insights into the constructive nature of episodic memory, but also some clues regarding the functional basis of constructive memory processes. Although memory errors such as false recognition may at first seem highly dysfunctional, especially given the havoc that memory distortions can wreak in real-world contexts (Loftus 1993; Schacter 2001), we have seen that they sometimes reflect the ability of a normally functioning memory system to store and retrieve general similarity or gist information, and that false recognition errors often recruit some of the same processes that support accurate memory decisions. Indeed, several researchers have argued that the memory errors involving forgetting or distortion serve an adaptive role (cf. Bjork & Bjork 1988; Anderson & Schooler 1991; Schacter 1999, 2001). For example, Anderson & Schooler (1991) contend that memory is adapted to retain information that is most likely to be needed in the environment in which it operates. Since we do not frequently need to remember all the exact details of our experiences, an adapted system need not slavishly preserve all such details as a default option; instead, it should record and preserve such details over time only when circumstances indicate that they are likely to be needed, as human memory tends to do. Similarly, memory for gist, which is sometimes responsible for false recognition, is also crucial for such adaptive capacities as categorization and comprehension and may facilitate transfer and generalization across tasks (McClelland 1995).
We attempt to build on this type of argument by suggesting that the constructive nature of episodic memory is highly adaptive for performing a major function of this system: to draw on past experiences in a way that allows us to imagine and simulate episodes that might occur in our personal futures. Thinking about the future plays a critical role in mental life (Gilbert 2006), and students of brain function have long recognized the important role of frontal cortex in allowing individuals to anticipate or plan for the future (e.g. Ingvar 1985; Stuss & Benson 1986; Fuster 1989; Shallice & Burgess 1996; Mesulam 2002). Tulving (1983, 2002, 2005) has argued that episodic memory affords the ability to engage in ‘mental time travel’, which involves projecting oneself into both the past and the future. From this perspective, representations of both past and future events may be richly detailed, vivid and contextually specific. Furthermore, a number of investigators have recognized that information about past experiences is useful only to the extent that it allows us to anticipate what may happen in the future (e.g. Atance & O'Neill 2001, 2005; Suddendorf & Busby 2003, 2005; Hancock 2005; Buckner & Carroll 2007). Indeed, Anderson & Schooler's (1991) analysis of adaptive forgetting supports the idea that information about the past is retained when it is likely to be useful in the future.
However, future events are rarely, if ever, exact replicas of past events. Thus, a memory system that simply stored rote records of what happened in the past would not be well suited to simulating future events, which will probably share some similarities with past events while differing in other respects. We think that a system built along the lines of the constructive principles that we and others have attributed to episodic memory is better suited to the job of simulating future happenings. Such a system can draw on elements of the past and retain the general sense or gist of what has happened. Critically, it can flexibly extract, recombine and reassemble these elements in a way that allows us to simulate, imagine or ‘pre-experience’ (Atance & O'Neill 2001) events that have never occurred previously in the exact form in which we imagine them. We will refer to this idea as the constructive episodic simulation hypothesis: the constructive nature of episodic memory is attributable, at least in part, to the role of the episodic system in allowing us to mentally simulate our personal futures (for similar perspectives, see Suddendorf & Corballis 1997; Suddendorf & Busby 2003; Dudai & Carruthers 2005).
The constructive episodic simulation hypothesis does not imply that the only function of episodic memory is to allow us to simulate future events, nor do we believe that its role in simulation of the future constitutes the sole reason why episodic memory is primarily constructive rather than reproductive. Episodic memory also functions to help us make sense of the past and the present. Furthermore, considerations such as economy of storage are no doubt relevant to understanding why the system does not simply preserve rote records of all experience: compressing information into a gist-like representation may protect the memory system from overload (Schacter 2001). We nonetheless endorse Suddendorf & Busby's (2003, p. 393) suggestion that ‘episodic reconstruction is just an adaptive feature of the future planning system’. Moreover, exploring the possible link between constructive aspects of memory and simulation of the future may help to provide fresh perspectives on such fundamental questions as why imagination is sometimes confused with memory and, more generally, why memories can be badly mistaken.
If the constructive episodic simulation hypothesis has merit, then remembering the past and imagining the future should show a number of similar characteristics and depend on some of the same neural substrates. We next consider cognitive, neuropsychological, psychopathological and neuroimaging data that bear on this hypothesis.
6. Cognitive studies of past and future events
In contrast to the extensive cognitive literature on episodic memory of past experiences, there is little evidence concerning simulation of future episodes and a virtual absence of direct comparisons between remembering the past and imagining the future. While there has been a great deal of research concerning prospective memory—remembering to do things in the future (e.g. Brandimonte et al. 1996)—this line of research has been concerned with such topics as the formulation and retention of intentions (e.g. Goschke & Kuhl 1993) or differences between event-based versus time-based prospective memory (e.g. Einstein & McDaniel 1990) and has not focused specifically on episodic simulation and imagining of future events. Research on the topic of affective forecasting—which examines how people predict, and often mispredict, future happiness (Gilbert 2006)—has revealed important interactions between memory of past events and predictions of future happiness. For example, Morewedge et al. (2005) found that people sometimes base predictions of future happiness on atypical past experiences that are highly memorable but not highly predictive of what is likely to occur in the future.
More directly related to the constructive episodic simulation hypothesis, D'Argembeau & Van der Linden (2004) directly compared ‘re-experiencing’ past episodes and ‘pre-experiencing’ episodes in the future. They investigated how the valence of events and their temporal distance from the present affect phenomenological qualities of past and future autobiographical events. Subjects were asked to either remember a specific event from their past or imagine a specific event that could plausibly happen to them in the future. For each of several past and future events that participants provided, they rated a number of phenomenological qualities using a variant of the memory characteristics questionnaire (Johnson et al. 1988), including perceptual details, valence and intensity of emotions involved, and clarity of spatial information. Participants also indicated the nature of their visual perspective on the event: observer (i.e. they ‘saw’ themselves in their representation of the event) or field (i.e. they saw the scene from their own perspective). Subjects were also asked to date past events and estimate the temporal proximity of future events.
D'Argembeau and van der Linden found that remembered past events were associated with richer and more vivid sensory and contextual details than were imagined future events, consistent with previous observations concerning phenomenological qualities of remembered versus imagined events (e.g. Johnson et al. 1988). Importantly, however, they also reported several notable commonalities between remembering the past and imagining the future. When compared with negative events, positive events were associated with subjective ratings of greater re-experiencing for past events and greater pre-experiencing for future events. Temporally close events in either the past or future included more sensory and contextual details, and were associated with greater feelings of re-experiencing and pre-experiencing, than temporally distant events (cf. Trope & Liberman 2003). Furthermore, participants were more likely to adopt a field than observer perspective for temporally close than temporally distant events in both the past and the future. More recently, D'Argembeau & Van der Linden (2006) extended these results by showing that individual differences in imagery ability and emotion regulation strategies are similarly related to past and future events. Overall, these results are consistent with the constructive episodic simulation hypothesis inasmuch as they highlight strong similarities between remembering the past and imagining the future.
7. Past and future events in Neuropsychological and psychopathological patients
It is well known that patients with damage to the hippocampus and related structures in the MTL have impairments of episodic memory (e.g. Squire et al. 2004). Much less is known about the capacity of amnesic patients to imagine future experiences. However, consistent with the constructive episodic simulation hypothesis, the existing evidence indicates that at least some amnesics have great difficulty imagining their personal futures. Some early observations along these lines were reported concerning patient K. C., who suffered from total loss of episodic memory as a result of closed head injury that produced damage to a number of brain regions, including the medial temporal and frontal lobes (Tulving et al. 1988; Rosenbaum et al. 2005). K. C. was unable to provide a description of his personal future for any time period asked about: ‘this afternoon’; ‘tomorrow’; or ‘next summer’. Instead, K. C. provided the same response when asked to think about any part of his personal future or past, describing his mental state as ‘blank’ (Tulving 1985; Tulving et al. 1988).
A later investigation in another patient, D. B., who became amnesic as a result of cardiac arrest and consequent anoxia revealed that he, like K. C., exhibited deficits in both retrieving past events and imagining future events (Klein & Loftus 2002). Klein and Loftus developed a 10-item questionnaire in which they probed past and future events that were matched for temporal distance from the present (e.g. What did you do yesterday? What are you going to do tomorrow?). One problem with assessing responses to questions about the personal future is that it is not entirely clear what constitutes a correct answer. Klein and Loftus evaluated D. B.'s responses in light of information provided by his family. Thus, when D. B. was asked ‘When will be the next time you see a doctor?’, his response (‘Sometime in the next week’) was judged correct because his daughter confirmed that he did have a doctors' appointment the next week. However, when D. B. was asked ‘Who are you going to see this evening?’, and indicated that he was going to visit his mother, this response was judged to be confabulatory because his mother had died nearly two decades earlier.
D. B. was highly impaired on both the past and future versions of this task. In fact, he provided only 2 of 10 responses on the future task that were judged correct by family members, providing five confabulatory responses and three ‘don't know’ responses to the other items. Control subjects provided correct responses to all questions regarding their personal pasts and futures. D. B.'s deficit in thinking about the future seemed specific to his personal future: he had little difficulty imagining possible future developments in the public domain (e.g. political events and issues), performing similar to control subjects. Note, however, that many of the items concerning the public domain did not inquire about specific events, so the evidence for a personal/public distinction is somewhat equivocal. Moreover, little information was provided concerning the precise location of D. B.'s lesion.
A more recent study by Hassabis et al. (2007) examined the ability of five patients with documented bilateral hippocampal amnesia to imagine new experiences. Patients and matched control subjects were cued to construct everyday imaginary experiences such as ‘Imagine you are lying on a white sandy beach in a beautiful tropical bay’. Subjects were specifically instructed not to provide a memory of a past event, but to construct something new. Participants described their imaginary scenarios in the presence of a cue card to remind them of the task, and experimenters occasionally probed subjects for further details and elaboration. Protocols were scored based on the content, spatial coherence and subjective qualities of the participants' imagined scenarios. Overall, the constructions of the hippocampal patients were greatly reduced in richness and content when compared with those of controls. The impairment was especially pronounced for the measure of spatial coherence, indicating that the constructions of the hippocampal patients tended to consist of isolated fragments of information rather than connected scenes. Four of the five patients showed an impaired ability to imagine new experiences; the one patient who performed normally exhibited some residual hippocampal sparing that might have supported intact performance.
In a related line of research, Dalla Barba et al. (1997, 1999) have found that patients who confabulate about their personal pasts also confabulate about their personal futures. Taken together, the pattern of deficits in these patients suggests that imagining personal future events may involve processes above and beyond the general processes involved in constructing non-personal events and generating images, and shares common processes with episodic remembering.
Studies of another population exhibiting episodic memory impairments—suicidally depressed individuals—also reveal commonalities between remembering the past and imagining the future (Williams et al. 1996). When given word cues and instruction to recall an episode from the past or imagine a future episode, depressed patients showed reduced specificity in their retrieval of both past and future autobiographical events. Importantly, the reduction in specificity of past and future events was significantly correlated. Moreover, Williams and colleagues demonstrated that in healthy individuals, manipulations that reduced the specificity of past events (e.g. instructions or cues which induce a general retrieval style) also reduced the specificity of subsequently generated future events.
8. Neuroimaging of past and future events
Cognitive and patient studies provide evidence, suggesting that retrieving past events and simulating future events rely on common processes. Three recent neuroimaging studies have demonstrated that past and future events engage common neural regions (Okuda et al. 2003; Addis et al. 2007; Szpunar et al. 2007), providing further support for the constructive episodic simulation hypothesis.
In the first of these studies, Okuda et al. (2003) instructed participants to talk freely about their past or future during a PET scan, with the only constraint being the time period to report on: either the near (i.e. the last or next few days) or the distant (i.e. the last or next few years) past or future. Regardless of time period, both the past and future conditions elicited shared activity in bilateral frontopolar cortex, probably reflecting the self-referential nature of both types of event representations (Craik et al. 1999; Gusnard et al. 2001). Further, there was evidence of common MTL activity, and Okuda et al. interpreted this outcome as reflecting the retrieval of past events during both tasks; as explicitly required by the past event task, and as arguably necessary for the simulation of future episodic events. The effect of temporal distance on neural activity in these two regions was also examined, and remarkably, in eight out of the nine foci the same neural response to temporal distance (i.e. either an increase or a decrease with increasing distance) was evident for both past and future events. The only region exhibiting an interaction between temporal direction (i.e. past versus future) and distance (i.e. near versus distant) was an inferior region in left parahippocampal gyrus (BA 36). Despite these marked similarities, Okuda et al. (2003) also demonstrated that right frontopolar activity exhibited strong positive correlations with the amount of intentional information produced during the future task, consistent with studies implicating this region in prospective memory (Bechara et al. 1994; Okuda et al. 1998; Burgess et al. 2001b).
Although participants in this study talked about their personal past or future, it is unclear whether these events were episodic in nature, i.e. unique events specific in time and place (Tulving 1983), rather than reflecting general or semantic information about one's past or future. Moreover, even if specific episodic events were localizable within a participant's narrative, the use of a block design, as necessitated by PET, prevented analysis of neural activity associated with specific events. Given that others have shown that specificity of past events can alter neural activity during retrieval (Addis et al. 2004), the specificity of events in Okuda et al.'s study, or lack thereof, may have influenced the pattern of results.
More recent fMRI studies have attempted to overcome this limitation using event-related designs to yield information regarding the neural bases of specific past and future events. For instance, Szpunar et al. (2007) instructed participants to remember specific past events, imagine specific future events or imagine specific events involving a familiar individual (Bill Clinton) in response to event cues (e.g. past birthday, retirement party). Again, there was striking overlap in activity associated with past and future events in the bilateral frontopolar and MTL regions reported by Okuda et al. (2003), as well as posterior cingulate cortex. Importantly, these regions were not activated to the same magnitude when imagining events involving Bill Clinton, demonstrating a neural signature that is unique to the construction of events in one's personal past or future and is not shared by the construction of event representations per se. This result dovetails with the suggestive findings considered earlier from amnesic patients who cannot remember or imagine events in their personal past or future despite some ability to remember and imagine non-personal information. Together, these data suggest that there is a core network of neural structures that commonly supports the generation of event representations from one's personal past or future, in line with the constructive episodic simulation hypothesis.
A direct comparison of activity associated with past and future events identified several regions that were significantly more active for future relative to past events, including bilateral premotor cortex and left precuneus. The authors argue that this pattern of findings may reflect a more active type of imagery processing required by future events. One must not only construct and maintain the image, but also manipulate the image to create a novel scenario.
In a study from our laboratory, Addis et al. (2007) divided the past and future tasks into two phases: (i) an initial construction phase during which participants generated a past or future event in response to an event cue (e.g. ‘dress’) and made a button press when they had an event in mind and (ii) an elaboration phase during which participants generated as much detail as possible about the event (for related evidence from an electrophysiological study of remembered and imagined events that also distinguished between construction and elaboration phases, see Conway et al. 2003). We argued that specific cognitive processes contributing to the completion of such past and future tasks could be differentially engaged during the different phases of the task. Thus, if a particular neural difference between past and future events is only evident during one phase, collapsing across both phases in a block design or sampling neural activity during another phase in an event-related design could potentially obscure such differences. The same logic also applies to the search for common neural activity, if the common network is engaged during only one, but not another, phase of the task. Furthermore, we confirmed that past and future events were of equivalent phenomenology with both objective and subjective measures, thus enabling the interpretation of past–future differences as reflecting differences in temporal orientation and engagement of task-specific processes. We compared activity during the past and future tasks with control tasks that required semantic and imagery processing, respectively.
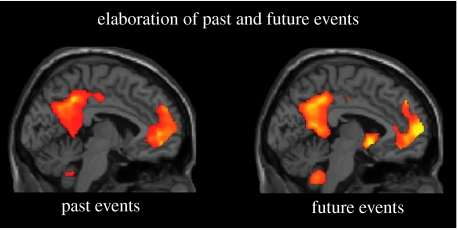
Consistent with the constructive episodic simulation hypothesis, there was indeed striking overlap between the past and future tasks. This overlap was most apparent during the elaboration phase, when participants are focused on generating details about the remembered or imagined event (figures 3 and 4). For instance, both event types were associated with activity in left anterior temporal cortex, a region thought to mediate conceptual and semantic information about the self and one's life (e.g. familiar people, common activities, Graham et al. 2003; Addis et al. 2004). Event representations also contained episodic and contextual imagery, perhaps related to activation of precuneus (e.g. Fletcher et al. 1995) and parahippocampal/retrosplenial cortices (e.g. Bar & Aminoff 2003), respectively. There was common activity in the left frontopolar cortex, reflecting the self-referential nature of past and future events (e.g. Craik et al. 1999), and in the left hippocampus, possibly reflecting the retrieval and/or integration of additional event details into the representation. Interestingly, this common past–future network is remarkably similar to the network consistently implicated in the retrieval of episodic memories of past autobiographical events (Maguire 2001), again consistent with the constructive episodic simulation hypothesis.
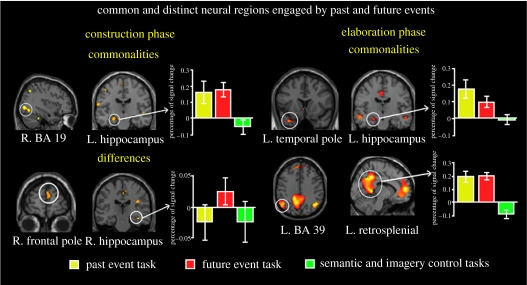
Figure 3.
Common and distinct regions engaged by the construction and elaboration of past and future events (Addis et al. 2007). A conjunction analysis of activity during the construction of past and future events revealed a few regions exhibiting common activity, such as left hippocampus and right occipital gyrus (BA 19). Contrast analyses identified a number of regions exhibiting differentially more activity for future events, including the right frontal pole and hippocampus. The elaboration phase was marked by striking overlap between past and future events, including left hippocampus, left temporal pole, bilateral parietal lobule (BA 39) and retrosplenial cortex. Plots of per cent signal change during the past event, future event and control (semantic and imagery) tasks are also shown. BA, Brodmann area.
Figure 4.
Sagittal slice (x=−4) illustrating the striking commonalities in the medial left prefrontal and parietal regions engaged when (a) remembering the past and (b) imagining the future (adapted from Addis et al. 2007). These marked similarities of activation were also evident in areas of the medial temporal lobe (bilateral parahippocampal gyrus) and lateral cortex (left temporal pole and left bilateral inferior parietal cortex). This extensive pattern of common activity was not present during the construction of past and future events (figure 4); it only emerged during the elaboration of these events (shown here, relative to elaboration phase of a semantic and an imagery control task).
The construction phase was associated with some common past–future activity in posterior visual regions and left hippocampus, which may reflect the initial interaction between visually presented cues and hippocampally mediated pointers to memory traces (Moscovitch 1992). Even so, this phase was characterized by considerable neural differentiation of past and future events. In particular, higher levels of activity during the future task were evident in the right frontopolar cortex, consistent with the association of this region with prospective memory (Burgess et al. 2001b; see also Burgess et al. 2007), and in the left inferior frontal gyrus, a region mediating generative processing (Poldrack et al. 1999). Furthermore, the right hippocampus was differentially engaged by the future event task, which may reflect the novelty of future events and/or additional relational processing required when one must recombine disparate details into a coherent event. This latter finding fits nicely with the observations noted earlier from Hassabis et al. (2007), indicating that hippocampal amnesics have difficulty imagining new experiences: the hippocampus may play a key role in recombining details of previous experiences into a coherent new imagined construction.
Notably, in all regions exhibiting significant past–future differences, future events were associated with more activity than past events, as also observed by Szpunar et al. (2007). We propose that this apparent regularity across neural regions and across studies reflects the more intensive constructive processes required by imagining future events relative to retrieving past events. Both past and future event tasks require the retrieval of information from memory, engaging common memory networks. However, only the future task requires that event details gleaned from various past events are flexibly recombined into a novel future event and, further, that this event is plausible given one's intentions for the future. Thus, additional regions supporting these processes are recruited by the future event task.
Many questions remain to be addressed regarding the nature of brain activity related to past and future events. For example, some of the regions that we found to be strongly activated when people imagine future events, including hippocampus and parahippocampal cortex, have been linked with imagery for spatial scenes (e.g. Burgess et al. 2001a; Byrne et al. in press). According to the constructive episodic simulation hypothesis, the adaptive nature of such activity is specifically related to its role in simulating the future. But to what extent do the activations associated with simulating future events specifically reflect the requirement to imagine a future event, as opposed to general imaginings that are not linked to a particular time frame? A critical task for research in this area is to attempt to distinguish between the specifically temporal component of episodic simulations and more general imaginative activity.
9. Concluding comments
Much research has focused on elucidating the constructive nature of episodic memory, and a growing number of recent investigations have recognized the close relationship between remembering the past and imagining the future. However, the possible relationship between constructive memory and past–future issues remains almost entirely unexplored. A major purpose of the present paper is to emphasize that this relationship constitutes a promising area for research (see also, Suddendorf & Corballis 1997; Dudai & Carruthers 2005; Hassabis et al. 2007). For example, according to the constructive episodic simulation hypothesis, it should be possible to document a direct link between processes underlying memory distortion and those underlying mental simulations of the future. It is already well known that imagining experiences can result in various kinds of memory distortions (e.g. Johnson et al. 1988, 1993; Garry et al. 1996; Goff & Roediger 1998; Loftus 2003); we think it will be quite informative to focus specifically on the link between imagining future events and memory distortion. Indeed, the scope of this research is probably even broader than that covered here. In a thoughtful review that elucidates the relationship between, and neural basis of, remembering the past and thinking about the future, Buckner & Carroll (2007) point out that neural regions that show common activation for past and future tasks closely resemble those that are activated during ‘theory of mind’ tasks, where individuals simulate the mental states of other people (e.g. Saxe & Kanwisher 2003). Buckner & Carroll note that such findings suggest that the commonly activated regions may be ‘specialized for, and engaged by, mental acts that require the projection of oneself in another time, place, or perspective’, resembling what Tulving (1985) referred to as autonoetic consciousness. Such observations highlight the importance of thinking broadly about the functions of episodic memory in constructing our personal and social worlds.
Acknowledgments
Preparation of this paper was supported by grants from the NIA (AG08441) and NIMH (MH060941). We thank Moshe Bar, Randy Buckner, Dan Gilbert, Itamar Kahn, Jason Mitchell and Gagan Wig for comments on the paper, and Alana Wong for invaluable aid in preparation of the manuscript.
Footnotes
One contribution of 14 to a Discussion Meeting Issue ‘Mental processes in the human brain’.
References
- Addis D.R, McIntosh A.R, Moscovitch M, Crawley A.P, McAndrews M.P. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. doi:10.1016/j.neuroimage.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Addis D.R, Wong A.T, Schacter D.L. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. doi:10.1016/j.neuropsychologia.2006.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.R, Schooler L.J. Reflections of the environment in memory. Psychol. Sci. 1991;2:396–408. doi:10.1111/j.1467-9280.1991.tb00174.x [Google Scholar]
- Atance C.M, O'Neill D.K. Episodic future thinking. Trends Cogn. Sci. 2001;5:533–539. doi: 10.1016/s1364-6613(00)01804-0. doi:10.1016/S1364-6613(00)01804-0 [DOI] [PubMed] [Google Scholar]
- Atance C.M, O'Neill D.K. The emergence of episodic future thinking in humans. Learn. Motiv. 2005;36:126–144. doi:10.1016/j.lmot.2005.02.003 [Google Scholar]
- Balota D.A, Cortese M.J, Duchek J.M, Adams D, Roediger H.L, McDermott K.B, Yerys B.E. Veridical and false memories in healthy older adults and in dementia of the Alzheimer's type. Cogn. Neuropsychol. 1999;16:361–384. doi:10.1080/026432999380834 [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. doi:10.1016/S0896-6273(03)00167-3 [DOI] [PubMed] [Google Scholar]
- Bartlett F.C. Cambridge University Press; Cambridge, UK: 1932. Remembering. [Google Scholar]
- Bechara A, Damasio A.R, Damasio H, Anderson S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. doi:10.1016/0010-0277(94)90018-3 [DOI] [PubMed] [Google Scholar]
- Bjork R.A, Bjork E.L. On the adaptive aspects of retrieval failure in autobiographical memory. In: Gruneberg M.M, Morris P.E, Sykes R.N, editors. Practical aspects of memory: current research and issues. Wiley; Chichester, UK: 1988. [Google Scholar]
- Brainerd C.J, Reyna V.F. When things that were never experienced are easier to “remember” than things that were. Psychol. Sci. 1998;9:484–489. doi:10.1111/1467-9280.00089 [Google Scholar]
- Brainerd C.J, Reyna V.F. Oxford University Press; New York, NY: 2005. The science of false memory. [Google Scholar]
- Brandimonte M, Einstein G.O, McDaniel M.A. Erlbaum; Mahwah, NJ: 1996. Prospective memory: theory and applications. [Google Scholar]
- Buckner, R. L. & Carroll, D. C. 2007 Self-projection and the brain. Trends Cogn. Sci 11, 49–57. (doi:10.1096/j.tics.2006.11.004) [DOI] [PubMed]
- Budson A.E, Daffner K.R, Desikan R, Schacter D.L. When false recognition is unopposed by true recognition: gist-based memory distortion in Alzheimer's disease. Neuropsychology. 2000;14:277–287. doi: 10.1037//0894-4105.14.2.277. doi:10.1037/0894-4105.14.2.277 [DOI] [PubMed] [Google Scholar]
- Budson A.E, Desikan R, Daffner K.R, Schacter D.L. Perceptual false recognition in Alzheimer's disease. Neuropsychology. 2001;15:230–243. doi:10.1037/0894-4105.15.2.230 [PubMed] [Google Scholar]
- Budson A.E, Sullivan A.L, Daffner K.R, Schacter D.L. Semantic versus phonological false recognition in aging and Alzheimer's disease. Brain Cogn. 2003;51:251–261. doi: 10.1016/s0278-2626(03)00030-7. doi:10.1016/S0278-2626(03)00030-7 [DOI] [PubMed] [Google Scholar]
- Budson A.E, Todman R.W, Schacter D.L. Gist memory in Alzheimer's disease: evidence from categorized pictures. Neuropsychology. 2006;20:113–122. doi: 10.1037/0894-4105.20.1.113. doi:10.1037/0894-4105.20.1.113 [DOI] [PubMed] [Google Scholar]
- Burgess P.W, Shallice T. Confabulation and the control of recollection. Memory. 1996;4:359–411. doi: 10.1080/096582196388906. doi:10.1080/096582196388906 [DOI] [PubMed] [Google Scholar]
- Burgess N, Becker S, King J.A, O'Keefe J. Memory for events and their spatial context: models and experiments. Phil. Trans. R. Soc. B. 2001a;356:1493–1503. doi: 10.1098/rstb.2001.0948. doi:10.1098/rstb.2001.0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P.W, Quayle A, Frith C.D. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001b;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. doi:10.1016/S0028-3932(00)00149-4 [DOI] [PubMed] [Google Scholar]
- Burgess P.W, Sam J.G, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Phil. Trans. R. Soc. B. 2007;362:887–899. doi: 10.1098/rstb.2007.2095. doi:10.1098/rstb.2007.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, P., Becker, S. & Burgess, N. In press. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev [DOI] [PMC free article] [PubMed]
- Cabeza R, Rao S.M, Wagner A.D, Mayer A.R, Schacter D.L. Can medial temporal lobe regions distinguish true from false? An event-related fMRI study of veridical and illusory recognition memory. Proc. Natl Acad. Sci. USA. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. doi:10.1073/pnas.081082698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Ghetti S, Frattarelli M, Ladavas E. When true memory availability promotes false memory: evidence from confabulating patients. Neuropsychologia. 2006;44:1866–1877. doi: 10.1016/j.neuropsychologia.2006.02.008. doi:10.1016/j.neuropsychologia.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Conway M.A, Pleydall-Pearce C.W, Whitecross S.E, Sharpe H. Neurophysiological correlates of memory for experienced and imagined events. Neuropsychologia. 2003;41:334–340. doi: 10.1016/s0028-3932(02)00165-3. doi:10.1016/S0028-3932(02)00165-3 [DOI] [PubMed] [Google Scholar]
- Craik F.I, Moroz T.M, Moscovitch M, Stuss D.T, Winocur G, Tulving E, Kapur S. In search of the self: a positron emission tomography study. Psychol. Sci. 1999;10:26–34. doi:10.1111/1467-9280.00102 [Google Scholar]
- Dab S, Claes T, Morais J, Shallice T. Confabulation with a selective descriptor process impairment. Cogn. Neuropsychol. 1999;16:215–242. doi:10.1080/026432999380771 [Google Scholar]
- Dalla Barba G, Cappelletti Y.J, Signorini M, Denes G. Confabulation: remembering “another” past, planning “another” future. Neurocase. 1997;3:425–436. doi:10.1093/neucas/3.6.425 [Google Scholar]
- Dalla Barba G, Nedjam Z, Dubois B. Confabulation, executive functions, and source memory in Alzheimer's disease. Cogn. Neuropsychol. 1999;16:385–398. doi:10.1080/026432999380843 [Google Scholar]
- D'Argembeau A, Van der Linden M. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: influence of valence and temporal distance. Conscious. Cogn. 2004;13:844–858. doi: 10.1016/j.concog.2004.07.007. doi:10.1016/j.concog.2004.07.007 [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Van der Linden M. Individual differences in the phenomenology of mental time travel. Conscious. Cogn. 2006;15:342–350. doi: 10.1016/j.concog.2005.09.001. doi:10.1016/j.concog.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. J. Exp. Psychol. 1959;58:17–22. doi: 10.1037/h0046671. doi:10.1037/h0046671 [DOI] [PubMed] [Google Scholar]
- Delbecq-Derouesné J, Beauvois M.F, Shallice T. Preserved recall versus impaired recognition. Brain. 1990;113:1045–1074. doi: 10.1093/brain/113.4.1045. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Carruthers M. The Janus face of mnemosyne. Nature. 2005;434:823–824. doi: 10.1038/434567a. doi:10.1038/434823a [DOI] [PubMed] [Google Scholar]
- Einstein G.O, McDaniel M.A. Normal aging and prospective memory. J. Exp. Psychol. Learn. Mem. Cogn. 1990;16:717–726. doi: 10.1037//0278-7393.16.4.717. doi:10.1037/0278-7393.16.4.717 [DOI] [PubMed] [Google Scholar]
- Fletcher P, Frith C, Baker S.C, Shallice T, Frackowiak R.S, Dolan R. The mind's eye—precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. doi:10.1006/nimg.1995.1025 [DOI] [PubMed] [Google Scholar]
- Fuster J.M. Raven Press; New York, NY: 1989. The prefrontal cortex: anatomy, physiology, and the frontal lobe. [Google Scholar]
- Gallo D.A. Taylor & Francis; New York, NY: 2006. Associative illusions of memory. [Google Scholar]
- Garoff-Eaton R.J, Slotnick S.D, Schacter D.L. Not all false memories are created equal: the neural basis of false recognition. Cereb. Cortex. 2006;16:1645–1652. doi: 10.1093/cercor/bhj101. doi:10.1093/cercor/bhj101 [DOI] [PubMed] [Google Scholar]
- Garry M, Manning C.G, Loftus E.F, Sherman S.J. Imagination inflation: Imagining a childhood event inflates confidence that it occurred. Psychon. Bull. Rev. 1996;3:208–214. doi: 10.3758/BF03212420. [DOI] [PubMed] [Google Scholar]
- Gilbert D.T. Alfred A. Knopf; New York, NY: 2006. Stumbling on happiness. [Google Scholar]
- Gilboa A, Alain C, Stuss D.T, Melo B, Miller S, Moscovitch M. Mechanisms of spontaneous confabulations: a strategic retrieval account. Brain. 2006;129:1399–1414. doi: 10.1093/brain/awl093. doi:10.1093/brain/awl093 [DOI] [PubMed] [Google Scholar]
- Goff L.M, Roediger H.L. Imagination inflation for action events: repeated imaginings lead to illusory recollections. Mem. Cogn. 1998;26:20–33. doi: 10.3758/bf03211367. [DOI] [PubMed] [Google Scholar]
- Goschke T, Kuhl J. The representation of intentions: persisting activation in memory. J. Exp. Psychol. Learn. Mem. Cogn. 1993;19:1211–1226. doi:10.1037/0278-7393.19.5.1211 [Google Scholar]
- Graham K.S, Lee A.C, Brett M, Patterson K. The neural basis of autobiographical and semantic memory: new evidence from three PET studies. Cogn. Affect. Behav. Neurosci. 2003;3:234–254. doi: 10.3758/cabn.3.3.234. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A, Akbudak E, Shulman G.L, Raichle M.E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl Acad. Sci. USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. doi:10.1073/pnas.071043098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock P.A. Time and the privileged observer. KronoScope. 2005;5:176–191. doi:10.1163/156852405774858744 [Google Scholar]
- Hassabis D, Kumaran D, Vann S.D, Maguire E.A. Patients with hippocampal amnesia cannot imagine new experiences. Proc. Natl Acad. Sci. USA. 2007;104:1726–1735. doi: 10.1073/pnas.0610561104. doi:10.1073/pnas.0610561104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar D.H. ‘Memory of the future’: an essay on the temporal organization of conscious awareness. Hum. Neurobiol. 1985;4:127–136. [PubMed] [Google Scholar]
- Johnson M.K. Reality monitoring: evidence from confabulation in organic brain disease patients. In: Prigatano G.P, Schacter D.L, editors. Awareness of deficit after brain injury: clinical and theoretical issues. Oxford University Press; New York, NY: 1991. pp. 176–197. [Google Scholar]
- Johnson M.K, Foley M.A, Suengas A.G, Raye C.L. Phenomenal characteristics of memories for perceived and imagined autobiographical events. J. Exp. Psychol. Gen. 1988;117:371–376. doi:10.1037/0096-3445.117.4.371 [PubMed] [Google Scholar]
- Johnson M.K, Hashtroudi S, Lindsey D.S. Source monitoring. Psychol. Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. doi:10.1037/0033-2909.114.1.3 [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner A.D. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J. Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. doi:10.1523/JNEUROSCI.0624-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.B, Loftus J. Memory and temporal experience: the effects of episodic memory loss on an amnesic patient's ability to remember the past and imagine the future. Soc. Cogn. 2002;20:353–379. doi:10.1521/soco.20.5.353.21125 [Google Scholar]
- Koutstaal W, Schacter D.L, Verfaellie M, Brenner C, Jackson E.M. Perceptually-based false recognition of novel objects in amnesia: effects of category size and similarity to prototype. Cogn. Neuropsychol. 1999;16:317–342. doi:10.1080/026432999380816 [Google Scholar]
- Loftus E.F. The reality of repressed memories. Am. Psychol. 1993;48:518–537. doi: 10.1037//0003-066x.48.5.518. doi:10.1037/0003-066X.48.5.518 [DOI] [PubMed] [Google Scholar]
- Loftus E.F. Make-believe memories. Am. Psychol. 2003;58:867–873. doi: 10.1037/0003-066X.58.11.867. doi:10.1037/0003-066X.58.11.867 [DOI] [PubMed] [Google Scholar]
- Maguire E.A. Neuroimaging studies of autobiographical event memory. Phil. Trans. R. Soc. B. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. doi:10.1098/rstb.2001.0944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Henkel L.A, Johnson M.K. Evaluating characteristics of false memories: remember/know judgments and memory characteristics questionnaire compared. Mem. Cogn. 1997;25:826–837. doi: 10.3758/bf03211327. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Phil. Trans. R. Soc. B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. doi:10.1098/rstb.1971.0078 [DOI] [PubMed] [Google Scholar]
- McClelland J.L. Constructive memory and memory distortions: a parallel-distributed processing approach. In: Schacter D.L, editor. Memory distortion: how minds, brains and societies reconstruct the past. Harvard University Press; Cambridge, MA: 1995. pp. 69–90. [Google Scholar]
- McClelland J.L, McNaughton B.L, O'Reilley R.C. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. doi:10.1037/0033-295X.102.3.419 [DOI] [PubMed] [Google Scholar]
- McDermott K.B. Priming on perceptual implicit memory test can be achieved through presentation of associates. Psychon. Bull. Rev. 1997;4:582–586. [Google Scholar]
- McKone E, Murphy B. Implicit false memory: effects of modality and multiple study presentations on long lived semantic priming. J. Mem. Lang. 2000;43:89–109. doi:10.1006/jmla.1999.2702 [Google Scholar]
- Melo B, Winocur G, Moscovitch M. False recall and false recognition: an examination of the effects of selective and combined lesions to the medial temporal lobe/diencephalon and frontal lobe structures. Cogn. Neuropsychol. 1999;16:343–359. doi:10.1080/026432999380825 [Google Scholar]
- Mesulam M.M. The human frontal lobes: transcending the default mode through contingent encoding. In: Stuss D.T, Knight R.T, editors. Principles of frontal Lobe function. Oxford University Press; New York, NY: 2002. pp. 8–30. [Google Scholar]
- Miller M.B, Wolford G.L. The role of criterion shift in false memory. Psychol. Rev. 1999;106:398–405. doi:10.1037/0033-295X.106.2.398 [Google Scholar]
- Morewedge C.K, Gilbert D.T, Wilson T.D. The least likely of times: how remembering the past biases forecasts of the future. Psychol. Sci. 2005;16:626–630. doi: 10.1111/j.1467-9280.2005.01585.x. doi:10.1111/j.1467-9280.2005.01585.x [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: a component process model based on modules and central systems. J. Cogn. Neurosci. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Confabulation. In: Schacter D.L, editor. Memory distortion: how minds, brains, and societies reconstruct the past. Harvard University Press; Cambridge, MA: 1995. pp. 226–254. [Google Scholar]
- Moulin C.J.A, Conway M.A, Thompson R.G, James N, Jones R.W. Disordered memory awareness: recollective confabulation in two cases of persistent deja vecu. Neuropsychologia. 2005;43:1362–1378. doi: 10.1016/j.neuropsychologia.2004.12.008. doi:10.1016/j.neuropsychologia.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Norman K.A, O'Reilly R.C. Modeling hippocampal and neocortical contributions to recognition memory: a complementary learning systems approach. Psychol. Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. doi:10.1037/0033-295X.110.4.611 [DOI] [PubMed] [Google Scholar]
- Norman K.A, Schacter D.L. Implicit memory, explicit memory, and false recollection: a cognitive neuroscience perspective. In: Reder L.M, editor. Implicit memory and metacognition. Erlbaum; Hillsdale, NJ: 1996. pp. 229–257. [Google Scholar]
- Norman K.A, Schacter D.L. False recognition in young and older adults: exploring the characteristics of illusory memories. Mem. Cogn. 1997;25:838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Yamadori A, Kawashima R, Tsukiura T, Fukatsu R, Suzuki K, Itoh M, Fukuda H. Participation of the prefrontal cortices in prospective memory: evidence from a PET study in humans. Neurosci. Lett. 1998;253:127–130. doi: 10.1016/s0304-3940(98)00628-4. doi:10.1016/S0304-3940(98)00628-4 [DOI] [PubMed] [Google Scholar]
- Okuda J, et al. Thinking of the future and the past: the roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. doi:10.1016/S1053-8119(03)00179-4 [DOI] [PubMed] [Google Scholar]
- Ost J, Costall A. Misremembering Bartlett: a study in serial reproduction. Br. J. Psychol. 2002;93:243–255. doi: 10.1348/000712602162562. doi:10.1348/000712602162562 [DOI] [PubMed] [Google Scholar]
- Poldrack R, Wagner A.D, Prull M.W, Desmond J.E, Glover G.H, Gabrieli J.D. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. doi:10.1006/nimg.1999.0441 [DOI] [PubMed] [Google Scholar]
- Roediger H.L, McDermott K.B. Creating false memories: remembering words not presented in lists. J. Exp. Psychol. Learn. Mem. Cogn. 1995;21:803–814. doi:10.1037/0278-7393.21.4.803 [Google Scholar]
- Rosenbaum R.S, Kohler S, Schacter D.L, Moscovitch M, Westmacott R, Black S.E, Gao F, Tulving E. The case of K. C.: contributions of a memory-impaired person to memory theory. Neuropsychologia. 2005;43:989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. doi:10.1016/j.neuropsychologia.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. doi:10.1016/S1053-8119(03)00230-1 [DOI] [PubMed] [Google Scholar]
- Schacter D.L. The seven sins of memory: insights from psychology and cognitive neuroscience. Am. Psychol. 1999;54:182–203. doi: 10.1037//0003-066x.54.3.182. doi:10.1037/0003-066X.54.3.182 [DOI] [PubMed] [Google Scholar]
- Schacter D.L. Houghton Mifflin; Boston, MA; New York, NY: 2001. The seven sins of memory: how the mind forgets and remembers. [Google Scholar]
- Schacter, D. L. & Addis, D. R. 2007. The ghosts of past and future. Nature445, 27. (doi:10.1038/445027a) [DOI] [PubMed]
- Schacter D.L, Slotnick S.D. The cognitive neuroscience of memory distortion. Neuron. 2004;44:149–160. doi: 10.1016/j.neuron.2004.08.017. doi:10.1016/j.neuron.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Schacter D.L, Curran T, Galluccio L, Milberg W, Bates J. False recognition and the right frontal lobe: a case study. Neuropsychologia. 1996a;34:793–808. doi: 10.1016/0028-3932(95)00165-4. doi:10.1016/0028-3932(95)00165-4 [DOI] [PubMed] [Google Scholar]
- Schacter D.L, Reiman E, Curran T, Sheng Yun L, Bandy D, McDermott K.B, Roediger H.L. Neuroanatomical correlates of veridical and illusory recognition memory: evidence from positron emission tomography. Neuron. 1996b;17:1–20. doi: 10.1016/s0896-6273(00)80158-0. doi:10.1016/S0896-6273(00)80158-0 [DOI] [PubMed] [Google Scholar]
- Schacter D.L, Verfaellie M, Pradere D. The neuropsychology of memory illusions: false recall and recognition in amnesic patients. J. Mem. Lang. 1996c;35:319–334. doi:10.1006/jmla.1996.0018 [Google Scholar]
- Schacter D.L, Verfaellie M, Anes M.D. Illusory memories in amnesic patients: conceptual and perceptual false recognition. Neuropsychology. 1997;11:331–342. doi: 10.1037//0894-4105.11.3.331. doi:10.1037/0894-4105.11.3.331 [DOI] [PubMed] [Google Scholar]
- Schacter D.L, Norman K.A, Koutstaal W. The cognitive neuroscience of constructive memory. Annu. Rev. Psychol. 1998a;49:289–318. doi: 10.1146/annurev.psych.49.1.289. doi:10.1146/annurev.psych.49.1.289 [DOI] [PubMed] [Google Scholar]
- Schacter D.L, Verfaellie M, Anes M, Racine C. When true recognition suppresses false recognition: evidence from amnesic patients. J. Cogn. Neurosci. 1998b;10:668–679. doi: 10.1162/089892998563086. doi:10.1162/089892998563086 [DOI] [PubMed] [Google Scholar]
- Schacter D.L, Cendan D.L, Dodson C.S, Clifford E.R. Retrieval conditions and false recognition: testing the distinctiveness heuristic. Psychon. Bull. Rev. 2001;8:827–833. doi: 10.3758/bf03196224. [DOI] [PubMed] [Google Scholar]
- Schacter D.L, Dobbins I.G, Schnyer D.M. Specificity of priming: a cognitive neuroscience perspective. Nat. Rev. Neurosci. 2004;5:853–862. doi: 10.1038/nrn1534. doi:10.1038/nrn1534 [DOI] [PubMed] [Google Scholar]
- Schnider A. Spontaneous confabulation and the adaptation of thought to ongoing reality. Nat. Rev. Neurosci. 2003;4:662–671. doi: 10.1038/nrn1179. doi:10.1038/nrn1179 [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and the temporal organization of behaviour. Phil. Trans. R. Soc. B. 1996;351:1405–1411. doi: 10.1098/rstb.1996.0124. doi:10.1098/rstb.1996.0124 [DOI] [PubMed] [Google Scholar]
- Slotnick S.D, Dodson C.S. Support for a continuous (single-process) model of recognition memory and source memory. Mem. Cogn. 2005;33:151–170. doi: 10.3758/bf03195305. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D, Schacter D.L. A sensory signature that distinguishes true from false memories. Nat. Neurosci. 2004;7:664–672. doi: 10.1038/nn1252. doi:10.1038/nn1252 [DOI] [PubMed] [Google Scholar]
- Slotnick S.D, Schacter D.L. The nature of memory related activity in early visual areas. Neuropsychologia. 2006;44:2874–2886. doi: 10.1016/j.neuropsychologia.2006.06.021. doi:10.1016/j.neuropsychologia.2006.06.021 [DOI] [PubMed] [Google Scholar]
- Squire L.R, Stark C.E, Clark R.E. The medial temporal lobe. Annu. Rev. Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. doi:10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- Stuss D.T, Benson D.F. Raven Press; New York, NY: 1986. The frontal lobes. [Google Scholar]
- Suddendorf T, Busby J. Mental time travel in animals? Trends Cogn. Sci. 2003;7:391–396. doi: 10.1016/s1364-6613(03)00187-6. doi:10.1016/S1364-6613(03)00187-6 [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Busby J. Making decisions with the future in mind: developmental and comparative identification of mental time travel. Learn. Motiv. 2005;36:110–125. doi:10.1016/j.lmot.2005.02.010 [Google Scholar]
- Suddendorf T, Corballis M.C. Mental time travel and the evolution of the human mind. Genet. Soc. Gen. Psychol. Monogr. 1997;123:133–167. [PubMed] [Google Scholar]
- Szpunar K.K, Watson J.M, McDermott K.B. Neural substrates of envisioning the future. Proc. Natl Acad. Sci. USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. doi:10.1073/pnas.0610082104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.F. In search of memory traces. Annu. Rev. Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. doi:10.1146/annurev.psych.56.091103.070239 [DOI] [PubMed] [Google Scholar]
- Trope Y, Liberman N. Temporal construal. Psychol. Rev. 2003;110:401–421. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- Tulving E. Clarendon Press; Oxford, UK: 1983. Elements of episodic memory. [Google Scholar]
- Tulving E. Memory and consciousness. Can. Psychol. 1985;26:1–12. [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu. Rev. Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. doi:10.1146/annurev.psych.53.100901.135114 [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory and autonoesis: uniquely human? In: Terrace H.S, Metcalfe J, editors. The missing link in cognition: origins of self-reflective consciousness. Oxford University Press; New York, NY: 2005. pp. 3–56. [Google Scholar]
- Tulving E, Schacter D.L, McLachlan D.R, Moscovitch M. Priming of semantic autobiographical knowledge: a case study of retrograde amnesia. Brain Cogn. 1988;8:3–20. doi: 10.1016/0278-2626(88)90035-8. doi:10.1016/0278-2626(88)90035-8 [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Schacter D.L, Cook S.P. The effect of retrieval instructions on false recognition: exploring the nature of the gist memory impairment in amnesia. Neuropsychologia. 2002;40:2360–2368. doi: 10.1016/s0028-3932(02)00074-x. doi:10.1016/S0028-3932(02)00074-X [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Page K, Orlando F, Schacter D.L. Impaired implicit memory for gist information in amnesia. Neuropsychology. 2005;19:760–769. doi: 10.1037/0894-4105.19.6.760. doi:10.1037/0894-4105.19.6.760 [DOI] [PubMed] [Google Scholar]
- Ward J, Parkin A.J, Powell G, Squires E.J, Townshend J, Bradley V. False recognition of unfamiliar people: “Seeing film stars everywhere”. Cogn. Neuropsychol. 1999;16:293–315. doi:10.1080/026432999380807 [Google Scholar]
- Williams J.M, Ellis N.C, Tyers C, Healy H, Rose G, MacLeod A.K. The specificity of autobiographical memory and imageability of the future. Mem. Cogn. 1996;24:116–125. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]
- Wixted J.T, Stretch V. The case against a criterion-shift account of false memory. Psychol. Rev. 2000;107:368–376. doi: 10.1037/0033-295x.107.2.368. doi:10.1037/0033-295X.107.2.368 [DOI] [PubMed] [Google Scholar]