Abstract
Neurotrophins, secreted in an activity-dependent manner, are thought to be involved in the activity-dependent refinement of synaptic connections. Here we demonstrate that in hippocampal neurons and the rat pheochromocytoma cell line PC12 application of exogenous neurotrophins induces secretion of neurotrophins, an effect that is mediated by the activation of tyrosine kinase neurotrophin receptors (Trks). Like activity-dependent secretion of neurotrophins, neurotrophin-induced neurotrophin secretion requires mobilization of calcium from intracellular stores. Because neurotrophins are likely to be released from both dendrites and axons, neurotrophin-induced neurotrophin release represents a potential positive feedback mechanism, contributing to the reinforcement and stabilization of synaptic connections.
The modulation of activity-dependent neuronal plasticity is a recent facet in the spectrum of biological functions of neurotrophins (1–5). In the peripheral nervous system the availability of limited amounts of neurotrophins regulate the extent of survival and differentiation of specific populations of neurons (6, 7). In the central nervous system the survival of a given population of neurons generally is supported by more than one neurotrophic factor (8). However, individual neurotrophins nevertheless are crucially involved in the modulation of activity-dependent neuronal plasticity. The evidence for the physiological importance of this role of neurotrophins evolved from observations that the local administration of neurotrophins or their corresponding blocking agents [anti-nerve growth factor (NGF) antibodies or TrkB-IgG receptor bodies] have dramatic effects on the activity-dependent development of ocular dominance in the visual cortex of various species (3–5). The visual cortex as a complex system does not lend itself favorably to the direct analysis of the underlying mechanisms at the cellular and molecular level. However, complementary information became available through the analysis of various “reductionistic systems,” and neurotrophins were shown to modulate synaptic transmission by both presynaptic and postsynaptic effects (9–17). At the presynaptic site they enhance the secretion of neurotransmitters as reflected by an increase in the frequency of miniature synaptic events both at frog neuromuscular junctions or synapses of cultured hippocampal neurons (9, 12). There is evidence that the enhanced transmitter release results from tyrosine kinase neurotrophin receptor (Trk)-mediated Ca2+ influx. In cultured hippocampal neurons, brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) induced a rapid rise in intracellular calcium (18, 19). At Xenopus neuromuscular junctions, BDNF-induced potentiation of neurotransmitter secretion is accompanied by an increase of calcium within the presynaptic terminal (20). Postsynaptically neurotrophins enhance the transmission through N-methyl-d-aspartic acid (NMDA) receptors (14) and promote the phosphorylation of the NMDA receptor subunit 1 (16).
Similar to the activity-dependent refinement of synaptic connections in the visual cortex, the availability of neurotrophins appears to be critical for the ability of synapses in the hippocampus to undergo long-term potentiation (LTP). In BDNF −/− mice, LTP at Schaffer collateral/CA1 synapses was found to be markedly impaired (21, 22). Surprisingly, a similar impairment already was observed in heterozygous BDNF (+/−) mice, indicating that BDNF must be available in critical quantities. This interpretation is supported by the observation that both the adenovirus-mediated re-expression of BDNF in the CA1 region (23) and the bath application of BDNF (22) largely restituted the defective LTP formation in hippocampal slices from BDNF knockout mice. In agreement with these observations treatment of hippocampal slices with TrkB-IgGs inhibited the generation of tetanus-induced LTP at Schaffer collateral/CA1 synapses (24). During development these synapses gain the ability to undergo LTP at a time, when BDNF expression dramatically increases (25). Before this developmental stage, exogenous BDNF permitted the induction of LTP (24), demonstrating that the availability of a minimal quantity of this neurotrophin is apparently a crucial factor for this form of neuronal plasticity.
To achieve a comprehensive understanding of the modulatory role of neurotrophins in integrated neuronal systems in vivo, information on the regionally differential expression of neurotrophins and their receptors together with the identification of the mechanisms and sites of regulation of synthesis and secretion of neurotrophins are of crucial importance.
The first evidence for the idea that neurotrophins may be involved in activity-dependent neuronal plasticity originated from studies showing that the synthesis of these factors is tightly regulated by neuronal activity (26–29). In the hippocampus, NGF and BDNF synthesis is regulated by the interplay between excitatory and inhibitory synaptic transmission, mediated by the neurotransmitters glutamate and acetylcholine on the one hand, and γ-aminobutyric acid on the other. Increased excitatory synaptic activity results in a rapid up-regulation of NGF and BDNF expression, whereas inhibitory synaptic activity results in repression (29, 30). Of particular interest was the observation that the synthesis of these neurotrophins increases in paradigms used to study LTP (31–33) or central synaptic activity after physiological sensory stimulation (34–39). Thus, these regulatory mechanisms were brought into play not only by extreme experimental procedures such as initiation of seizures (26, 27, 40–42), but they also became clearly apparent by rapid changes in the visual input (34), monocular deprivation (35), mechanical stimulation of whiskers (36), augmented physical exercise on the running wheel (37), osmotic stress (38), or even changing the environment (39).
In addition to the rate, extent, and site of regulation of the synthesis the functional availability of neurotrophins also is determined by the mechanism and site of secretion. Neurotrophins have all of the structural characteristics of secreted molecules (6, 7). In hippocampal slices and primary hippocampal neuronal cultures NGF and BDNF secretion follows a constitutive (43, 47) and a regulated pathway (43–47). The latter is activated by glutamate or depolarization with high concentrations of potassium. Neurotrophin secretion is blocked by the intracellular, high-affinity calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid pentaacetoxymethyl ester (BAPTA-AM) and by blockade or depletion of intracellular calcium stores by dantrolene or thapsigargin, respectively, indicating that it is dependent on intact Ca2+ stores (43, 44, 47). Thus, the intracellular calcium release is the triggering step for regulated neurotrophin secretion.
In the present study we examined an additional feature of the regulation of the secretion of neurotrophins, namely the capability of neurotrophins to regulate their own secretion, which is thought to occur from both dendrites and axons (44). In hippocampal slices and dissociated neurons in culture, exogenous application of neurotrophins rapidly enhanced secretion of neuronally expressed neurotrophins, requiring the activation of neurotrophin receptors of the Trk gene family. Neurotrophin-induced secretion of neurotrophins occurred independently of extracellular calcium, but required mobilization of calcium from intracellular stores, thus resembling the mechanism described for activity-dependent release. Neurotrophin-induced neurotrophin secretion may provide a positive feedback mechanism for the selective stabilization of synaptic connections.
MATERIALS AND METHODS
Cell Culture.
Hippocampal neurons were prepared from E17 rat embryos according to Zafra and coworkers (27). Briefly, hippocampi were incubated for 20 min at 37°C in PBS without Ca2+ and Mg2+, containing 10 mM glucose, 1 mg/ml BSA (Sigma), 1 μg/ml DNase (Sigma), and 12 μg/ml papain (Sigma), dissociated with a plastic pipette, and centrifuged (5 min at 1,000 revolutions per min). Cells were resuspended in DMEM (GIBCO) containing 10% fetal calf serum (Hyclone). Neurons were plated at a density of 3 × 105/0.8 cm2 or 2 × 106/1.8 cm2 on glass coverslips coated with poly(dl-ornithine) (Sigma), and after 3 hr the medium was changed to defined medium.
Rat pheochromocytoma cells (PC12) were maintained in DMEM supplemented with 5% fetal calf serum and 10% horse serum (Hyclone) and incubated at 37°C and 10% CO2.
Acute Hippocampal Slices.
Slices (350 μm) were prepared from hippocampus of adult Wistar rats in cold, oxygenated modified Hanks buffer (125 mM NaCl/5 mM KCl/1.2 mM NaH2PO4/1 mM CaCl2/1.2 mM MgCl2/1 μM ZnCl2/10 mM glucose/25 mM Hepes/0.25% BSA, pH 7.4) by using a MacIlwain tissue chopper. Slices obtained from one hippocampus were placed in a perfusion chamber (Minucell and Minutissue Bad Abbach, Germany) and constantly perfused with Hanks’ buffer equilibrated with 95% O2 and 5% CO2 with a flow rate of 0.1 ml/min for 30 min.
Calcium Imaging.
For measurements of free intracellular calcium ([Ca2+]i) (48) hippocampal neurons were loaded for 40 min (37°C, 10% CO2) with 2 μM [1-[2-(5-carboxyoxazol-2-yl)-6-aminobenzofuran-5-oxy]-2-(2′-amino-5′methylphenoxy)-ethane-N,N,N′,N′-tetraacetic acid pentaacetoxymethyl ester (Fura-2AM; Calbiochem) dissolved in dimethyl sulfoxide/10% pluronic F-127 (Molecular Probes), rinsed, and incubated in fresh culture medium for 10 min before the measurement. During [Ca2+]i imaging, cells were kept in modified Hanks’ buffer. Cells were visualized with a Zeiss Fluar 40×/1,30 oil objective by using an inverted microscope (Axiovert 100, Zeiss). Fluorescence was determined at the excitation wavelengths of 340 and 380 nm with an intensified charge coupled device camera (C24000–87, Hamamatsu, Middlesex, U.K.), and images were processed with the Argus 50/CA software (Hamamatsu) to calculate the respective fluorescence ratios. Images were taken at a sampling rate of 0.75/s, and for each image eight frames were averaged.
Adenoviral Vectors.
Viral vectors were constructed by homologous recombination in 293 cells (49, 50). AdCMV-NGF contained the sequence of mouse preproNGF and AdCMV-BDNF the sequence of mouse preproBDNF. AdCMV-BDNF-myc contained the sequence of mouse preproBDNF, which was tagged with 9E10 myc epitope (51) at the C terminus of the BDNF coding region. To eliminate a potential proteolytic cleavage site, the last three amino acids of the BDNF coding region were removed (J. Klose, personal communication). The neurotrophin cDNAs were cloned into the shuttle plasmid pXCJL1/2. Shuttle plasmids and the plasmid pJM17, which contains the whole circularized genome of human adenovirus type 5, were cotransfected into 293 cells. After homologous recombination, recombinant clones were isolated by plaque purification and amplified. Stocks were prepared with titers ranging from 5 × 109 to 2 × 1011 plaque-forming units/ml.
Release Experiments.
After 7 days in culture hippocampal neurons or undifferentiated PC12 cells plated on 0.8-cm2 coverslip were infected with about 5 × 106 plaque-forming units of either AdCMV-NGF AdCMV-BDNF or AdCMV-BDNFmyc in a reduced volume of 300 μl complete medium. On the next day neurons were used for the release experiments. Cultured hippocampal neurons or hippocampal slices were perfused with modified Hanks’ buffer at a perfusion rate of 0.1 ml/min at 37°C as described (43). Viability of neurons before or after the release experiments was evaluated by using fluorescein-diacetate and propidium iodide staining (52). After 30 min of equilibration samples were collected at 5-min intervals. Glutamate (50 μM) or 100 ng/ml neurotrophins (BDNF, NT-4/5, NGF, and NT-3) were applied over a 5-min period. Treatment with specific inhibitors (10 μM BAPTA; 10 μM BAPTA-AM; 200 nM K252a; 50 μM 6-cyano-7-nitroquinoxaline-2,3-dione) started 35 min after beginning the perfusion and was maintained during the entire collection period. The amount of BDNF or NGF in each fraction subsequently was determined by ELISA.
Release experiments also were performed under conditions without constant perfusion (static condition) as described by Goodman and coworkers (46), by using 2 × 106/1.8 cm2 hippocampal neurons or PC12 cells after infection according to the above described procedure. After 20 min of equilibration samples of a volume of 200 μl were collected every 10 min.
ELISA.
Neurotrophins were quantified by using a two-site immunoassay (ref. 43; R.K., unpublished results). For NGF, microtiter plates were coated with a mAb (27/21) in 0.05 sodium carbonate buffer (pH 9.7) and incubated overnight at 4°C. After washing with 0.05 M Tris⋅HCl, pH 7.0/0.2 M NaCl/0.1% Triton X-100 buffer and blocking with 1% BSA in washing buffer, standards (2–500 pg/ml) and samples were incubated overnight at 4°C. A third overnight incubation step with the 27/21 antibody conjugated to β-galactosidase in washing buffer, 1% BSA was performed. The assay finally was developed by using 4-methylumbelliferyl-β-d-galactoside freshly dissolved in substrate buffer (0.4 mM in 0.1 M sodium phosphate, 1 mM MgCl2). The amount of NGF was calculated by using a standard curve determined on the same plate.
For BDNF, microtiter plates were coated with the mAb BDNF1 and incubated overnight at 4°C. After blocking, standards (2–500 pg/ml) and samples were incubated overnight at 4°C together with the mAb BDNF9 conjugated to peroxidase. The assay was developed by using BM Blue peroxidase substrate (Boehringer Mannheim).
For BDNF-myc, microtiter plates were coated with purified antibodies against a myc epitope and incubated overnight at 4°C. After washing and blocking with PBS 1% BSA, 0.1% Triton X-100 samples were incubated overnight at 4°C. A third overnight incubation step with a BDNF9 antibody conjugated to peroxidase was performed. The assay was developed by using BM Blue peroxidase substrate (Boehringer Mannheim).
The analysis of the data was performed with at least four independent experiments.
RESULTS
Trk Activation Results Both in Influx of Extracellular Calcium and Calcium Mobilization from Intracellular Stores.
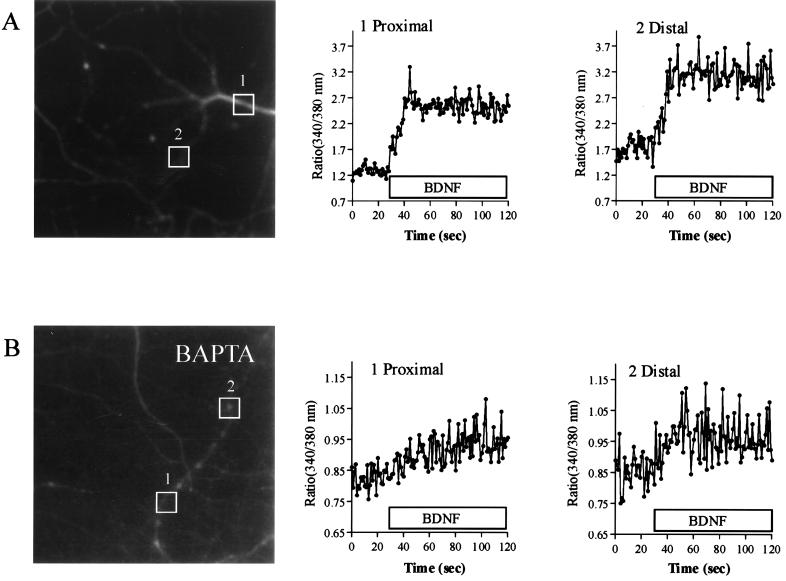
Initial experiments were designed to characterize neurotrophin-induced calcium signaling in hippocampal neurons in culture. Bath application of 100 ng/ml BDNF induced a rapid rise in the concentration of [Ca2+]i, both in dendritic processes and cell bodies of hippocampal neurons (Fig. 1 and see Fig. 7B). In calcium-free perfusion medium (0 calcium, 10 μM BAPTA) (Fig. 1B and see Fig. 7B) a substantial calcium signal remained, which disappeared after loading of hippocampal neurons with BAPTA-AM (see Fig. 7B), indicating that part of the signal was caused by calcium mobilization from intracellular stores. All of these effects of neurotrophins were blocked by 200 nM K252a, a protein kinase inhibitor with preference for Trk (53) (data not shown). Because calcium mobilization from intracellular stores previously has been shown to mediate glutamate-induced release of NGF from hippocampal neurons, the question arose whether neurotrophins regulate their own secretion in an autocrine or paracrine manner.
Figure 1.
BDNF induces [Ca2+]i elevation in hippocampal neurons. (A) Effect of bath application of 100 ng/ml BDNF on [Ca2+]i of hippocampal neurons. Neurons were loaded with Fura-2/AM, and [Ca2+]i was estimated from the respective ratio of the fluorescence at 340 and 380 nm excitation wavelength. After BDNF bath application, [Ca2+]i raises in both the proximal part (area 1) and the distal part (area 2) of the dendritic process. (B) Effect of bath application of BDNF on the [Ca2+]i of hippocampal neurons in absence of extracellular calcium (0 calcium, 10 μM BAPTA). After BDNF bath application, [Ca2+]i raises in both the proximal part (area 1) and the distal part (area 2) of the dendritic process.
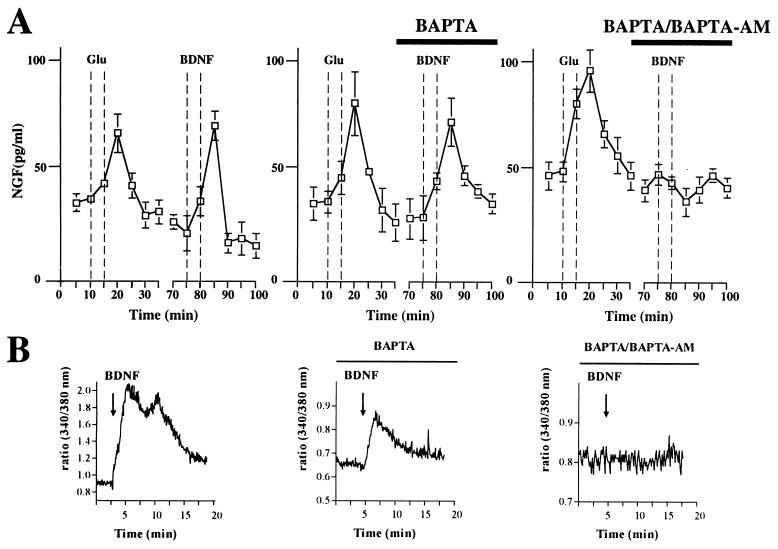
Figure 7.
BDNF-induced NGF release occurs independently of extracellular calcium, but requires mobilization of calcium from intracellular stores. (A) Cultured hippocampal neurons, which had been transduced with AdCMV-NGF, were perfused continuously. BDNF-induced secretion of NGF was unaffected by removal of extracellular calcium and the presence of BAPTA, but was fully abolished by intracellular chelation of calcium after loading of hippocampal neurons with BAPTA-AM. Standard errors are referred to four independent experiments. (B) BDNF mobilizes calcium from intracellular stores. Cultured hippocampal neurons were loaded with Fura-2/AM and the effect of bath application of 100 ng/ml BDNF on [Ca2+]i was measured in the cell body. Graphs depict representative responses. In the absence of extracellular calcium a [Ca2+]i elevation still was observed, albeit reduced, indicating that BDNF can mobilize calcium from intracellular stores. Intracellular chelation of calcium by previous loading of the neurons with BAPTA-AM abolished any change in the fluorescence ratio.
Comparison between Glutamate and Neurotrophin-Mediated Neurotrophin Release in Hippocampal Cultures and Hippocampal Slices.
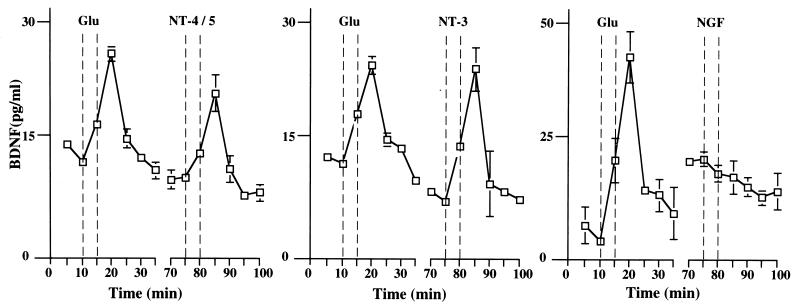
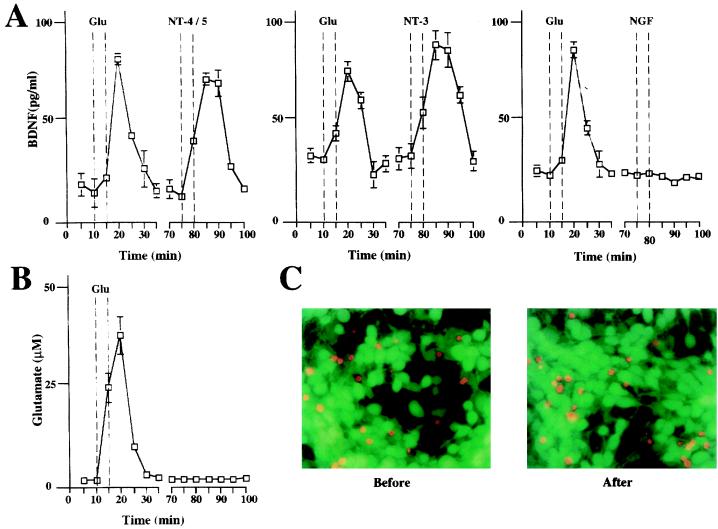
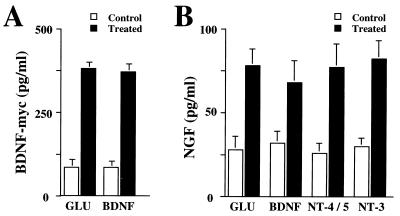
To study the effect of neurotrophins on the secretion of BDNF, hippocampal slices from adult rats were placed in a perfusion chamber and the perfusate was collected at 5-min intervals (Fig. 2). The quantity of BDNF in each fraction then was determined by a two-site immunoassay. NT-4/5 and NT-3 (100 ng/ml) rapidly induced release of BDNF, with maximum levels in the perfusate being reached 5 min after the beginning of neurotrophin administration. The time course and the extent of BDNF secretion elicited by NT-4/5 and NT-3 were similar to that observed after treatment with 50 μM glutamate (Fig. 2). Similar experiments were performed with primary cultures of hippocampal neurons, in which BDNF was overexpressed by using adenovirus-mediated gene transfer, as endogenous BDNF levels were too low to be detected reliably (47). The characteristics of basal and glutamate-induced secretion of BDNF apparently were not changed by transduction with an adenovirus encoding BDNF, as shown in Fig. 3. Glutamate, NT-4/5, and NT-3 induced BDNF release with a similar time course. The amount of glutamate present in each fraction of the perfusate was measured to compare it with the corresponding amount of secreted BDNF. Glutamate and BDNF levels peaked in the same fraction (Fig. 3B). Because the release of BDNF induced by BDNF itself is of particular interest, we studied BDNF secretion from hippocampal neurons in culture, which had been infected with an adenovirus encoding myc-tagged BDNF. Indeed, BDNF induced release of BDNF-myc (Fig. 4A). Similarly, BDNF-myc release also was evoked by NT-4/5 and NT-3 (data not shown). In contrast, NGF did not elicit an increase in BDNF release, neither from hippocampal slices nor cultured hippocampal neurons (Fig. 2 and 3A). This is in agreement with the failure of NGF to increase [Ca2+]i in hippocampal neurons (18), reflecting the fact that hippocampal neurons do not express functional TrkA at this developmental stage.
Figure 2.
Neurotrophins induce BDNF release from acute hippocampal slices. Time course of BDNF release from hippocampal slices before, during, and after application of 50 μM glutamate or 100 ng/ml neurotrophins (NT-4/5, NT-3, or NGF). Fractions were collected every 5 min, and BDNF contents were measured with a two-site ELISA. A recovery phase of 30 min was allowed between different stimulations. The 5-min delay between the onset of stimulation and the peak of BDNF secretion is caused by the dead volume of the perfusion system (approximately 0.5 ml) corresponding to 5 min of perfusion time. Standard errors are referred to four independent experiments.
Figure 3.
Neurotrophins induce BDNF release from hippocampal neuron in culture. (A) Time course of BDNF release from hippocampal primary culture infected with an adenovirus expressing BDNF before, during, and after application of 50 μM glutamate or 100 ng/ml neurotrophins (NT-4/5, NT-3, and NGF). Hippocampal neurons in culture (3 × 105) overexpressing BDNF were perfused as described in Fig. 2. Standard errors are referred to four independent experiments. (B) Time course of glutamate recovered from hippocampal cultures. Aliquots of each fraction were analyzed for glutamate contents. The same set of experiments as that used to study the effect of NT-4/5. The dashed lines indicate the time of 50 μM glutamate application. (C) Cell viability was evaluated by labeling living (green) and dead (red) cells by using fluorescein-diacetate and propidium iodide before and after each release experiment.
Figure 4.
(A) BDNF induces BDNF-myc release from hippocampal neurons in culture. Release experiments were conducted under conditions without constant perfusion, “static conditions” for which 2 × 106/1.8 cm2 hippocampal neurons had been transduced by AdCMV-BDNF-myc. Release of BDNF-myc was induced either by 50 μg/ml glutamate or 100 ng/ml BDNF, and BDNF-myc content was determined by a two-site immuno-assay. (B) NGF release from hippocampal neuron in culture. Hippocampal neurons (3 × 105) were perfused continuously, and NGF release from cultured hippocampal neurons infected with AdCMV-NGF was induced either by 50 μM glutamate or 100 ng/ml neurotrophins (NT-4/5, NT-3, and BDNF). Standard errors are referred to four independent experiments.
Similar to BDNF, NGF release from cultured hippocampal neurons, transduced by an adenovirus encoding NGF, was increased after administration of either BDNF, NT-4/5, or NT-3, providing evidence that the secretory mechanisms are similar for all neurotrophins (Fig. 4B).
The Release of Neurotrophins Depends on Neurotrophin-Mediated Activation of Trk.
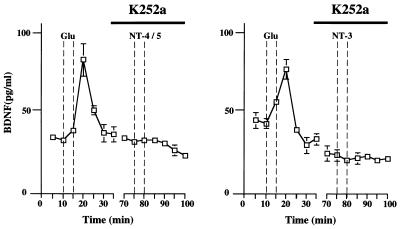
At relatively low concentrations, the protein kinase inhibitor K252a predominantly inhibits the tyrosine kinase activity of Trk. In the presence of 200 nM of K252a (Fig. 5) BDNF release induced by either NT-4/5 or NT-3 was completely blocked. Likewise, neurotrophin-induced release of NGF was fully abolished under these experimental conditions (data not shown).
Figure 5.
Neurotrophin-induced BDNF release from hippocampal neurons requires Trk activation. In perfusion medium containing 200 nM K252a, 100 ng/ml neurotrophin (NT-4/5 or NT-3)-induced release of BDNF was abolished completely. Cultured hippocampal neurons had been transduced with an adenovirus gene transfer system expressing BDNF. Standard errors are referred to four independent experiments.
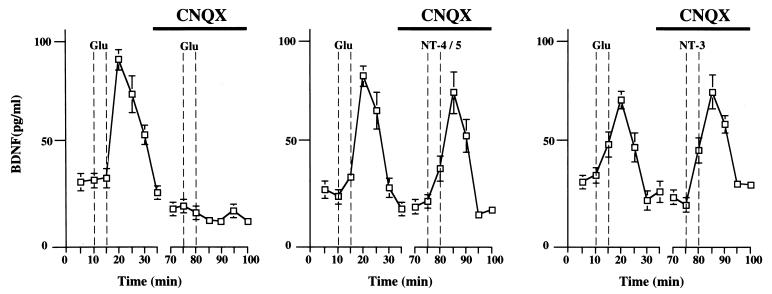
Stimulation with neurotrophins may have led to increased release of glutamate from presynaptic nerve terminals, which in turn may have induced neurotrophin release by activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionat (AMPA)-type glutamate receptors. Although the release of BDNF elicited by glutamate from hippocampal neurons was prevented in the presence of the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione, it occurred upon NT-4/5 or NT-3 stimulation (Fig. 6), demonstrating that neurotrophin-induced neurotrophin release results from a direct activation of Trk by neurotrophins rather than indirectly via the release of glutamate.
Figure 6.
Neurotrophin-induced BDNF release from hippocampal neurons does not require activation of non-NMDA type glutamate receptors. 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μM), an inhibitor of non-NMDA type glutamate receptors, inhibits glutamate-induced release of BDNF but had no effect on NT-4/5-induced or NT-3-induced release from hippocampal neurons in culture transduced with an adenovirus gene transfer system expressing BDNF. Standard errors are referred to four independent experiments.
Neurotrophin-Induced Neurotrophin Release Depends on Calcium Release from Intracellular Calcium Stores.
Omission of calcium from the perfusion medium in the presence of 10 μM BAPTA had no effect on the extent of BDNF-induced NGF release, indicating that neurotrophin-induced neurotrophin secretion occurs independently from extracellular calcium. In contrast, loading of hippocampal neurons with BAPTA-AM fully abolished secretion of NGF from cultured hippocampal neurons transduced with NGF adenovirus (Fig. 7A). The same results were obtained under experimental conditions without constant perfusion, “static condition” (data not shown). As demonstrated by calcium imaging, the ability of BDNF to induce NGF secretion was dependent on its ability to mobilize intracellular calcium (Fig. 7B). Furthermore, depletion of intracellular calcium stores by caffeine and/or thapsigargin caused a marked impairment of BDNF-induced NGF release or NT-4/5-mediated BDNF release, whereas acute application of caffeine induced release of NGF or BDNF (data not shown), indicating that calcium release from intracellular stores is necessary and sufficient for neurotrophin-induced neurotrophin secretion.
Characterization of Neurotrophin-Induced Neurotrophin Release in PC12 Cells.
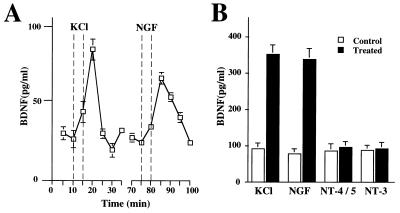
We infected PC12 cells with adenovirus expressing BDNF and compared the release of BDNF elicited by high potassium with that induced by NGF, NT-4/5, or NT-3 (Fig. 8 A and B). In agreement with the exclusive expression of TrkA in PC12 cells, BDNF secretion was elicited only by NGF, to a similar extent as by 50 mM KCl. In contrast, NT-3 or NT-4/5 failed to cause BDNF release from PC12 cells. This data indicate that an involvement of the pan-neurotrophin receptor (P75NTR) in neurotrophin-induced release of neurotrophins is unlikely, but that it requires activation of Trk. Indeed in mutant PC12 cell line PC12NNR5, a variant that only expresses P75NTR but no TrkA, NGF failed to elicit release of BDNF (data not shown).
Figure 8.
NGF induces BDNF release from PC12 cells. (A) Time course of BDNF release from undifferentiated PC12 cells before, during, and after application of 50 mM KCl or 100 ng/ml NGF. PC12 cells (3 × 105) infected with an adenovirus gene transfer system expressing BDNF were perfused as described in Fig. 2. BDNF contents, expressed in pg/ml, was measured with a two-site ELISA system. (B) Release experiments were conducted under conditions without constant perfusion, “static condition” by using 2 × 106/1.8 cm2 confluent PC12 cells. Cells were infected with about 5 × 106 plaque-forming units of an adenovirus gene transfer system expressing BDNF. After washing, the release of BDNF was initiated either by 50 mM KCl or 100 ng/ml neurotrophins (NGF, NT-4/5 or, NT-3) and BDNF content was determined by ELISA. Standard errors are referred to four independent experiments.
DISCUSSION
For the elucidation of cellular and molecular mechanisms responsible for the modulatory role of neurotrophins in activity-dependent neuronal plasticity, information on the regionally differential expression of neurotrophins or their receptors and the identification of the mechanisms and sites of release of neurotrophins are of crucial importance. We report here that neurotrophins can regulate their own release via activation of Trk. Neurotrophin-induced neurotrophin secretion is independent of extracellular calcium, but requires mobilization of [Ca2+]i. Interestingly, both the extent and time course of this release resembled that elicited by glutamate or depolarization with high concentrations of potassium. That these effects were mediated via Trk rather than the P75NTR was concluded from the following observation: BDNF and NT-4/5, both ligands of Trk B and NT-3, the preferred ligand of TrkC, induced release of neurotrophins from hippocampal neurons, respectively (Figs. 2 and 3). To date there is little evidence for the expression of TrkA in hippocampus (54) although a PCR product coding for TrkA recently has been demonstrated from mRNA extract of dendritic process of hippocampal neurons (55). However, under our experimental conditions NGF did not induce neurotrophin release in these neurons (Figs. 2 and 3) but elicited neurotrophin release from PC12 cells (Fig. 8), which express TrkA. Moreover, in a mutant PC12 cell line (PC12NNR5), which only expresses P75NTR but no TrkA, NGF failed to induce neurotrophin release. The latter indicates that an involvement of P75NTR is very unlikely to occur although a cooperative interaction between p75NTR and TrkA (56) that further modulates neurotrophin-induced neurotrophin release cannot be excluded. Finally, all neurotrophin-induced neurotrophin release was blocked by previous treatment with K252a, a protein kinase inhibitor with high preference for Trk (Fig. 5).
A critical step in the neurotrophin-induced neurotrophin release downstream of Trk activation consists in the generation of a transient calcium signal. Loading of hippocampal neurons with the intracellular high-affinity calcium chelator BAPTA-AM completely abolished the neurotrophin-induced neurotrophin secretion, whereas omission of calcium and presence of the extracellular calcium chelator BAPTA did not interfere with the secretion pointing to calcium release from intracellular stores as the triggering step for neurotrophin secretion (Fig. 7A). The involvement of intracellular calcium stores was supported further by the observation that depletion of intracellular calcium stores by pretreatment with thapsigargin or caffeine impaired neurotrophin-induced neurotrophin secretion. In fact, direct imaging of the intracellular calcium concentration with the indicator Fura-2 revealed that BDNF is capable of mobilizing intracellular calcium in the absence of extracellular calcium, although the calcium signal was reduced (Figs. 1 and 7B). The residual calcium signal was eliminated by the intracellular loading with BAPTA-AM (Figs. 1 and 7B).
Mechanistically, neurotrophin-induced secretion of neurotrophins thus resembles neurotrophin secretion elicited by glutamate, therefore the question arises whether neurotrophin-induced neurotrophin release may result indirectly as an exclusive consequence of the release of glutamate. However, this possibility can be ruled out, because the non-NMDA type glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione, which is known to block glutamate-induced neurotrophin secretion, did not abolish neurotrophin secretion elicited by neurotrophins. Therefore, neurotrophin-induced secretion of neurotrophins is likely to be a direct consequence of Trk activation, which is known to activate phospholipase C-γ-1, leading to the formation of inositol 1,4,5 trisphosphate and calcium mobilization from the endoplasmic reticulum (57, 58).
The nature of the secretory machinery responsible for the regulated neurotrophin release to date has remained elusive. The puzzling observation that release of calcium from intracellular stores is an essential step for triggering neurotrophin secretion whereas influx of extracellular calcium contributes little, if anything, to the calcium increase required for secretion in hippocampal neurons, suggests that the localization of the calcium sensor associated with the secretory machinery must be fairly distant from the sites of calcium influx, but close to the sites of intracellular calcium release. This is in distinct contrast to the activity-dependent release of neurotransmitters resulting from the exocytosis of synaptic vesicles, which are closely associated with voltage-gated calcium channels, whose activation results in a sufficiently high calcium concentration necessary to trigger the exocytosis (62–64). Fura-2 imaging revealed that BDNF mobilizes calcium within dendritic processes, also reflecting the sites of neurotrophin release. Immunohistochemical studies have demonstrated that NGF and BDNF are colocalized with markers of the endoplasmic reticulum (ref. 44; A. Gärtner, personal communication). On the basis of the currently available information it seems reasonable to assume that the calcium sensor is localized at or close to the endoplasmic reticulum, which is also an important site of the intracellular calcium storage. One therefore may hypothesize neurotrophins are released from a subcompartment of the endoplasmic reticulum located close to the plasma membrane.
Neurotrophins elicit changes in synaptic efficacy through both presynaptic and postsynaptic effects. As a presynaptic modification, neurotrophins have been shown to augment transmitter release, resulting in an increased frequency of miniature synaptic events (9, 12). Although the mechanism responsible for the increased transmitter release has not yet been clarified in detail, it may involve potentiation of voltage-gated calcium channels, as the effect depends on extracellular calcium (20). As a postsynaptic modification, BDNF and NT-4/5 were shown to stimulate the phosphorylation of NMDA receptors (16), resulting in increased postsynaptic charge transfer (14). In agreement with these findings, BDNF potentiates calcium influx in dendritic varicosities during spontaneous synaptic activity (59). Conversely, BDNF attenuates the transmission via γ-aminobutyric acidA receptors (60). The effect of BDNF on inhibitory synaptic events was completely abolished by loading of hippocampal neurons with BAPTA or previous treatment with an inhibitor of phospholipase C, suggesting that these effects of BDNF required calcium mobilization from intracellular stores. Recently, it has been shown that presynaptic stimulation indeed can induce neurotrophin release from the postsynaptic cell, resulting in synaptic potentiation (61). When Xenopus spinal neurons innervating myocytes overexpressing NT-4/5 were stimulated at high frequency, evoked postsynaptic responses were rapidly potentiated, an effect that was blocked in the presence of TrkB-IgGs, whereas synapses formed on control myocytes showed use-dependent depression.
Because neurotrophins are likely to be released both from dendrites and axons, they may, depending on the expression pattern of their respective Trks, enhance their own release in an autocrine or paracrine manner. At synapses, activity-dependent release of neurotrophins might become additionally amplified via a neurotrophin-dependent component, thereby further regulating the efficacy with which neurotrophins can modulate synaptic transmission.
Acknowledgments
We thank Yves-Alain Barde and Tobias Bonhoeffer for critically reading this manuscript and Jens Richter and Claudia Huber for excellent technical assistance. B.B. was supported by a fellowship from the Human Frontier Science Program Organization. G.C. was supported by a fellowship from Societa′ Italiana di Farmacologia.
ABBREVIATIONS
- NGF
nerve growth factor
- BDNF
brain-derived neurotrophic factor
- NT-3 and NT-4/5
neurotrophin-3, -4/5
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid pentaacetoxymethyl ester
- NMDA
N-methyl-d-aspartic acid
- LTP
long-term potentiation
- Trk
tyrosine kinase neurotrophin receptor
- p75NTR
pan-neurotrophin receptor
- [Ca2+]i
free intracellular calcium
- Fura-2AM
1-[2-(5-carboxyoxazol-2-yl)-6-aminobenzofuran-5-oxy]-2-(2′-amino-5′methylphenoxy)-ethane-N,N,N′,N′-tetraacetic acid pentaacetoxymethyl ester
References
- 1.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 2.Bonhoeffer T. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 3.Cellerino A, Maffei L. Prog Neurobiol. 1996;49:53–63. doi: 10.1016/0301-0082(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 4.Cabelli R J, Hohn A, Shatz C J. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 5.Cabelli R J, Shelton D L, Segal R A, Shatz C J. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 6.Bothwell M. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 7.Lewin G R, Barde Y-A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 8.Lindholm D, Carroll P, Tzimagiogis G, Thoenen H. Eur J Neurosci. 1996;8:1452–1460. doi: 10.1111/j.1460-9568.1996.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 9.Lohof A M, Ip N Y, Poo M-M. Nature (London) 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 10.Knipper M, Berzaghi M P, Blöchl A, Thoenen H, Lindholm D. Eur J Neurosci. 1994;6:668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 11.Knipper M, Leung L S, Zhao D, Rylett R. NeuroReport. 1994;5:2433–2436. doi: 10.1097/00001756-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Lessmann V, Gottmann K, Heumann R. NeuroReport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 13.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 14.Levine E S, Dreyfus C F, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berninger B, Poo M M. Curr Opin Neurobiol. 1996;6:324–330. doi: 10.1016/s0959-4388(96)80115-2. [DOI] [PubMed] [Google Scholar]
- 16.Suen P C, Wu K, Levine E S, Mount H T J, Xu J L, Lin S Y, Black I B. Proc Natl Acad Sci USA. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blöchl A, Sirrenberg C. J Biol Chem. 1996;271:21100–21107. doi: 10.1074/jbc.271.35.21100. [DOI] [PubMed] [Google Scholar]
- 18.Berninger B, García D E, Inagaki N, Hahnel C, Lindholm D. NeuroReport. 1993;4:1303–1306. doi: 10.1097/00001756-199309150-00004. [DOI] [PubMed] [Google Scholar]
- 19.Marsh H N, Palfrey H C. J Neurochem. 1996;67:952–963. doi: 10.1046/j.1471-4159.1996.67030952.x. [DOI] [PubMed] [Google Scholar]
- 20.Stoop R, Poo M-M. J Neurosci. 1996;16:3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson S L, Abel T, Deuel T A S, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 23.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figurov A, Pozzo-Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 25.Maisonpierre P C, Belluscio L, Friedman B, Alderson R F, Wiegand S J, Furth M E, Lindsay R M, Yancopoulos G D. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 26.Gall C M, Isackson P J. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- 27.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu B, Yokoyama M, Dreyfus C F, Black I B. Proc Natl Acad Sci USA. 1991;88:6289–6292. doi: 10.1073/pnas.88.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindholm D, Castrén E, Berzaghi M, Blöchl A, Thoenen H. J Neurobiol. 1994;25:1362–1372. doi: 10.1002/neu.480251105. [DOI] [PubMed] [Google Scholar]
- 30.Marty S, Berzaghi M P, Berninger B. Trends Neurosci. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- 31.Patterson S L, Grover L M, Schwartzkroin P A, Bothwell M. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 32.Castrén E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen P J. NeuroReport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Dragunow M, Beilharz E, Mason B, Lawlor P, Abraham W, Gluckman P. Neurosci Lett. 1993;160:232–236. doi: 10.1016/0304-3940(93)90420-p. [DOI] [PubMed] [Google Scholar]
- 34.Castrén E, Zafra F, Thoenen H, Lindholm D. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bozzi Y, Pizzorusso T, Cremisi F, Rossi F M, Barsacchi G, Maffei L. Neuroscience. 1995;69:1133–1144. doi: 10.1016/0306-4522(95)00321-9. [DOI] [PubMed] [Google Scholar]
- 36.Rocamora N, Welker E, Pascual M, Soriano E. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neeper S A, Gomez-Pinilla F, Choi J, Cotman C. Nature (London) 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 38.Castrén E, Thoenen H, Lindholm D. Neuroscience. 1995;64:71–80. doi: 10.1016/0306-4522(94)00386-j. [DOI] [PubMed] [Google Scholar]
- 39.Falkenberg T, Mohammed A K, Henriksson B, Persson H, Winblad B, Lindefors N. Neurosci Lett. 1992;138:153–156. doi: 10.1016/0304-3940(92)90494-r. [DOI] [PubMed] [Google Scholar]
- 40.Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- 41.Isackson P J, Huntsman M M, Murray K D, Gall C M. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- 42.Dugich-Djordjevic M M, Tocco G, Lapchak P A, Pasinetti G M, Najm I, Baudry M, Hefti F. Neuroscience. 1996;47:303–315. doi: 10.1016/0306-4522(92)90246-x. [DOI] [PubMed] [Google Scholar]
- 43.Blöchl A, Thoenen H. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 44.Blöchl A, Thoenen H. Mol Cell Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- 45.Heymach J, Jr, Kruttgen A, Suter U, Shooter E M. J Biol Chem. 1996;271:25430–25437. doi: 10.1074/jbc.271.41.25430. [DOI] [PubMed] [Google Scholar]
- 46.Goodman L J, Valverde J, Lim F, Geschwind M D, Federoff H J, Geller A I, Hefti F. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 47.Griesbeck O, Blöchl A, Carnaham J F, Nawa H, Thoenen H. Soc Neurosci Abstr. 1995;417:1046. [Google Scholar]
- 48.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 49.McGrory W J, Bautista D S, Graham F L. Virology. 1989;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 50.Graham F L, Prevec L. Methods Mol Biol. 1991;7:109–128. doi: 10.1385/0-89603-178-0:109. [DOI] [PubMed] [Google Scholar]
- 51.Evan G I, Lewis G K, Ramsaz G, Bishop J M. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones K H, Senft J A. J Histochem Cytochem. 1985;33:77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- 53.Knusel B, Hefti F. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- 54.Holtzman D M, Kilbridge J, Li Y, Cunningham E T, Lenn N J, Clar D O, Reichardt L F, Mobley W C. J Neurosci. 1995;15:1567–1576. doi: 10.1523/JNEUROSCI.15-02-01567.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crino P B, Eberwine J. Neuron. 1996;17:1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 56.Chao M V, Hempstead B L. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- 57.Tinhofer I, Maly K, Dietl P, Hochholdinger F, Mayr S, Obermeier A, Grunicke H H. J Biol Chem. 1996;271:30505–30509. doi: 10.1074/jbc.271.48.30505. [DOI] [PubMed] [Google Scholar]
- 58.Obermeier A, Tinhofer I, Grunicke H H, Ullrich A. EMBO J. 1996;15:73–82. [PMC free article] [PubMed] [Google Scholar]
- 59.Berninger B, Thoenen H. Soc Neurosci Abstr. 1996;397:995. [Google Scholar]
- 60.Tanaka T, Saito H, Matsuki N. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, X-h. & Poo, M.-M. (1997) Neuron, in press. [DOI] [PubMed]
- 62.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 63.Burgoyne R D, Morgan A. Trends Neurosci. 1995;18:191–196. doi: 10.1016/0166-2236(95)93900-i. [DOI] [PubMed] [Google Scholar]
- 64.Calakos N, Scheller R H. Physiol Rev. 1996;76:1–29. doi: 10.1152/physrev.1996.76.1.1. [DOI] [PubMed] [Google Scholar]