Abstract
The immediacy and directness of our subjective visual experience belies the complexity of the neural mechanisms involved, which remain incompletely understood. This review focuses on how the subjective contents of human visual awareness are encoded in neural activity. Empirical evidence to date suggests that no single brain area is both necessary and sufficient for consciousness. Instead, necessary and sufficient conditions appear to involve both activation of a distributed representation of the visual scene in primary visual cortex and ventral visual areas, plus parietal and frontal activity. The key empirical focus is now on characterizing qualitative differences in the type of neural activity in these areas underlying conscious and unconscious processing. To this end, recent progress in developing novel approaches to accurately decoding the contents of consciousness from brief samples of neural activity show great promise.
Keywords: consciousness, vision, functional magnetic resonance imaging, awareness
1. Introduction
The subjective experience of conscious mental contents is central to our everyday life. Whether the content of such subjective experiences can be reliably decoded and predicted from patterns of brain activity is an empirical question. Techniques for non-invasive measurement of human brain activity such as functional magnetic resonance imaging (fMRI), positron emission tomography, electroencephalography (EEG) and magnetoencephalography (MEG) can reveal the neural substrates of both conscious and unconscious processing underlying these subjective experiences. This review focuses on recent attempts to identify experimentally the neural correlates of the contents of consciousness related to visual perception in humans.
Consciousness and awareness of a stimulus are used interchangeably in this review to indicate the ability of an observer to report either the presence of that stimulus or its identity. In contrast, unconscious or invisible stimuli fail to be reported successfully or are associated with responses that objectively indicate failure to discriminate the presence or identity of the stimulus (e.g. d′∼=0). The experiments reviewed here relate these differences in the reportability of stimuli (reflecting differences in the subjective contents of consciousness) to brain activity measured using non-invasive imaging techniques. In general, such studies to date have found consistently that both visible and invisible or unreportable stimuli nevertheless undergo processing in both anatomically early and higher levels of the human visual system. This makes a simple division of sensory areas into those supporting conscious or unconscious processing, respectively, increasingly untenable. Instead, the key empirical question is now to establish what quantitative and qualitative differences in brain activity distinguish conscious and unconscious processing (Frith et al. 1999). Such efforts have benefited from recent developments in analysing fMRI data that focus not only on focal changes in brain activity, but also on decoding the information contained within local spatial patterns of activity (Haynes & Rees 2006; Norman et al. 2006). While the application of these techniques is only just beginning, they place new emphasis on determining how much information about mental states can be decoded from functional brain images; and, in turn, they raise the challenge of specifying what information would be sufficient to decode consciousness.
2. Unconscious processing in human visual cortex
A prerequisite for appreciating how the contents of consciousness might be represented in patterns of brain activity is an understanding of which brain structures and psychological processes are associated with unconscious processing (see also Kouider & Dehaene 2007). Delineating the quantitative and qualitative differences between conscious and unconscious processing is central to identifying the neural basis of consciousness. There is now substantial evidence that processing of sensory stimuli outside awareness can influence behaviour (e.g. Marcel 1983; Naccache et al. 2002, though see Holender & Duscherer (2004) for a sceptical critique); but the neural bases of such processing remain under active exploration.
At the anatomically earliest stages of cortical processing, activity in primary visual cortex (V1) can readily be identified even in the absence of visual stimulation. For example, saccadic eye movements or blinks made in complete darkness nevertheless evoke generalized activity in retinotopically defined human primary visual cortex (Bodis-Wollner et al. 1999; Bristow et al. 2005; Sylvester et al. 2005; Sylvester & Rees 2006). Such signals, presumably representing ‘efference copy’ of the motor command, are usually not associated with any perception of phenomenal content (though see Enoch et al. 2003). Activation of V1 per se is therefore insufficient for awareness. Activation without awareness in early retinotopic visual areas can be confined to the retinotopic location of an invisible target. When a simple target such as an achromatic disc is briefly flashed, robust activity is elicited in the corresponding retinotopic location of V1 (plus V2 and V3) even when the target is rendered completely invisible by a surrounding mask (Haynes et al. 2005b). These experiments establish that invisible stimuli can evoke retinotopically specific activity in early visual cortex. However, they do not determine whether the specific neuronal processes elicited within these regions differ for visible and invisible stimuli. Such a determination requires a more direct way of measuring the neuronal representations associated with stimulus presentation.
Neurons in early visual cortex are sensitive to a number of visual features, such as orientation and direction of motion. It is well established that orientation-selective after-effects can result from exposure to grating stimuli that are too fine to be consciously perceived (He & MacLeod 2001), suggesting that orientation-selective but unconscious activation of visual cortex is possible. Direct physiological measurement of such unconscious feature-selective processing in human V1 has proven elusive due to the relatively low spatial resolution (several millimetres) of functional neuroimaging methods when compared with the size of orientation columns in visual cortex (hundreds of microns). However, it has recently become possible to use fMRI even at conventional resolutions (typically, voxels measuring 3×3×3 mm) to obtain a direct measure of orientation-selective processing in V1 (Haynes & Rees 2005a; Kamitani & Tong 2005). Many individual fMRI voxels in V1 show subtle but reproducible biases in their activity when differently oriented stimuli are presented to the experimental subject. This is thought to reflect biased sampling by the (relatively) low spatial resolution voxels of the underlying columnar organization of orientation-specific neuronal populations, due to an uneven distribution of different orientation specificities across the cortical surface (for more detailed explanations, see Kamitani & Tong 2005 or Haynes & Rees 2005a). Importantly, this information can be efficiently accumulated across the whole of V1 using multivariate pattern recognition analyses (for reviews, see Haynes & Rees 2006; Norman et al. 2006). Such multivoxel pattern analysis can successfully predict which one of the two oriented stimuli a participant is viewing, even when masking renders that stimulus invisible (figure 1a,b; Haynes & Rees 2005a). This indicates the presence of feature-selective processing in early visual cortex, even for invisible stimuli. Such analyses thus open up the possibility of probing different levels of neuronal representation associated with conscious or unconscious processing.
Figure 1.
Unconscious and conscious processing in V1. (a) An oriented target annulus can be effectively rendered invisible by subsequently presenting a contrast-inverted mask with no specific orientation. (b) However, pattern-based decoding applied to V1 activity measured using fMRI can still successfully determine significantly greater than chance which target orientation was presented to a subject. Decoding is not significantly different from chance in V2 and V3. This demonstrates that unconscious information about target orientation must be present in the fMRI signals in V1. Data are from Haynes & Rees (2005a). (c) Apparent motion on a curved path can be perceived by alternating appearance of tilted line inducers on the horizontal or vertical meridians. (d) Perception of apparent motion is associated with significantly enhanced feedback effective connectivity (as assessed with dynamic causal modelling) from V5/MT to the retinotopic location in V1 on the path of apparent motion. Data are from Sterzer et al. (2006).
Beyond primary visual cortex, the human ventral visual pathway comprises multiple functionally specialized visual areas that respond to different attributes of the visual scene. Activation of such areas (albeit modest in amplitude) is consistently identified for invisible words, faces and objects. For example, masked and invisible words can activate the ‘temporal word form area’ (Dehaene et al. 2001). Dichoptically masked and therefore invisible object and face stimuli can nevertheless activate functionally specialized areas of ventral visual cortex despite their invisibility (Moutoussis & Zeki 2002). Such observations are not restricted to different types of visual masking, as unconscious activation of the ventral visual pathway during the ‘attentional blink’ can reflect object category (Marois et al. 2004) and semantic analysis of visually presented words (Luck et al. 1996). Visual motion rendered invisible through ‘crowding’ can still activate V5/MT (Moutoussis & Zeki 2006). Changes in an object that are not perceived due to introduction of visual flicker between changes nevertheless lead to category-specific activity in the ventral visual pathway that can precede conscious change detection (Niedeggen et al. 2001).
Unconscious sensory activation by complex visual stimuli is not confined to the ventral visual pathway, but extends to both dorsal and subcortical structures. Dorsal cortical areas show activation for different types of objects even when observers are completely unaware of their identity through binocular suppression (Fang & He 2005). Moreover, the amygdala can be activated by fearful face stimuli rendered invisible through masking (Morris et al. 1999), in response to the emotional content of invisible words (Naccache et al. 2005) or during suppression in binocular rivalry (Pasley et al. 2004).
Unconscious activation of ventral visual cortex can also be identified following parietal damage causing visual extinction. Patients with visual extinction show deficient awareness for contralesional visual stimuli, particularly when a competing stimulus is also present ipsilesionally. When visual stimuli are presented to patients with visual extinction, areas of both primary and extrastriate visual cortex that are activated by a seen left visual field stimulus are also activated by an unseen and extinguished left visual field stimulus (Rees et al. 2000, 2002b; Vuilleumier et al. 2001). The unconscious processing of an extinguished face stimulus extends even to face selective cortex in the fusiform face area (FFA; Rees et al. 2002b; see also Vuilleumier & Driver 2007).
Taken together, these data present a picture of modest (in terms of the level of activity evoked) but pervasive unconscious processing of visual stimuli throughout the dorsal and ventral visual pathways, plus subcortical structures. In particular, all visually responsive cortical areas appear to show evidence for unconscious visual processing.
3. Conscious processing in human visual cortex
Signals recorded from most, if not all, parts of the human visual pathways can therefore show signals associated with processing visual stimuli that do not reach awareness. However, evidence will now be reviewed indicating that signals recorded from these regions can also show signals strongly correlated with the contents of consciousness.
At the anatomically earliest stages of visual processing, signals in V1 scale linearly with the magnitude of change in retinal illumination, as do subjects' subjective ratings of the perceived brightness of the stimuli (Haynes et al. 2004). Such a close correspondence between fMRI signals and phenomenal perception is consistent with a role for these areas in representing conscious contents. More direct evidence has been provided by studies of sensory stimulation at perceptual threshold. For simple grating stimuli, a stimulus that is successfully detected by a subject evokes significantly greater activity in V1 when compared with identical stimuli that do not reach awareness (Ress & Heeger 2003). Such conscious detection of threshold-level stimuli is associated with very early differential electrical signals at posterior electrodes (Pins & Ffytche 2003), suggesting that changes in conscious contents can be associated with activity that is both temporally and anatomically early in processing. However, it is important to note that it is not necessarily the case that conscious and unconscious processing can always be distinguished early in time. In some experimental situations, conscious and unconscious processing can only be dissociated at a much later stage, after several hundred milliseconds (Vogel et al. 1998; Sergent et al. 2005). Neither need it be the case that such late divergence does not reflect V1 involvement, as such temporally delayed correlates of conscious perception can be identified in monkey V1 (Super et al. 2001), supporting theoretical suggestions that conscious perception correlates not with the ‘feed-forward’ sweep of information processing following stimulation processing but rather with later feedback or recurrent signals, perhaps to V1 (Lamme & Roelfsema 2000).
Retinotopic activity in V1 can reflect conscious perception of illusory features. When a moving grating is divided by a large gap, observers report seeing a moving ‘phantom’ in the gap and there is enhanced activity in the locations in early retinotopic visual cortex corresponding to the illusory percept (Meng et al. 2005). Moreover, when phantom-inducing gratings are paired with competing stimuli that induce binocular rivalry, spontaneous fluctuations in conscious perception of the phantom occur together with changes in early visual activity. Similarly, V1 activation can be found on the path of apparent motion (Muckli et al. 2005) and is associated with strengthened feedback connections to that retinotopic location from cortical area V5/MT (Sterzer et al. 2006; see figure 1c,d here). Finally, when two objects subtending identical angles in the visual field are made to appear of different sizes using three-dimensional context, the spatial extent of activation in the V1 retinotopic map reflects the perceived rather than actual angular size of the objects (Murray et al. 2006). These data thus show a rather close correspondence between either the level or spatial extent of V1 activation and the perceived phenomenal properties of the visual world.
Such close correspondence between V1 activity and conscious contents also extends to cross-modal influences on visual perception. Irrelevant auditory stimulation can lead to illusory perception of a single flash as two flashes, and primary visual cortex shows enhanced activity when compared with physically identical stimulation that is veridically perceived (Watkins et al. 2006). Moreover, these alterations in the contents of visual consciousness are associated with very early modulation of MEG responses over posterior occipital sensors (Shams et al. 2005). Responses in human V1 can therefore be altered by sound, and can reflect subjective perception rather than the physically present visual stimulus.
V1 is not the only area in the human visual pathway whose activity can reflect the contents of consciousness. Visually presented objects can be made difficult to identify by degrading them, and in such circumstances, occipitotemporal activity shows a close correlation with recognition performance (Grill-Spector et al. 2000). Similarly, conscious detection of changes in a visually presented object is associated with enhanced activity in ventral visual cortex (Beck et al. 2001). There are many other examples of similarly close correspondence between the level of activity in the ventral visual pathway and conscious perception. For example, visual imagery activates category-specific areas of visual cortex (O'Craven & Kanwisher 2000), and category-selective neurons in the human medial temporal lobe (Kreiman et al. 2000). Contingent after-effects based on colour or motion lead to activation of either V4 (Sakai et al. 1995; Barnes et al. 1999) or V5/MT (Tootell et al. 1995; He et al. 1998), respectively, and the time course of such activation reflects phenomenal experience. Perception of illusory or implied motion in a static visual stimulus is associated with activation of V5/MT (Zeki et al. 1993; Kourtzi & Kanwisher 2000; Senior et al. 2000), whereas perception of illusory contours activates extrastriate cortex (Hirsch et al. 1995; Halgren et al. 2003). Differential activity in word-processing areas is present when subjects are consciously aware of the presence and identity of visually presented words, but can be absent when they are not (Rees et al. 1999). Patients with schizophrenia who experience visual and auditory hallucinations show activity in modality-specific cortex during hallucinatory episodes (Silbersweig et al. 1995). Similarly, patients with damage to the visual system who experience hallucinations with specific phenomenal content show activity in functionally specialized areas of visual cortex corresponding to the contents of their hallucinations (Ffytche et al. 1998).
Common to all these diverse paradigms are changes in the contents of consciousness without corresponding changes in physical stimulation. Corresponding changes in activity in ventral visual cortex are seen in areas known to represent the attributes represented in the contents of consciousness. Thus, activity in the visual pathway can reflect conscious processing of visual stimuli; but the studies reviewed earlier also indicate that stimuli that are not consciously perceived can also evoke some activation of the same cortical areas, sometimes with comparable amplitude to consciously perceived stimuli (e.g. Rees et al. 2000). However, common involvement of a cortical area in both conscious and unconscious processing, as indicated by blood-oxygen-level-dependent (BOLD) signals from large neuronal populations, does not imply that all underlying neuronal processes in that area must be identical for the two conditions. Taken together, these somewhat divergent findings therefore demonstrate the importance of now trying to characterize in more detail whether visible and invisible stimuli elicit qualitatively different types of neuronal activity.
Pattern-based decoding approaches have recently been applied to fMRI signals from human ventral visual cortex. These techniques have the potential to reveal qualitative differences in neural processes underlying conscious and unconscious processing in a cortical area. Orientation and direction of motion of simple visual stimuli can be decoded from the local pattern of brain activity (Haynes & Rees 2005a; Kamitani & Tong 2005) and the identity of more complex objects (Haxby et al. 2001). Moreover, if subjects are asked to attend one of the two overlapping orientations or motion directions, then patterns of activity in early visual cortex can be used to predict which one is attended (Kamitani & Tong 2005, 2006). These data suggest that reliable decoding of the subjective contents of consciousness, at least under controlled viewing conditions, may be a realistic prospect. Moreover, the ability to provide information about the underlying specificities of the neuronal populations may now provide a future basis for characterizing whether conscious and unconscious stimuli elicit different types of activity in a single cortical region.
The experiments reviewed thus far typically focus on characterizing neural activity evoked by individual visual stimuli that either reach awareness or on other occasions remain invisible. In contrast, when a single ambiguous visual stimulus has more than one possible perceptual interpretation, conscious perception alternates spontaneously and unpredictably between each individual interpretation. Such bistable perceptual phenomena elicit dynamic ongoing fluctuations in the contents of visual awareness, but without any changes in physical stimulation. They have proven extremely popular for studying the neural correlates of consciousness and so relevant empirical data from this particular class of paradigm will now be reviewed separately.
4. Neural correlates of bistable perception and binocular rivalry
Binocular rivalry is a particularly well-known bistable phenomenon, and a popular and enduring paradigm for studying the neural correlates of consciousness (Tong et al. 2006). When dissimilar images are presented to the two eyes, they compete for perceptual dominance so that each image is consciously visible in turn for a few seconds while the other is perceptually suppressed. Such binocular rivalry is associated with relative suppression of local, eye-based representations that can also be modulated by high-level influences such as perceptual grouping. Since perceptual transitions between each monocular view occur spontaneously without any change in the physical stimulus, neural correlates of consciousness may be distinguished from neural correlates attributable to stimulus characteristics. All stages of visual processing show such activity changes associated with rivalrous fluctuations (Tong et al. 2006), which will now be reviewed in more detail.
Even at the earliest subcortical stages of visual processing, signals recorded from the human lateral geniculate nucleus (LGN) exhibit fluctuations in activity during binocular rivalry (Haynes et al. 2005a; Wunderlich et al. 2005). Regions of the LGN that show strong eye preference independently show strongly reduced activity during binocular rivalry when the stimulus presented in their preferred eye is perceptually suppressed. The human LGN is thus the earliest stage of visual processing that reflects changes in the contents of consciousness, even when physical stimulation is unchanged. Primary visual cortex shows a similar pattern of changes in activity correlated with changes in the contents of consciousness (Polonsky et al. 2000; Tong & Engel 2001; Lee & Blake 2002; Lee et al. 2005). In general (though see Tong & Engel 2001), such fluctuations in activity are about half as large as those evoked by non-rivalrous stimulus alternation. This indicates that the suppressed image during rivalry undergoes a considerable degree of unconscious processing.
Further along the ventral visual pathway, responses in FFA during rivalry are larger than those in V1 and equal in magnitude to responses evoked by non-rivalrous stimuli (Tong et al. 1998). This suggests that neural competition during rivalry has been resolved by these later stages of visual processing, and activity in FFA thus reflects the contents of consciousness rather than the retinal stimulus. However, such an account is inconsistent with the finding that binocularly suppressed faces can nevertheless still activate the FFA (Moutoussis & Zeki 2002). Moreover, Philipp Sterzer, John Haynes and myself have recently re-examined the local spatial patterns of brain activity in FFA when faces are rendered invisible by binocular suppression. Neither invisible faces nor invisible houses ‘activated’ FFA when fMRI signals were averaged across the whole of FFA, consistent with previous findings. But when local fine-grained spatial patterns of activity were taken into account using multivariate decoding, it was now possible to accurately decode whether the invisible stimulus was a face or house (figure 2). These data demonstrate that even during suppression and phenomenal invisibility in rivalry, sufficient information is encoded at high levels of the human visual system to determine the identity of the suppressed stimulus. Future research should now focus on characterizing the nature and extent of such information and whether it differs from conscious perception.
Figure 2.
Unconscious representation of face-specific information in FFA. Prediction accuracy of a multivoxel pattern-based decoder for discriminating the presentation of either a face or house from activity recorded in the fusiform face area (FFA) or parahippocampal place area (PPA), respectively. Average prediction accuracies across participants (n=5) for visible faces versus houses are denoted by filled circles (±s.e.m.) and for invisible faces versus houses by empty circles. Performance was uniformly high and significantly above chance level (50%, dotted line) for all pairwise comparisons across participants.
Other forms of bistable perception do not necessarily involve binocular competition. Nevertheless, a repeated finding is that these paradigms lead to activation of visual cortical structures that correspond to the attributes of whichever competing percept the observer is currently aware of (Kleinschmidt et al. 1998; Sterzer et al. 2002, 2003). This is consistent with the more general observation, reviewed earlier, that visible stimuli often give rise to greater activation in functionally specialized regions of visual cortex corresponding to stimulus type than corresponding invisible stimuli.
Remarkably, the information encoded in early visual cortex during binocular rivalry (as revealed by fMRI) can be sufficient to reconstruct the dynamic stream of consciousness. Information that is contained in the multivariate pattern of responses to stimulus features in V1–V3 as recorded using fMRI can be used to accurately predict, and therefore track, changes in conscious contents during rivalry (Haynes & Rees 2005b). Accurate decoding is possible for extended periods of time during rivalry, while awareness undergoes many spontaneous changes (figure 3). In that study, successful prediction of rivalry from primary visual cortex activity primarily reflected eye-based signals, whereas prediction in higher areas reflected the colour of the percept. Furthermore, accurate prediction during binocular rivalry could be established in that study on the basis of classifying signals originally recorded during stable monocular viewing, showing that prediction generalizes across different viewing conditions and does not require or rely on motor responses. It is therefore possible to predict the dynamically changing time course of subjective experience using brain activity alone. This raises the possibility that more complex dynamic changes in consciousness might be decoded, though this in turn raises important questions about whether such an approach will be able to generalize to novel mental states (Haynes & Rees 2006).
Figure 3.
Decoding the stream of consciousness from activity in human V1. A multivoxel pattern-based decoder trained on activity from V1, while participants experience binocular rivalry can successfully decode the stream of consciousness over several minutes. Data are from Haynes & Rees (2005b) and show participants' reported percepts (dotted lines) alternating between each monocular view. Blind predictions of conscious contents made from patterns of V1 activity by the decoder are shown (solid lines). A close correspondence between decoded and actual perceptual state is apparent.
5. Necessary and sufficient correlates of conscious perception in human visual cortex
fMRI and EEG/MEG studies in normal subjects, such as those discussed above, reveal the correlation between particular contents of consciousness and specific types of neural activity. However, they can neither determine whether this neural activity plays a causal role in determining the contents of consciousness, nor ascertain with certainty the necessary and sufficient correlates of consciousness. In order to do this, neural activity must be manipulated either experimentally (e.g. Vuilleumier & Driver 2007) or as a consequence of neurological disease causing brain damage.
In individuals who are blind due to retinal damage, phosphenes can still be elicited by transcranial magnetic stimulation (TMS) of visual cortex (just as in sighted individuals). This suggests that the retina is not necessary for conscious visual experience. Similarly, visual experiences of varying complexity can be elicited by direct electrical stimulation of cortical structures in the ventral visual pathway (Lee et al. 2000), suggesting that the LGN may also not be necessary for consciousness. Whether V1 activity is necessary is more controversial. TMS of visual cortex does not elicit phosphenes when blindness results from damage to V1 (Cowey & Walsh 2000), and activation of extrastriate cortex without awareness occurs when the blind visual field is stimulated in patients with damage to V1 (Ptito et al. 1999; Goebel et al. 2001). These data suggest that structural integrity of V1 is necessary for at least some types of conscious visual experience, and extrastriate cortical activity alone is not sufficient. But in patients with V1 damage, conscious vision may return over time in previously blind regions of the visual field. Stimulation of such locations in the visual field that gives rise to awareness can be associated with activation of perilesional V1 in some, but not all, patients (Kleiser et al. 2001), suggesting that V1 activity is not necessary for conscious awareness in all cases.
One possibility is that some functional aspect of V1 activity, such as its overall level or precise timing, plays a role in determining the contents of visual awareness. Consistent with this, awareness of motion is impaired if feedback signals from V5/MT to V1 are disrupted by TMS (Pascual-Leone & Walsh 2001; Silvanto et al. 2005). Similarly, using occipital TMS to disrupt processing of a metacontrast mask presented after a target can lead to unmasking and corresponding visibility of the original target (Ro et al. 2003). These data suggest that temporally late signals in V1 representing feedback from other ventral visual (or higher cortical) areas may be required for at least some forms of visual awareness. Findings reviewed earlier that conscious and non-conscious processing can be distinguished by signals occurring several hundred milliseconds after stimulus presentation (Vogel et al. 1998; Sergent et al. 2005) are consistent with such a role for feedback signals. Similarly, coupling is disrupted between the V1 representation of a target and higher visual areas when the target is rendered invisible by masking (Haynes et al. 2005b).
6. The role of frontal and parietal cortex in visual awareness
Although visual cortex plays a central role in representing the contents of visual consciousness, it has long been recognized that deficits in visual consciousness can result from damage to regions outside visual cortex (see also Vuilleumier & Driver 2007). For example, damage to frontal and parietal cortex is commonly associated with visual neglect, where patients often do not perceive or respond to any type of visual stimulus placed in one-half of the visual field, particularly in the presence of competitor stimuli in the other hemifield (Driver & Mattingley 1998). This deficit in the representation of conscious contents is consistent with a general involvement of these structures in representing many different types of conscious contents. Activation of visual cortex or subcortical structures by visual stimuli is insufficient to result in awareness in visual neglect patients (Driver et al. 2001). In visual extinction following parietal damage, a proportion of visually presented stimuli reach awareness and in such cases, awareness is associated not only with activation of visual cortical areas (Rees et al. 2000, 2002b), but also with enhanced covariation of activity between visual cortical areas representing the visual stimulus and undamaged parietal and prefrontal regions (Vuilleumier et al. 2001; see also Vuilleumier & Driver 2007).
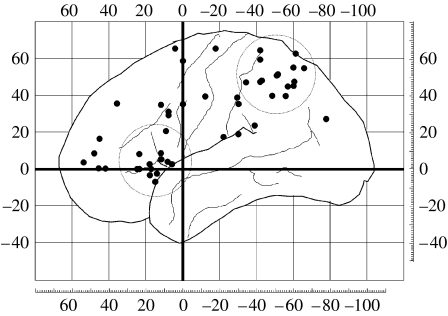
Supporting the notion that regions outside visual cortex play a role in visual awareness, a striking and consistent finding from neuroimaging experiments over the last decade is a strong association of activity in frontal and parietal cortex with changes in the contents of visual consciousness (figure 4; see also Kouider & Dehaene 2007). For example, activity during transitions in binocular rivalry, and other forms of bistable perception, is time-locked to frontal and parietal cortex activity (Kleinschmidt et al. 1998; Lumer et al. 1998; Sterzer et al. 2002). Fronto-parietal activity is not simply associated with the requirement that observers report their experience. When binocular rivalry occurs in the absence of behavioural reports, there is a close coupling between activity in early visual cortical areas representing the rivalling stimuli and multiple regions of frontal and parietal cortex previously associated with the report of conscious transitions (Lumer & Rees 1999). Such fronto-parietal involvement in rivalry therefore appears independent of the requirement to make motor reports.
Figure 4.
Fronto-parietal activation associated with awareness. Areas of parietal and prefrontal cortex that show activation correlated with changes in visual awareness (Kleinschmidt et al. 1998; Lumer et al. 1998; Sterzer et al. 2002) are plotted on a template brain. Each circle is placed at the centre of a cluster of activation; overlapping loci from the same study are omitted for clarity. There is prominent clustering of activations in superior parietal and dorsolateral prefrontal cortex, highlighted by large, dotted circles.
Strikingly, frontal and parietal activity is also associated with spontaneous changes in the contents of consciousness in a variety of perceptual paradigms, such as stereo pop-out (Portas et al. 2000), the perception of fragmented figures (Eriksson et al. 2004), the detection of change in a visually presented object (Beck et al. 2001), conscious perception of flicker (Carmel et al. 2006) and successful conscious identification of visually masked words (Dehaene et al. 2001). Electrical activity over parietal sensors is associated with the detection of a simple threshold-level stimulus (Pins & Ffytche 2003). Moreover, changes in the contents of consciousness during bistable perception are associated with distributed changes in synchronous electrical oscillations measured on the scalp (Tononi et al. 1998; Srinivasan et al. 1999; Struber & Herrmann 2002).
These data are consistent with the notion that signals from parietal and prefrontal cortex are necessary for normal conscious perception (Driver & Mattingley 1998; Rees et al. 2002a). Further direct evidence for such a hypothesis comes from the observation that conscious detection of change is impaired when frontal and parietal cortex is transiently disrupted using TMS (Turatto et al. 2004; Beck et al. 2006).
These data strongly suggest the involvement of prefrontal and parietal cortex in visual awareness, but do not determine the precise functional role of such structures nor clarify the nature of the underlying neural processes. One promising line of future enquiry will be to use the pattern-based decoding approaches that have been used to study representations in ventral visual cortex (reviewed previously here; see also Haynes & Rees 2006) to now probe the nature of the neural representations in frontal and parietal cortex associated with visual awareness.
7. Conclusion
The most parsimonious account of currently available data is that the current contents of visual consciousness consist of a representation in primary visual cortex and ventral visual pathway corresponding to the attributes represented in consciousness, together with related activity in specific parietal (and perhaps) prefrontal structures. Information contained within signals in the ventral visual pathway is sufficient to permit accurate decoding both of stable contents of visual awareness and dynamic changes in the stream of consciousness, at least for simple visual paradigms. The challenge for the future is to specify more precisely the interactions between and causal role for each of these areas, and to hence further refine our ability to decode consciousness.
Acknowledgments
The Wellcome Trust supported this work.
Footnotes
One contribution of 14 to a Discussion Meeting Issue ‘Mental processes in the human brain’.
References
- Barnes J, Howard R.J, Senior C, Brammer M, Bullmore E.T, Simmons A, David A.S. The functional anatomy of the McCollough contingent colour after-effect. Neuroreport. 1999;10:195–199. doi: 10.1097/00001756-199901180-00037. doi:10.1097/00001756-199901180-00037 [DOI] [PubMed] [Google Scholar]
- Beck D.M, Rees G, Frith C.D, Lavie N. Neural correlates of change detection and change blindness. Nat. Neurosci. 2001;4:645–650. doi: 10.1038/88477. doi:10.1038/88477 [DOI] [PubMed] [Google Scholar]
- Beck D.M, Muggleton N, Walsh V, Lavie N. Right parietal cortex plays a critical role in change blindness. Cereb. Cortex. 2006;16:712–717. doi: 10.1093/cercor/bhj017. doi:10.1093/cercor/bhj017 [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Bucher S.F, Seelos K.C. Cortical activation patterns during voluntary blinks and voluntary saccades. Neurology. 1999;53:1800–1805. doi: 10.1212/wnl.53.8.1800. [DOI] [PubMed] [Google Scholar]
- Bristow D, Haynes J.D, Sylvester R, Frith C.D, Rees G. Blinking suppresses the neural response to unchanging retinal stimulation. Curr. Biol. 2005;15:1296–1300. doi: 10.1016/j.cub.2005.06.025. doi:10.1016/j.cub.2005.06.025 [DOI] [PubMed] [Google Scholar]
- Carmel D, Lavie N, Rees G. Conscious awareness of flicker in humans involves frontal and parietal cortex. Curr. Biol. 2006;16:907–911. doi: 10.1016/j.cub.2006.03.055. doi:10.1016/j.cub.2006.03.055 [DOI] [PubMed] [Google Scholar]
- Cowey A, Walsh V. Magnetically induced phosphenes in sighted, blind and blindsighted observers. Neuroreport. 2000;11:3269–3273. doi: 10.1097/00001756-200009280-00044. doi:10.1097/00001756-200009280-00044 [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan D.L, Mangin J.F, Poline J.B, Rivière D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 2001;4:752–758. doi: 10.1038/89551. doi:10.1038/89551 [DOI] [PubMed] [Google Scholar]
- Driver J, Mattingley J.B. Parietal neglect and visual awareness. Nat. Neurosci. 1998;1:17–22. doi: 10.1038/217. doi:10.1038/217 [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P, Eimer M, Rees G. Functional magnetic resonance imaging and evoked potential correlates of conscious and unconscious vision in parietal extinction patients. Neuroimage. 2001;14:S68–S75. doi: 10.1006/nimg.2001.0842. doi:10.1006/nimg.2001.0842 [DOI] [PubMed] [Google Scholar]
- Enoch J.M, Choi S.S, Kono M, Schwartz D, Bearse M. Utilization of eye-movement phosphenes to help understand transient strains at the optic disc and nerve in myopia. Ophthalmic Physiol. Opt. 2003;23:377–381. doi: 10.1046/j.1475-1313.2003.00120.x. doi:10.1046/j.1475-1313.2003.00120.x [DOI] [PubMed] [Google Scholar]
- Eriksson J, Larsson A, Riklund A.K, Nyberg L. Visual consciousness: dissociating the neural correlates of perceptual transitions from sustained perception with fMRI. Conscious Cogn. 2004;13:61–72. doi: 10.1016/S1053-8100(03)00050-3. doi:10.1016/S1053-8100(03)00050-3 [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat. Neurosci. 2005;8:1380–1385. doi: 10.1038/nn1537. doi:10.1038/nn1537 [DOI] [PubMed] [Google Scholar]
- Ffytche D.H, Howard R.J, Brammer M.J, David A, Woodruff P, Williams S. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat. Neurosci. 1998;1:738–742. doi: 10.1038/3738. doi:10.1038/3738 [DOI] [PubMed] [Google Scholar]
- Frith C, Perry R, Lumer E. The neural correlates of conscious experience: an experimental framework. Trends Cogn. Sci. 1999;3:105–114. doi: 10.1016/s1364-6613(99)01281-4. doi:10.1016/S1364-6613(99)01281-4 [DOI] [PubMed] [Google Scholar]
- Goebel R, Muckli L, Zanella F.E, Singer W, Stoerig P. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients. Vision Res. 2001;41:1459–1474. doi: 10.1016/s0042-6989(01)00069-4. doi:10.1016/S0042-6989(01)00069-4 [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Malach R. The dynamics of object-selective activation correlate with recognition performance in humans. Nat. Neurosci. 2000;3:837–843. doi: 10.1038/77754. doi:10.1038/77754 [DOI] [PubMed] [Google Scholar]
- Halgren E, Mendola J, Chong C.D, Dale A.M. Cortical activation to illusory shapes as measured with magnetoencephalography. Neuroimage. 2003;18:1001–1009. doi: 10.1016/s1053-8119(03)00045-4. doi:10.1016/S1053-8119(03)00045-4 [DOI] [PubMed] [Google Scholar]
- Haxby J.V, Gobbini M.I, Furey M.L, Ishai A, Schouten J.L, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. doi:10.1126/science.1063736 [DOI] [PubMed] [Google Scholar]
- Haynes J.D, Rees G. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nat. Neurosci. 2005a;8:686–691. doi: 10.1038/nn1445. doi:10.1038/nn1445 [DOI] [PubMed] [Google Scholar]
- Haynes J.D, Rees G. Predicting the stream of consciousness from activity in human visual cortex. Curr. Biol. 2005b;15:1301–1307. doi: 10.1016/j.cub.2005.06.026. doi:10.1016/j.cub.2005.06.026 [DOI] [PubMed] [Google Scholar]
- Haynes J.D, Rees G. Decoding mental states from brain activity in humans. Nat. Rev. Neurosci. 2006;7:523–534. doi: 10.1038/nrn1931. doi:10.1038/nrn1931 [DOI] [PubMed] [Google Scholar]
- Haynes J.D, Lotto R.B, Rees G. Responses of human visual cortex to uniform surfaces. Proc. Natl Acad. Sci. USA. 2004;101:4286–4291. doi: 10.1073/pnas.0307948101. doi:10.1073/pnas.0307948101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J.D, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005a;438:496–499. doi: 10.1038/nature04169. doi:10.1038/nature04169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J.D, Driver J, Rees G. Visibility reflects dynamic changes of effective connectivity between V1 and fusiform cortex. Neuron. 2005b;46:811–821. doi: 10.1016/j.neuron.2005.05.012. doi:10.1016/j.neuron.2005.05.012 [DOI] [PubMed] [Google Scholar]
- He S, MacLeod D.I. Orientation-selective adaptation and tilt after-effect from invisible patterns. Nature. 2001;411:473–476. doi: 10.1038/35078072. doi:10.1038/35078072 [DOI] [PubMed] [Google Scholar]
- He S, Cohen E.R, Hu X. Close correlation between activity in brain area MT/V5 and the perception of a visual motion aftereffect. Curr. Biol. 1998;8:1215–1218. doi: 10.1016/s0960-9822(07)00512-x. doi:10.1016/S0960-9822(07)00512-X [DOI] [PubMed] [Google Scholar]
- Hirsch J, DeLaPaz R.L, Relkin N.R, Victor J, Kim K, Li T, Borden P, Rubin N, Shapley R. Illusory contours activate specific regions in human visual cortex: evidence from functional magnetic resonance imaging. Proc. Natl Acad. Sci. USA. 1995;92:6469–6473. doi: 10.1073/pnas.92.14.6469. doi:10.1073/pnas.92.14.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holender D, Duscherer K. Unconscious perception: the need for a paradigm shift. Percept. Psychophys. 2004;66:872–881. doi: 10.3758/bf03194980. discussion 888–895. [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat. Neurosci. 2005;8:679–685. doi: 10.1038/nn1444. doi:10.1038/nn1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. Decoding seen and attended motion directions from activity in the human visual cortex. Curr. Biol. 2006;16:1096–1102. doi: 10.1016/j.cub.2006.04.003. doi:10.1016/j.cub.2006.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A, Buchel C, Zeki S, Frackowiak R.S. Human brain activity during spontaneously reversing perception of ambiguous figures. Proc. Biol. Sci. 1998;265:2427–2433. doi: 10.1098/rspb.1998.0594. doi:10.1098/rspb.1998.0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiser R, Wittsack J, Niedeggen M, Goebel R, Stoerig P. Is V1 necessary for conscious vision in areas of relative cortical blindness? Neuroimage. 2001;13:654–661. doi: 10.1006/nimg.2000.0720. doi:10.1006/nimg.2000.0720 [DOI] [PubMed] [Google Scholar]
- Kouider S, Dehaene S. Levels of processing during non-conscious perception: a critical review of visual masking. Phil. Trans. R. Soc. B. 2007;362:857–875. doi: 10.1098/rstb.2007.2093. doi:10.1098/rstb.2007.2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Activation in human MT/MST by static images with implied motion. J. Cogn. Neurosci. 2000;12:48–55. doi: 10.1162/08989290051137594. doi:10.1162/08989290051137594 [DOI] [PubMed] [Google Scholar]
- Kreiman G, Koch C, Fried I. Imagery neurons in the human brain. Nature. 2000;408:357–361. doi: 10.1038/35042575. doi:10.1038/35042575 [DOI] [PubMed] [Google Scholar]
- Lamme V.A, Roelfsema P.R. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. doi:10.1016/S0166-2236(00)01657-X [DOI] [PubMed] [Google Scholar]
- Lee S.H, Blake R. V1 activity is reduced during binocular rivalry. J. Vis. 2002;2:618–626. doi: 10.1167/2.9.4. doi:10.1167/2.9.4 [DOI] [PubMed] [Google Scholar]
- Lee H.W, Hong S.B, Seo D.W, Tae W.S, Hong S.C. Mapping of functional organization in human visual cortex: electrical cortical stimulation. Neurology. 2000;54:849–854. doi: 10.1212/wnl.54.4.849. [DOI] [PubMed] [Google Scholar]
- Lee S.H, Blake R, Heeger D.J. Traveling waves of activity in primary visual cortex during binocular rivalry. Nat. Neurosci. 2005;8:22–23. doi: 10.1038/nn1365. doi:10.1038/nn1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S.J, Vogel E.K, Shapiro K.L. Word meanings can be accessed but not reported during the attentional blink. Nature. 1996;383:616–618. doi: 10.1038/383616a0. doi:10.1038/383616a0 [DOI] [PubMed] [Google Scholar]
- Lumer E.D, Rees G. Covariation of activity in visual and prefrontal cortex associated with subjective visual perception. Proc. Natl Acad. Sci. USA. 1999;96:1669–1673. doi: 10.1073/pnas.96.4.1669. doi:10.1073/pnas.96.4.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumer E.D, Friston K.J, Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. doi:10.1126/science.280.5371.1930 [DOI] [PubMed] [Google Scholar]
- Marcel A.J. Conscious and unconscious perception: experiments on visual masking and word recognition. Cognitive Psychol. 1983;15:197–237. doi: 10.1016/0010-0285(83)90009-9. doi:10.1016/0010-0285(83)90009-9 [DOI] [PubMed] [Google Scholar]
- Marois R, Yi D.J, Chun M.M. The neural fate of consciously perceived and missed events in the attentional blink. Neuron. 2004;41:465–472. doi: 10.1016/s0896-6273(04)00012-1. doi:10.1016/S0896-6273(04)00012-1 [DOI] [PubMed] [Google Scholar]
- Meng M, Remus D.A, Tong F. Filling-in of visual phantoms in the human brain. Nat. Neurosci. 2005;8:1248–1254. doi: 10.1038/nn1518. doi:10.1038/nn1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.S, Ohman A, Dolan R.J. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc. Natl Acad. Sci. USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. doi:10.1073/pnas.96.4.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. The relationship between cortical activation and perception investigated with invisible stimuli. Proc. Natl Acad. Sci. USA. 2002;99:9527–9532. doi: 10.1073/pnas.142305699. doi:10.1073/pnas.142305699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. Seeing invisible motion: a human fMRI study. Curr. Biol. 2006;16:574–579. doi: 10.1016/j.cub.2006.01.062. doi:10.1016/j.cub.2006.01.062 [DOI] [PubMed] [Google Scholar]
- Muckli L, Kohler A, Kriegeskorte N, Singer W. Primary visual cortex activity along the apparent-motion trace reflects illusory perception. PLoS Biol. 2005;3:e265. doi: 10.1371/journal.pbio.0030265. doi:10.1371/journal.pbio.0030265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S.O, Boyaci H, Kersten D. The representation of perceived angular size in human primary visual cortex. Nat. Neurosci. 2006;9:429–434. doi: 10.1038/nn1641. doi:10.1038/nn1641 [DOI] [PubMed] [Google Scholar]
- Naccache L, Blandin E, Dehaene S. Unconscious masked priming depends on temporal attention. Psychol. Sci. 2002;13:416–424. doi: 10.1111/1467-9280.00474. doi:10.1111/1467-9280.00474 [DOI] [PubMed] [Google Scholar]
- Naccache L, Gaillard R, Adam C, Hasboun D, Clemenceau S, Baulac M, Dehaene S, Cohen L. A direct intracranial record of emotions evoked by subliminal words. Proc. Natl Acad. Sci. USA. 2005;102:7713–7717. doi: 10.1073/pnas.0500542102. doi:10.1073/pnas.0500542102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedeggen M, Wichmann P, Stoerig P. Change blindness and time to consciousness. Eur. J. Neurosci. 2001;14:1719–1726. doi: 10.1046/j.0953-816x.2001.01785.x. doi:10.1046/j.0953-816x.2001.01785.x [DOI] [PubMed] [Google Scholar]
- Norman K.A, Polyn S.M, Detre G.J, Haxby J.V. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn. Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. doi:10.1016/j.tics.2006.07.005 [DOI] [PubMed] [Google Scholar]
- O'Craven K.M, Kanwisher N. Mental imagery of faces and places activates corresponding stimulus-specific brain regions. J. Cogn. Neurosci. 2000;12:1013–1023. doi: 10.1162/08989290051137549. doi:10.1162/08989290051137549 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast back projections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Pasley B.N, Mayes L.C, Schultz R.T. Subcortical discrimination of unperceived objects during binocular rivalry. Neuron. 2004;42:163–172. doi: 10.1016/s0896-6273(04)00155-2. doi:10.1016/S0896-6273(04)00155-2 [DOI] [PubMed] [Google Scholar]
- Pins D, Ffytche D. The neural correlates of conscious vision. Cereb. Cortex. 2003;13:461–474. doi: 10.1093/cercor/13.5.461. doi:10.1093/cercor/13.5.461 [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger D.J. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat. Neurosci. 2000;3:1153–1159. doi: 10.1038/80676. doi:10.1038/80676 [DOI] [PubMed] [Google Scholar]
- Portas C.M, Strange B.A, Friston K.J, Dolan R.J, Frith C.D. How does the brain sustain a visual percept? Proc. Biol. Sci. 2000;267:845–850. doi: 10.1098/rspb.2000.1080. doi:10.1098/rspb.2000.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptito M, Johannsen P, Faubert J, Gjedde A. Activation of human extrageniculostriate pathways after damage to area V1. Neuroimage. 1999;9:97–107. doi: 10.1006/nimg.1998.0390. doi:10.1006/nimg.1998.0390 [DOI] [PubMed] [Google Scholar]
- Rees G, Russell C, Frith C.D, Driver J. Inattentional blindness versus inattentional amnesia for fixated but ignored words. Science. 1999;286:2504–2507. doi: 10.1126/science.286.5449.2504. doi:10.1126/science.286.5449.2504 [DOI] [PubMed] [Google Scholar]
- Rees G, Wojciulik E, Clarke K, Husain M, Frith C, Driver J. Unconscious activation of visual cortex in the damaged right hemisphere of a parietal patient with extinction. Brain. 2000;123:1624–1633. doi: 10.1093/brain/123.8.1624. doi:10.1093/brain/123.8.1624 [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat. Rev. Neurosci. 2002a;3:261–270. doi: 10.1038/nrn783. doi:10.1038/nrn783 [DOI] [PubMed] [Google Scholar]
- Rees G, Wojciulik E, Clarke K, Husain M, Frith C, Driver J. Neural correlates of conscious and unconscious vision in parietal extinction. Neurocase. 2002b;8:387–393. doi: 10.1076/neur.8.4.387.16190. doi:10.1093/neucas/8.5.387 [DOI] [PubMed] [Google Scholar]
- Ress D, Heeger D.J. Neuronal correlates of perception in early visual cortex. Nat. Neurosci. 2003;6:414–420. doi: 10.1038/nn1024. doi:10.1038/nn1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro T, Breitmeyer B, Burton P, Singhal N.S, Lane D. Feedback contributions to visual awareness in human occipital cortex. Curr. Biol. 2003;13:1038–1041. doi: 10.1016/s0960-9822(03)00337-3. doi:10.1016/S0960-9822(03)00337-3 [DOI] [PubMed] [Google Scholar]
- Sakai K, Watanabe E, Onodera Y, Uchida I, Kato H, Yamamoto E, Koizumi H, Miyashita Y. Functional mapping of the human colour centre with echo-planar magnetic resonance imaging. Proc. Biol. Sci. 1995;261:89–98. doi: 10.1098/rspb.1995.0121. doi:10.1098/rspb.1995.0121 [DOI] [PubMed] [Google Scholar]
- Senior C, Barnes J, Giampietro V, Simmons A, Bullmore E.T, Brammer M, David A.S. The functional neuroanatomy of implicit-motion perception or representational momentum. Curr. Biol. 2000;10:16–22. doi: 10.1016/s0960-9822(99)00259-6. doi:10.1016/S0960-9822(99)00259-6 [DOI] [PubMed] [Google Scholar]
- Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat. Neurosci. 2005;8:1391–1400. doi: 10.1038/nn1549. doi:10.1038/nn1549 [DOI] [PubMed] [Google Scholar]
- Shams L, Iwaki S, Chawla A, Bhattacharya J. Early modulation of visual cortex by sound: an MEG study. Neurosci. Lett. 2005;378:76–81. doi: 10.1016/j.neulet.2004.12.035. doi:10.1016/j.neulet.2004.12.035 [DOI] [PubMed] [Google Scholar]
- Silbersweig D.A, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. doi:10.1038/378176a0 [DOI] [PubMed] [Google Scholar]
- Silvanto J, Cowey A, Lavie N, Walsh V. Striate cortex (V1) activity gates awareness of motion. Nat. Neurosci. 2005;8:143–144. doi: 10.1038/nn1379. doi:10.1038/nn1379 [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Russell D.P, Edelman G.M, Tononi G. Increased synchronization of neuromagnetic responses during conscious perception. J. Neurosci. 1999;19:5435–5448. doi: 10.1523/JNEUROSCI.19-13-05435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Russ M.O, Preibisch C, Kleinschmidt A. Neural correlates of spontaneous direction reversals in ambiguous apparent visual motion. Neuroimage. 2002;15:908–916. doi: 10.1006/nimg.2001.1030. doi:10.1006/nimg.2001.1030 [DOI] [PubMed] [Google Scholar]
- Sterzer P, Eger E, Kleinschmidt A. Responses of extrastriate cortex to switching perception of ambiguous visual motion stimuli. Neuroreport. 2003;14:2337–2341. doi: 10.1097/00001756-200312190-00010. doi:10.1097/00001756-200312190-00010 [DOI] [PubMed] [Google Scholar]
- Sterzer P, Haynes J.D, Rees G. Primary visual cortex activation on the path of apparent motion is mediated by feedback from hMT+/V5. Neuroimage. 2006;32:1308–1316. doi: 10.1016/j.neuroimage.2006.05.029. doi:10.1016/j.neuroimage.2006.05.029 [DOI] [PubMed] [Google Scholar]
- Struber D, Herrmann C.S. MEG alpha activity decrease reflects destabilization of multistable percepts. Brain Res. Cogn. Brain Res. 2002;14:370–382. doi: 10.1016/s0926-6410(02)00139-8. doi:10.1016/S0926-6410(02)00139-8 [DOI] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme V.A.F. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) Nat. Neurosci. 2001;4:304–310. doi: 10.1038/85170. doi:10.1038/85170 [DOI] [PubMed] [Google Scholar]
- Sylvester R, Rees G. Extraretinal saccadic signals in human LGN and early retinotopic cortex. Neuroimage. 2006;30:214–219. doi: 10.1016/j.neuroimage.2005.09.014. doi:10.1016/j.neuroimage.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Sylvester R, Haynes J.D, Rees G. Saccades differentially modulate human LGN and V1 responses in the presence and absence of visual stimulation. Curr. Biol. 2005;15:37–41. doi: 10.1016/j.cub.2004.12.061. doi:10.1016/j.cub.2004.12.061 [DOI] [PubMed] [Google Scholar]
- Tong F, Engel S.A. Interocular rivalry revealed in the human cortical blind-spot representation. Nature. 2001;411:195–199. doi: 10.1038/35075583. doi:10.1038/35075583 [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends Cogn. Sci. 2006;10:502–511. doi: 10.1016/j.tics.2006.09.003. doi:10.1016/j.tics.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Tong F, Nakayama K, Vaughan J.T, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron. 1998;21:753–759. doi: 10.1016/s0896-6273(00)80592-9. doi:10.1016/S0896-6273(00)80592-9 [DOI] [PubMed] [Google Scholar]
- Tononi G, Srinivasan R, Russell D.P, Edelman G.M. Investigating neural correlates of conscious perception by frequency-tagged neuromagnetic responses. Proc. Natl Acad. Sci. USA. 1998;95:3198–3203. doi: 10.1073/pnas.95.6.3198. doi:10.1073/pnas.95.6.3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R.B, Reppas J.B, Dale A.M, Look R.B, Sereno M.I, Malach R, Brady T.J, Rosen B.R. Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature. 1995;375:139–141. doi: 10.1038/375139a0. doi:10.1038/375139a0 [DOI] [PubMed] [Google Scholar]
- Turatto M, Sandrini M, Miniussi C. The role of the right dorsolateral prefrontal cortex in visual change awareness. Neuroreport. 2004;15:2549–2552. doi: 10.1097/00001756-200411150-00024. doi:10.1097/00001756-200411150-00024 [DOI] [PubMed] [Google Scholar]
- Vogel E.K, Luck S.J, Shapiro K.L. Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. J. Exp. Psychol. Hum. Percept. Perform. 1998;24:1656–1674. doi: 10.1037//0096-1523.24.6.1656. doi:10.1037/0096-1523.24.6.1656 [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Phil. Trans. R. Soc. B. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. doi:10.1098/rstb.2007.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Sagiv N, Hazeltine E, Poldrack R.A, Swick D, Rafal R.D, Gabrieli J.D.E. Neural fate of seen and unseen faces in visuospatial neglect: a combined event-related functional MRI and event-related potential study. Proc. Natl Acad. Sci. USA. 2001;98:3495–3500. doi: 10.1073/pnas.051436898. doi:10.1073/pnas.051436898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Shams L, Tanaka S, Haynes J.D, Rees G. Sound alters activity in human V1 in association with illusory visual perception. Neuroimage. 2006;31:1247–1256. doi: 10.1016/j.neuroimage.2006.01.016. doi:10.1016/j.neuroimage.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Schneider K.A, Kastner S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat. Neurosci. 2005;8:1595–1602. doi: 10.1038/nn1554. doi:10.1038/nn1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Watson J.D, Frackowiak R.S. Going beyond the information given: the relation of illusory visual motion to brain activity. Proc. Biol. Sci. 1993;252:215–222. doi: 10.1098/rspb.1993.0068. doi:10.1098/rspb.1993.0068 [DOI] [PubMed] [Google Scholar]