Abstract
The role of the frontal lobes has often been described as a ‘paradox’ or a ‘riddle’. Ascribed to this region has been the loftiest of functions (e.g. executive; seat of wisdom); others contested that the frontal lobes played no special role. There has also been controversy about the unity or diversity of functions related to the frontal lobes. Based on the analysis of the effects of lesions of the frontal lobes, we propose that there are discrete categories of functions within the frontal lobes, of which ‘executive’ functioning is one. Within the executive category, the data do not support the concept of an undifferentiated central executive/supervisory system. The results are better explained as impairments in a collection of anatomically and functionally independent but interrelated attentional control processes. Evidence for three separate frontal attentional processes is presented. For each process, we present an operational description, the data supporting the distinctiveness of each process and the evidence for impairments of each process after lesions in specific frontal regions. These processes and their coarse frontal localizations are energization—superior medial, task setting—left lateral and monitoring—right lateral. The strength of the findings lies in replication: across different tasks; across different cognitive modalities (e.g. reaction time paradigms, memory); and across different patient groups. This convergence minimizes the possibility that any of the findings are limited to a specific task or to a specific set of patients. Although distinct, these processes are flexibly assembled in response to context, complexity and intention over real time into different networks within the frontal regions and between frontal and posterior regions.
Keywords: frontal lobes, dysexecutive syndrome, attention, monitoring, energization, task setting
1. Introduction
(a) Evolution of the question and initial response
Their relatively large size, late evolutionary development and rich anatomical connectivity all strongly suggest a central role, or roles, for the frontal lobes in human cognition and emotion. That there are so many competing theories about frontal functions despite the many recent advances in lesion and imaging research illuminates the difficulty in understanding this complex region.
The difficulties in studying the frontal lobes are myriad. First, there is no particular predisposition for a neurological disorder to the frontal lobes. Although cerebrovascular disorders may damage only the frontal lobes, the number of individuals with focal frontal pathology is not particularly high. The pressures of publication, the time required to collect an adequate sample of focal frontal lobe lesions and the rapid change in theoretical positions during the period of data accumulation often predispose researchers to use more convenient samples (e.g. undifferentiated traumatic brain injury) as a proxy. Such studies have practical value for understanding the target population, but precise brain–behaviour relationships cannot be determined. Central roles for various frontal regions have been proposed for both cognitive and emotional functions, and both may be recruited for complex tasks—gambling decision, investment planning, etc. Lesions may disrupt either or both, depending on site. It may be the interaction of emotional status and cognition that determines many behaviours, but it is the cognitive aspect of tasks that are defined by executive functions.
Many prominent theoretical positions emphasized the dominant role of the frontal lobes in organizing cognition, thus such terms as supervisory system and central executive. A controversy within this approach has been related to the unity (Duncan & Miller 2002) versus diversity (Stuss & Benson 1986; Shallice 2002) of executive functions. In the early 1990s, we embarked on a research plan to examine whether such an executive system could be fractionated (Stuss et al. 1995). Our approach was different from that often used in neuropsychological research. Instead of selecting one test or one process that was considered executive, we took a ‘root and branches’ approach. We selected attention (the ‘root’) as the cognitive focus owing to its prominent role in many influential theories of frontal functions (e.g. Heilman & Watson 1977; Shallice 1982; Mesulam 1985; Norman & Shallice 1986; Posner & Petersen 1990; Knight 1991; Paus et al. 1997; Godefroy et al. 1999; Sturm & Willmes 2001). We elected to study patients with focal lesions to demonstrate that a region was essential for an attentional process, as opposed to simply being activated during a process (as, say, with functional magnetic resonance imaging, fMRI). All published studies addressing attentional deficits in patients with single focal frontal lesions were reviewed. The tasks used in the various studies could be grouped into a relatively small number of categories. Based on our review of the different papers, we proposed a limited number of distinct frontal lobe processes (the ‘branches’) which could explain the performance of each task. The reviewed papers also implied potential frontal localization of at least some of these processes. This initial review implied that there was no common central organizing role of the frontal lobes; rather, there were independent control processes related to different brain regions. We concluded that
If we are correct that there is no central executive, neither can there be a dysexecutive syndrome. The frontal lobes (in anatomical terms) or the supervisory system (in cognitive terms) do not function (in physiological terms) as a simple (inexplicable) homunculus. Monitoring, energizing, inhibition, etc.—these are processes that exist at many levels of the brain, including those more posterior ‘automatic’ processes. Owing to their extensive reciprocal connections with virtually all other brain regions, the frontal lobes may be unique in the quality of the processes that have evolved, and perhaps in the level of processing which might be labelled ‘executive’ or ‘supervisory’.
Bolstered by this review and using Norman & Shallice's (1986) supervisory system as our launching point, we undertook a programme of research to examine whether we could differentiate and define frontal processes within a supervisory system. Such processes had to be domain-general, in that they would be necessary for different cognitive modalities (e.g. language, memory) as well as basic attentional tasks such as reaction time (RT). Domain-general implies that different tasks in one modality or similar tasks in different modalities would show similar effects of specific lesions. Finally, the results had to be replicable across different groups of frontal lobe patients to ensure there was no particular subject group bias. Other researchers have embarked on a similar journey (e.g. Shallice & Burgess 1991; Burgess & Shallice 1994; Godefroy et al. 1994; Diaz et al. 1996; Robbins 1996, 2007; Burgess et al. 2007). This paper will necessarily focus on our own programme of research, but results from other laboratories will be presented where appropriate.
(b) Methodological philosophy
We began with three assumptions of what would be necessary for success. First, the history of research on ‘executive’ functions has conflated psychological theories with anatomical ones, so we focused our investigation on the effects of frontal injuries, not on an investigation of executive functions (which can be examined independently of any brain relationship). This required including only patients with purely focal frontal single lesions. Second, restricting the patients to those with vascular aetiology would profoundly limit the regional representation of frontal lesions, so patients with different aetiologies were accepted if they met specific conditions (see Stuss et al. 1995 for a review of these conditions). In addition, we and others have demonstrated several times that, under these conditions, the location is more important than the aetiology (Elsass & Hartelius 1985; Burgess & Shallice 1996; Stuss et al. 2005; Picton et al. 2006, in press). Third, in order not to confound acute diffuse problems with more focal impairments, we tested patients in the chronic stage of recovery, ideally after three months. As patients' lesions become more and more chronic, it is possible that brain–behaviour relationships are affected by brain plasticity and reorganization. The evolution of these relationships from acute to post-acute to chronic phases is probably interesting, but would require another programme of research following patients during the course of recovery, supported by imaging. Recent data do suggest that similar patterns of behaviour may be observable in both acute and chronic patients (Stuss et al. 1994; Alexander et al. 2003; Turner et al. in press).
A process must be isolated to demonstrate a specific brain–behaviour relationship. Process dissociation was used for the standard clinical tests where possible. In the experimental tests, the goal was to devise simple tests that probed single processes and then manipulate difficulty and context to probe more complex processes.
The next step was to devise a method to assign frontal lesions to specific frontal regions to determine whether there were any regional effects on each process. There are several different methods to achieve this (Stuss et al. 2002a). In this paper, we present data from two approaches. In some studies, the frontal patients are compared based on a coarse predominant location of the lesion: left lateral (LL); right lateral (RL); inferior medial (IM); and superior medial (SM). In addition, however, we were able to focus on much more precise architectonic regions with a ‘hotspotting’ method developed by us (Stuss et al. 2002a, 2005). The lesion for each patient is mapped onto the Petrides & Pandya (1994) architectonic template. For every patient, each architectonic region is identified as significantly damaged or not. Then, for the measurement in question, the performance of individuals who have damage in a particular region is compared with all those who do not have damage in that region. This hotspotting approach is open to criticism of too many comparisons and the risk of type I error. We have been cognizant of this problem, but given the substantial difficulties of lesion-based research and the potential benefits of identifying specific brain–behaviour relationships, this approach seemed reasonable, at least as a first approximation of focal effects. Furthermore, if the results can be replicated across different tests that demand a similar process, and across different patient groups, the summated evidence for that brain–behaviour relationship is strengthened. At the very least, having these findings in lesion research provides a plausible approach for verification in other studies, or for devising a more specific region of interest hypothesis.
Lesion research demonstrates that some structure within the lesion is critical for impairing a task. Comparing patients with similar, partly overlapping lesions allows increasingly fine identification of which structures are essential. Lesion studies are not equivalent to functional imaging studies that demonstrate activation of a region during defined tasks. The activation may or may not represent a critical role in the performance of the task. Nevertheless, there is considerable convergence of the neuroimaging and lesion studies.
(c) The basic paradigms
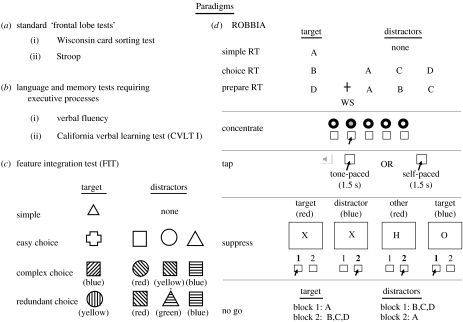
If executive functions are truly ‘superordinate’ and ‘domain-general’, it should be possible to determine their effects across a variety of tasks. Four different sets of data are used in support of our hypothesis of process fractionation within the frontal lobes. We used classic ‘frontal’ tasks (Wisconsin Card Sorting Test, WCST, Milner 1963; the Stroop test of interference, Stroop 1935; Comalli et al. 1962), other tasks with a control requirement but in different modalities (language—verbal fluency, Borkowski et al. 1967; memory—list learning, Delis et al. 1987), feature integration test (FIT; Stuss et al. 1989, 2002b) in which complexity of response distractions could be manipulated, and finally, a novel battery of tests (ROBBIA, ROtman-Baycrest Battery to Investigate Attention; Stuss et al. 2005), that systematically probe levels of attention and response control (all of the tests are shown and explained in figure 1 and appendix A). In each section below, we start with the more precisely defined ROBBIA tests, ending with the more complex clinical tests.
Figure 1.
The paradigms used in the various studies. (a) Based on commonly used neuropsychological tests of ‘frontal lobe’ functions: adapted from Wisconsin Card Sorting Test (WCST) and Stroop. (b) Language and memory tests that require executive processes: letter fluency and word list learning (CVLT I). (c) Three conditions of a FIT are described. (d) Finally, several tests from the ROtman-Baycrest Battery to Investigate Attention (ROBBIA) are presented. Test administration and the selected control measures are described in more detail in appendix A.
(d) Summary of approach
Our goal was to determine whether all focal frontal lesions produced a similar impairment in cognitive supervisory control or whether lesions in different regions produced specific impairments that might or might not appear on a task depending upon the particular demands of the task.1 There is currently evidence for at least three separate frontal processes related to attention, each related to a different region within the frontal lobes as illuminated by deficit profiles after injury. We have labelled these processes as energization, task setting and monitoring. For each process, we start with our conclusions, and present a current description of the process and data that support the existence of each distinct process. In some instances, we have reanalysed the original data to be consistent across the tasks. The possibility of a type I error is minimized by the replication of the findings: the replications often occur across the tasks, including tasks of different cognitive modalities (e.g. RT paradigms, memory); the same results can be demonstrated with different patient groups, minimizing the possibility that the results are unique to a specific set of patients2; in some cases, there is supporting evidence from other research laboratories.
(e) Definition of ‘energization’
Energization is the process of initiation and sustaining of any response. The basis for proposing an energization function comes from neurophysiological observations that there is an internal tendency for any neural activity to become quiescent in the absence of input. A natural extension of the supervisory system model is to assume that, in the absence of external triggers or motivational conditions to optimize responding, lower level perceptual or motor schemata would have to be energized or re-energized when activation becomes low, as would be required, for example, for detecting occasional stimuli or performing occasional motor acts. Without energization, setting and sustaining a specific selected response cannot occur and maintaining performance over prolonged periods of time will waver.
(f) Evidence for energization and putative frontal localization
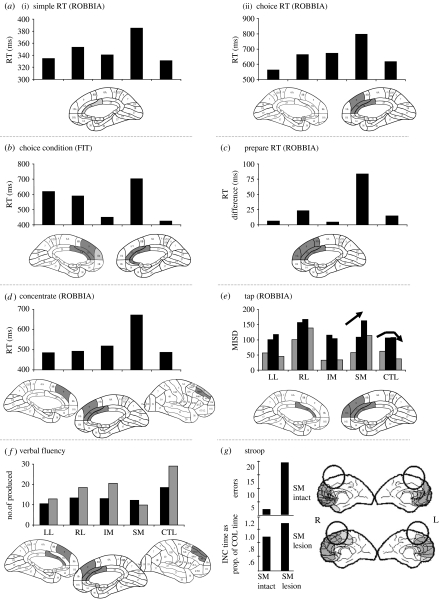
Deficient energization is most consistently associated with lesions in the SM region bilaterally, with some evidence for a more important role for the right SM area (figure 2).
Figure 2.
Quantitative data illustrated in graphs for coarse frontal anatomical groupings (most often, left lateral (LL), right lateral (RL), inferior medial (IM) and superior medial (SM)) compared with a matched control (CTL) group (see Stuss et al. 1998, for methods), for each illustrated paradigm in figures 2–4. Note that the baselines may differ from test to test for illustrative purposes. Corresponding to the quantitative data for each test, the architectonic ‘hotspots’ are depicted in the brain diagrams. Hotspots are those architectonic regions damaged in at least three patients who have significantly impaired performance compared with all other patients who do not have damage in those regions. In the coarse anatomical breakdown used for x-axis in graphs, the patient is assigned to a group based on the predominant localization; there may be some overlap with an adjoining region. For example, SM or IM patients may have some overlap into the lateral region. The hotspotting technique emphasizes the specific architectonic localization regardless of coarse grouping. ‘Energization’ is consistently impaired after damage to the SM region bilaterally, with some emphasis on the right SM area. All measures (except for g) are RTs. In the brain diagrams, only the medial regions are illustrated, unless the lateral regions identified by this hotspotting technique are continuous with the medial pathology. In (a(i), (ii) and b) the simple and choice RTs and (d) concentrate, only the RT of the SM group is significantly slower than the CTL group. For prepare RT (c), the measure depicts the difference between RTs for the 1 and 3 s warning conditions. The tap measure (e) is the mean intra-individual standard deviation (ISD). The light coloured bars indicate externally paced tapping via tones, the dark bars self-paced tapping. The CTL group exhibited greater variability of performance for the self-timed condition compared with the externally timed condition. The SM group increased in ISD in both condition replications and the slowing with replication is indicated by the direction of arrow. The IM and LL were comparable with the control group (see figure 4b for interpretation of the RL group). In the verbal fluency task (f), the number of words produced in the first 15 s (black bars) was compared with the last 45 s (grey bars). The architectonic hotspot was completed using the difference between these two measures. Analysis of the most important brain region in the Stroop task (g) was carried out by comparison of overlapping lesions. If the SM area was damaged, the result was a significantly higher number of errors and slower speed of task completion compared with the colour naming condition. INC, incongruent; COL, colour naming.
The ROBBIA simple and choice RT tests differed in that the choice RT tasks required an easy differentiation of the feature of the target stimulus and the presence of distractors (Stuss et al. 2005; see figure 1 and appendix A for further task details). In both the tests, there was a significant slowing of the SM patients only, with the hotspotting technique highlighting primarily areas 24 and 32 (figure 2a). The involvement of the SM region appeared to be more pronounced in the somewhat more demanding task. In the simple RT, the SM group was marginally slower than the control group (p=0.06); in the choice RT, the significant difference was p=0.007. The slowing was not a factor of lesion size, since the SM group was the slowest by far, and there was no relation of lesion size to RT in the SM group (e.g. p=0.6–0.9 for the different tasks). Lesion location is a better predictor of response slowing than lesion size. The RT results of the feature integration test (Stuss et al. 2002b), similar in design to the simple and choice RT tests of ROBBIA, yielded similar results (figure 2b).
In the prepare RT test (Stuss et al. 2005; figure 2c), we analysed whether a warning stimulus presented either 1 or 3 s prior to a choice RT affected speed of response. In all the three conditions (no warning, 1 s warning and 3 s warning), the overall significant SM slowing remained. All groups benefited from the 1 s warning, and all groups were slower on the 3 s warning condition compared with the 1 s warning. The 3 s warning RT was still faster than without a warning in all but one group, the SM group. Figure 2c shows the difference in RT between the 3 and 1 s warning conditions. The loss of benefit for the SM group from the longer warning interval is compatible with a deficit in sustaining energized attention and response systems. The fact that there were not significant differences in errors among the conditions suggests that this cannot be secondary to a deficit in noticing and reacting to signals.
The ROBBIA concentrate test perhaps illustrates the energization deficit most clearly (Alexander et al. 2005; figure 2d). It is very simple in structure but requires high levels of sustained attention. Only patients with lesions in SM frontal regions had significant RT slowness, and this was consistent across the entire test. This group's mean RT was 33% greater than the other patient groups and the control subjects. The prolonged RT was not simply due to fatigue or errors, as the slowness was evident from the beginning across 500 trials. We had initially hypothesized that the anterior cingulate gyrus (ACG) would be the critical region (Paus et al. 1997; Luu et al. 2000a). However, the critical region was larger and involved supplementary motor area (SMA) and the preSMA region (P & P areas 24, 32, 9 and 46d). The noted slowness with right SM lesions on this test requiring sustained concentration was interpreted as an insufficient energizing of attention to respond.
The ROBBIA tap test consisted of two simple timing tasks, one requiring tapping to an externally driven stimulus (every 1.5 s), and another demanding maintenance of the same regular response rhythm without any external stimulus (Picton et al. 2006). Normal performance is illustrated in the control group (see CTL in figure 2e). An increase in the variability of timing performance, as the task continued (see rising arrow), was noted in patients with lesions to the SM regions of the frontal lobe (figure 2e). The SM frontal area is necessary to maintain consistent timing performance over prolonged periods of time.
This energization function was also revealed in two standard clinical frontal lobe tests. An energization function should be applicable to any task. Evidence for this function was observed in a verbal fluency language task, requiring the generation of words beginning with a specific letter over 60 s (Stuss et al. 1998). All groups produced fewer words over time, but the total of the last 45 s (grey versus black bars in figure 2f) was greater in all but the SM group. The critical region again was the SM area (figure 2f).
We administered the classic Comalli et al. (1962) Stroop version with three conditions: word reading; colour naming of colour patches; and colour naming of colour words printed in a colour different from that of the word (interference; Stuss et al. 2001). Patients with frontal and non-frontal pathology were compared with normal control subjects. Patients with posterior lesions were not significantly deficient in any condition. Within the frontal patients, bilateral SM frontal as well as right superior posteromedial lesions were significantly associated with increased errors and slowness in response time for the incongruent condition (figure 2g), an impairment interpreted as failure of maintenance of consistent activation of the intended response in the incongruent Stroop condition. This inability to maintain an activated response mode appeared consistent with the rapid decline in preparatory activation from 1 to 3 s after a warning stimulus in the prepare RT.
(g) Interim summary for energization
Decreased facilitation (energizing) of the neural systems that are needed to make the decisions (contention scheduling) and initiate the responses (schemata) is impaired after bilateral SM frontal lesions, with suggestion of greater importance for the right SM region. Supportive data came from different RT tasks, from studies in different modalities (RT, language) and across different patients. The SM deficit was demonstrated by prolonged simple RT, proportionately greater prolongation of choice RT, inability to sustain preparation to respond, inability to maintain consistent short time-intervals in a task, diminished output in a verbal fluency test, and increased errors and slower speed in a Stroop test. The localization is similar in each study, although comparison of the different tests suggests that there was some relationship of the severity of the deficit with the demands of the test. The time course across the tasks (e.g. from a few seconds—prepare RT to 1 min—fluency) implies that this region is important for initial energization as well as sustaining of energization; future research might also unveil potential localization or context differences related to the initial energization versus sustaining.
Our data, and we believe our interpretation, appear to support other research and theories of the functions of this region (e.g. Luria 1973; Drewe 1975b; Leimkuhler & Mesulam 1985; Passingham 1993; Richer et al. 1993; Godefroy et al. 1994; Picard & Strick 1996; Paus 2001). It is also compatible with one theoretical explanation of Stroop interference performance, which emphasizes maintenance of the strength of the activated intention, a strength which can wax and wane along a gradient (Cohen et al. 1990; West & Baylis 1998; Kornblum et al. 1999).
These findings are also concordant with clinical observations. Bilateral damage to ACG and SMA produces akinetic mutism, a dramatic example of deficient energizing (Plum & Posner 1980; Devinsky et al. 1995; Alexander 2001). Changes in activity of the cingulate cortex occur as a function of sleep stages (Hofle et al. 1997), vigilance (Paus et al. 1997) and alertness (Luu et al. 2000a,b). We consider the energizing deficit in SM patients independent of general arousal as, in our studies, there was no correlation or interaction of slowness and reported sleepiness or level of motivation among groups (Stuss et al. 2005). We therefore think that energizing schemata is the process that allows subjects to maintain their concentration on a particular task. In the neurological literature, this would correspond to phasic attention (Stuss & Benson 1984, 1986); in the information processing literature, energization would correspond to the effort system of Hockey (1993).
(h) Definition of task setting
Each of the tests in which the task setting attentional process is demonstrated requires the ability to set a stimulus–response relationship. Task setting would be necessary in the initial stages of learning to drive a car or planning a wedding. This may be initiated a priori and is most often learned and consolidated through trial and error. In easier tasks, task setting would be more relevant in the early stages. Any deficit would be more evident under conditions that require continuous refreshing and suppression of more salient responses. The establishment of the connection between a stimulus and a response would require formation of a criterion to respond to a defined target with specific attributes, organization of the schemata necessary to complete a particular task and adjustment of contention scheduling, so that the automatic processes of moving through the steps of a task can work more smoothly. Owing to the role of the SM region in energization in some tasks addressing task setting (and monitoring below), there could be evidence of SM involvement. This is not illustrated.
(i) Evidence for the task setting process and putative frontal localization
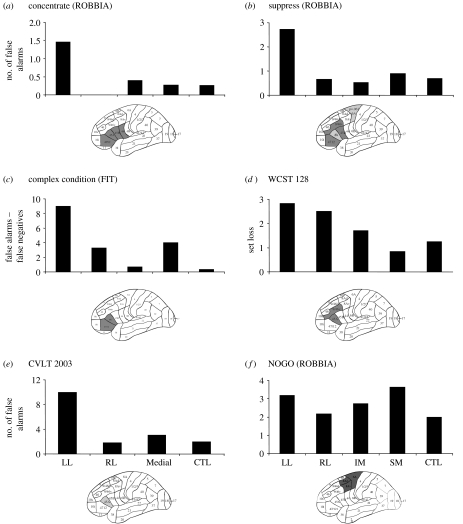
Task setting is consistently impaired after damage to the LL region of the frontal lobes, most often with a more ventrolateral distribution (figure 3).
Figure 3.
‘Task setting’ process consistently impaired after damage to the LL frontal region. Figure 3 is organized in a similar manner to figure 2. The tests are illustrated and explained in figure 1 and appendix A. There were no significant impairments after RL pathology. Where the measure is RT, there would also be involvement of the SM region, as described in figure 2. The measure for concentrate is the number of errors in the first 100 out of 500 trials. In suppress, a Stroop-like task, and in NOGO, a motor suppression task, false positives are the dependent measurement. In the complex condition of the feature integration task (FIT), the false positives were subtracted from the number of false negative responses to isolate task setting from monitoring. In the WCST, the coarse anatomical groupings used in the graphs indicate that set loss is evident after pathology in both RL and LL frontal regions. The hotspotting method, however, (brain diagrams) suggests that maximum involvement in this mode of WCST administration is related to the left ventrolateral region. A task setting deficit is evident in memory tasks as well, as noted in the number of false positive responses in the recognition recall of the CVLT. In this study, all patients with medial pathology were originally grouped together.
Errors in the ROBBIA concentrate (see figure 1 for task description) task were noted primarily in the first 100 out of 500 trials, and these were made maximally by patients with damage to left frontal P & P Areas 44, 45A and 45B and 47/12 (Alexander et al. 2005; figure 3a). This was not associated with decreased RT (no time–accuracy trade-off) or increased RT (no awareness and monitoring of errors). The difficulty was interpreted as defective setting of specific stimulus–response contingencies (see also Godefroy et al. 1994, 1999). Once in ‘task responding set’ (after the first 100 trials), there were no deficits in any subgroup of patients.
The suppress task in ROBBIA was a variant of the Stroop, a test which assesses the ability of patients to control intact cognitive operations under the condition of conflicting possible responses (Alexander et al. in press). Lesions of the left ventrolateral region (areas 44 and 45) produced an impairment in setting contingent response rules as indexed by the number of false alarms (figure 3b).
The importance of isolating the process is illustrated by an analysis of the feature integration test complex condition (three features). We had hypothesized that making false positive errors would be an index of task (criterion) setting to respond appropriately to the target as opposed to non-targets (Stuss et al. 2002b). The first architectonic analysis was surprising—the RL region appeared to be most associated with this type of error (cf. figure 4c). However, patients with damage to this RL region made errors of all kinds, which we interpreted as a monitoring impairment (see below for analysis of just false negative errors). We subtracted the false negative from the false positive responses to isolate the patients who made primarily the type of errors indicating that they could not set a task criterion to respond ‘yes’ (response bias—false positives). When the architectonic analysis was redone isolating the false positive responders, the major area of impairment was now focally left frontal (figure 3c).
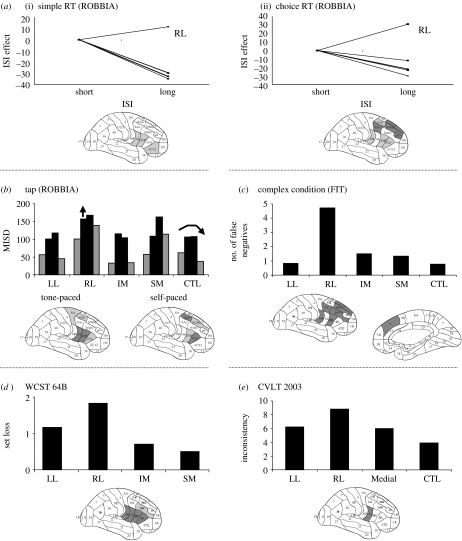
Figure 4.
‘Monitoring’ process consistently impaired after damage to the RL frontal area. The tests are illustrated and explained in figure 1 and appendix A. The dependent measures vary among tasks. The RTs for the short (3, 4 s) and long (6, 7 s) ISIs are compared among groups by setting the short ISIs for each group to zero, and then illustrating the difference in RT between the long and short ISIs. The measurement for tap is the same as in figure 2—the mean intra-individual variability across repeated taps. The arrow over the RL group indicates that this group has a significantly greater ISD across both conditions and replications. In the FIT complex (three features) condition, the greatest number of errors was associated with damage to the RL region. In this figure, emphasis is placed on the number of false negatives to differentiate from the effect of false positive errors, which reflects task setting also. Set loss for the WCST 128 condition is defined in figure 3. Inconsistency in recall for the CVLT word list learning test indicates the ‘in and out’ recall of words across the learning trials.
Another important example of process isolation, and the need to understand that the experimental manipulation may define what a specific measure may represent, occurred in the analysis of the WCST results (Stuss et al. 2000). In the 128 card condition, set loss would probably reflect primarily the potential trial and error learning of the correct sorting criterion, in a way similar to the early trial errors in concentrate, as well as some degree of monitoring. In the 64B condition, in which the subject had been informed of the three sorting criteria, what criterion to start with and when the criterion had changed (i.e. the task parameters had been quite specifically established), set loss errors more probably indexed the online monitoring and checking of performance, since the task had been set by the explicit instructions. The hotspot analysis confirmed this hypothesis. In the 128 condition, although set loss problems were apparent after both LL and RL damage, the identified most relevant area was the LL region (areas 9/46v, 45A; figure 3d); in 64B, set loss was related to damage in the RL region (areas 6B, 44, 45A and 45B; figure 4d).
Task setting impairment, as indexed by difficulty in establishing an appropriate response criterion, can also be observed in memory tasks. We have now administered three different word lists (one of them being the standardized California Verbal Learning Test, CVLT) to three different groups of frontal lobe patients. In the first study, the left frontal group was most impaired in the number of false positives (the hotspotting technique had not yet been developed; Stuss et al. 1994). The number of false positives in the CVLT study was associated with damage in the LL area 45A (Alexander et al. 2003; figure 3e).
One task resulted in a more caudal localization than the other task setting effects. In a response inhibition (NOGO) task, four equiprobable stimuli (letters A, B, C and D) were presented in two different conditions (Picton et al. in press). In the first condition, the subject responded to the letter A only; in the second condition, the subject responded to the letters B, C and D, and not to A. These two conditions were called the ‘improbable-go’ and the ‘improbable-nogo’. An increased number of false alarms (incorrect responses to the nogo stimulus) were found primarily in patients with lesions to left SM (6A) and LL regions' area (8B, area 9/46) of the frontal lobes (figure 3f). Although our patients were primarily right handed, the involvement of the LL and superior region in nogo motor control is probably independent of response hand (Talati & Hirsch 2005). Our localization results appear comparable to the original nogo study of Drewe (1975a,b), clinical studies (Verfaille & Heilman 1987) and other human and animal research (Brutkowski 1965; Iversen & Mishkin 1970). Some of the variability of the results in other go–nogo paradigms may derive from the differing cognitive requirements.
(j) Interim summary of task setting
If the task setting process can be isolated, there is consistent evidence that left frontal damage disturbs this process. The task setting–left frontal relationship was observed in different RT tests, the clinical WCST and word list learning. Different patient populations were examined in several of these studies. It is yet uncertain if any variation in task setting localization observed in our tasks is a reflection of inadequate sampling of different brain regions or a reflection of a more general left frontal function interacting with more specific task demands (e.g. response task setting). In general, our findings support Luria's (1966) postulate that damage to the left frontal lobes damages the patient's ability to use task instructions to direct behaviour (verbal regulation of behaviour), even when clearly able to comprehend their meaning.
Other lesion studies using similar patients attempted to identify regional frontal effects using the Stroop task and define underlying impaired neural mechanisms; with some discrepancies in localization details attributable to differences in patient populations and precision of lesion definition, they, in general, support our findings that LL lesions affect setting of stimulus–response contingencies (while SM lesions affect energizing attention to task; Perret 1974; Richer et al. 1993).
We believe that fMRI studies using variations of the standard Stroop paradigm also generally support our hypotheses. Derfus et al. (2005) completed a meta-analysis of all neuroimaging studies with sufficient data on task switching and the Stroop published from 2000 to 2004. The major localizations for the Stroop studies were in the left inferior frontal gyrus (IFG) (areas 44 and 6; with the next largest clusters in bilateral SM cortex, areas 32/6 and 32/9; see also Brass & von Cramon 2004). Localizations were similar if tasks had similar properties to the Stroop. Their conclusion was that the left VL region was the critical region for updating task representations.
(k) Definition of monitoring
Monitoring is the process of checking the task over time for ‘quality control’ and the adjustment of behaviour. Monitoring may occur at many levels: the ongoing activity in a task-specific schema; the timing of activity; anticipation of a stimulus actually occurring; detecting the occurrence of errors; and detecting discrepancies between the behavioural response and external reality. If an anomaly or problem is detected by monitoring, then an interrupt or explicit modulation of the ongoing programme would occur.
(l) Evidence for the monitoring process and putative frontal localization
Our results demonstrate that lesions of the RL prefrontal cortex critically impair monitoring as defined previously (figure 4).
Interstimulus intervals (ISI) in the ROBBIA simple and choice RT tests provided the opportunity to assess the ability of the different patient groups to monitor the interval between trials. The control group showed a normal foreperiod effect, namely a gradual decrease in RT with ISI (Niemi & Näätänen 1981). Only the RL group exhibited a reverse foreperiod effect, an increase in RT with increasing ISI as opposed to the decrease in the control group and all other patient groups (figure 4a). This was evident across both tests, although more evident in the more demanding choice RT test. The architectonic hotspotting of the difference in RT between late and short ISI revealed maximum impairment in areas 9, 9/46d and 9/46v. Vallesi et al. (in press), using TMS over right frontal lateral, left frontal lateral and right angular gyrus in healthy young adults, demonstrated an abnormal foreperiod effect independent of any sequential effect only after RL stimulation. Damage to the RL frontal region impairs the modulation of expectancy. Our study had suggested that this was related to time estimation, but Vallesi used much shorter ISIs (0.5–1.5 s) and revealed the same effect, indicating that the role of RL region in monitoring is not just over longer periods of time. The RL region may be involved in monitoring of temporal information which may be required explicitly as in time reproduction or discrimination tasks (e.g. Basso et al. 2003; Lewis & Miall 2003; Picton et al. in press) or implicitly as in the foreperiod effect.
In the tap experiment (Picton et al. in press), requiring tapping at a rate of once in every 1.5 s either in response to an external stimulus or self-timed, patients with lesions to the RL frontal lobe, particularly involving P & P area 45 and the subjacent regions of the basal ganglia, had an abnormally high intra-individual variability in both self- and tone-timed conditions (figure 4b). This impairment was interpreted as deficient monitoring of the passage of the intervals, although a potential role in generating time-intervals could not be excluded.
The monitoring deficit in relation to checking performance of errors was noted in the FIT (Stuss et al. 2002b). In contrast to the LL group who exhibited false positive errors in the complex three-feature integration condition, the RL group made errors of all kinds: false positives; false negatives; and omissions. Architectonic localization of the false negative errors alone, to minimize any task setting impact, reveals the importance of the RL region in monitoring performance (figure 4c).
Our modification of the WCST, moving from less (128 card) to more structure (64B condition), revealed that set loss errors were more associated with the LL frontal region, when the task was unstructured and subjects were learning what the criteria were and how to perform the task. But the same measure was associated with the RL region instead when the major task demands have been explained to the patients (informed of the three criteria that colour would be the first and the criterion would change after 10 consecutive correct trials), interpreted as deficient monitoring and checking of performance over time (figure 4d).
In our original study of word list learning, there was an association of RL pathology with two measures that we considered monitoring (Stuss et al. 1994). Double recalls were defined as the recall of a word that had already been recalled, but only after at least one intervening word. Inconsistency was a measure of the ‘in and out’ recall of the same word over different trials. In the replication of this study with the CVLT (Alexander et al. 2003), the hotspotting technique did support the predominantly RL (primarily ventrolateral) relationship with the measure of inconsistency. A more direct reflection of this ‘checking’ role of the RL region was achieved by Turner et al. (in press). Functional imaging of memory also indicates an RL role in monitoring of memory (Henson et al. 1999; Fletcher & Henson 2001).
(m) Interim summary of monitoring
Lesions of the RL frontal region produce impairments in monitoring and checking of performance over time. This has been shown by a failure to show a decrease in RT with variable foreperiods, contrasted to normally maintained energizing over a fixed warning interval. In essence, the patients fail to note that a stimulus has not yet occurred, hampering their preparedness to respond. The RL group also fail to note that an error has occurred and do not adjust their performance accordingly. These data and interpretation are compatible with imaging and lesion research in ‘vigilance’ and monitoring (Wilkins et al. 1987; Pardo et al. 1991; Rueckert & Grafman 1996; Coull et al. 1998; Henson et al. 1999; Fletcher & Henson 2001; Shallice 2001, 2002). Functional lesions using transcranial magnetic stimulation over short ISIs suggest that the monitoring is not necessarily limited to ‘vigilance’ over time in the classic sense (Vallesi et al. in press).
The difficulty in monitoring the ongoing passage of the ISI to prepare responsiveness over time is corroborated by the results of the TAP experiment. Although it is possible that prefrontal lesions impair time perception (e.g. Mangels et al. 1998), the variability in time perception throughout the entire experiment whether paced or unpaced promotes the idea of a deficit in monitoring a ‘clock’.
Comparing the SM and RL groups illuminates the type of regionally specific effects that we originally proposed. The RL group alone revealed an abnormal foreperiod effect (ISI effect) when ISI was manipulated. In the TAP test, the patients with RL pathology showed greater variability of performance throughout the test; the SM patients, on the other hand, showed a significant increase in the variability of the responses as the test continued. Taken together, one might hypothesize that the areas of the RL region may interact with the SM regions to initiate or maintain phasic arousal. The RL frontal lobe is crucially involved in the ongoing control of timed behaviour, either owing to its role in generating time-intervals or in monitoring the passage of these intervals. In contrast, the SM regions of the frontal lobe are necessary to maintain consistent timing performance over prolonged periods of time. Lesions to either of these areas may thus generally slow the responses, but for entirely different reasons that become clear only when the fundamental processes dependent on the different regions are explicitly measured.
2. An overview
(a) What of inhibition?
Our model appears to give no special place to a process—inhibition—that classic neuropsychology implies is a major function of the frontal lobes (see also Robbins 2007). The interpretation of our data, including the classic ‘inhibitory’ tasks such as the Stroop, did not have to rely on inhibition as an explanatory phenomenon. Apparent inhibitory processes can be explained by our triad of frontal processes: energization; task setting; and monitoring. Inhibition clearly does exist neurobiologically and neurochemically. Is it possible that inhibition exists only at the level of biology and not psychology? A different theoretical construct, proposed by Cohen and colleagues (Braver et al. 1999), also does not rely on inhibition at an explanatory level. In an earlier study (Stuss et al. 1999), in which we did not have the localizing power of later studies, we had suggested that not only are there frontal inhibitory processes, but also there are different frontal inhibitory processes. In retrospect, however, these data could be explained in light of our current triad. Clearly, this is an avenue of future exploration.
(b) Integrated systems and task demands
Demonstrating fractionation of frontal lobe functions does not imply a set of independent processes. These processes are flexibly assembled in response to context, complexity and intention over real time into different networks within the frontal regions (and between frontal and posterior regions; see also Vuilleumier & Driver 2007). Depending on task demands, there may be recruitment of processes within the frontal lobes. In some cases, ‘top-down’ recruitment of posterior processes is demanded. In tasks with simple demands, the more automatic non-frontal processes function independently, but with increase in task requirements, an increased involvement of different frontal (more ‘strategic’) regions may be required, even to the point where it appears that all frontal regions are involved.
This dynamic interaction can occur among the different domain-general frontal lobe processes, and between the frontal lobe domain-general and more posterior domain-specific functions (see Stuss et al. 1999, 2002a; Stuss 2006 for illustrations). Examples occur in how we learn (memory), pay attention and communicate. Take the example of language (Alexander 2006). The ‘executive’ or domain-general frontal lobe processes are not required for answering simple questions, requesting an object or recounting an old familiar story. However, telling a complex narrative, or composing a complicated response to a difficult question requires setting the task goal (what to include, what does the listener already know of the story and what is socially appropriate for this occasion), energization (to plan, and to activate and sustain the intention) and monitoring (keeping track of the objective and content and the listener's response). These frontal lobe operations also demand a series of posterior cognitive operations, and all of these must be recruited and interwoven at different levels and different times.
3. Conclusions
Research in patients with focal circumscribed chronic frontal lobe lesions demonstrates that specific, highly differentiated attentional deficits are associated with discrete regional frontal injuries and, at least in patients with single focal circumscribed lesions, there is no undifferentiated ‘frontal lobe syndrome’ or ‘dysexecutive’ disorder.
In the area of attention, three separate functions can be demonstrated consistently: energization, related to damage in the SM frontal area, possibly predominantly right; task setting—LL; and monitoring—RL. Further division within the lateral regions, perhaps related to posterior connectivity, has not yet been examined.
These processes are indeed ‘supervisory’, in that they are important for the control of lower-order processes. Since they interact with many other modular cognitive modalities, these processes are domain-general. The functioning of what has been called the central executive or supervisory system can be explained by the flexible assembly of these processes in response to context, complexity and intention over real time into different networks within the frontal regions, and between frontal and posterior regions. There is no overarching supervisory system—no ‘ghost in the machine’—that is higher in the hierarchy.
Acknowledgments
Funding for the research described in this study was provided primarily by the Canadian Institutes of Health Research Grants no. 108636, and MRC-GR-14974. Thanks to the co-authors on the various papers referenced, all of our staff who have worked on these studies over the years and the patients and subjects who contributed their time. We are especially indebted to Susan Gillingham for figure and manuscript preparation.
Appendix A. Descriptions of tests and measures
(a) Standard ‘frontal lobe’ tests
Two standard ‘frontal lobe’ tests were administered and they are described as follows:
(i) Wisconsin Card Sorting Test (WCST; Milner 1963)
In our study (Stuss et al. 2000), the test was administered three times in the order described below, each administration increasing in the amount of information and/or external structure provided.
128 cards: all 128 cards were administered, following the administration procedure of Milner (1963).
64 cards (64A): the subject was informed what the three sorting criteria were (colour, form and number), and an additional 64 cards were administered, again starting with colour as the first criterion.
64 cards (64B): the subject was again informed of the three sorting criteria, but now, in addition, was told that colour was the initial sorting criterion, and that the criterion would change after 10 consecutive responses.
The dependent measure used in this paper to illustrate the different attentional processes was ‘set loss’, defined in our research as the number of times an error is made after at least three consecutively correct responses, one of which has to be unambiguously correct to indicate that there had been experience with the correct sorting criterion. Set loss in conditions 128 and 64B are compared. In condition 128, the subject is hypothetically using trial and error to learn the criteria. Set loss in this condition would be more likely to reflect the discovery and establishment of the correct criterion. In condition 64B, the subject is provided with much more structure and information. Set loss in this case is more probably related to the monitoring of responses rather than setting the response.
(ii) Stroop (Comalli et al. 1962)
In our study (Stuss et al. 2001), we used the Comalli et al. (1962) version often used clinically. There were three conditions, 100 stimuli in each condition: reading colour words (red, blue and green) printed in black; naming colour patches (R, B and G); naming the colour of ink in which a colour name is printed when the colour is incongruent with the name (‘red’ printed in the colour green; the Stroop interference effect).
The dependent measurement for this study was the number of errors made in each condition.
(b) Language and memory tests requiring executive processes
(i) Verbal fluency (Borkowski et al. 1967)
We analysed the performance of patients with focal frontal lesions on verbal fluency (Stuss et al. 1998). For letter fluency, subjects were required to generate words (no proper names), beginning with letters F, A, and then S, over a duration of 1 min per letter.
For this paper, we compared the number of correct words produced. To isolate the energization process, the time was divided into first 15 and last 45 s. The loss of energization was evaluated by comparing the number of words generated in the first 15 s to that generated in the last 45 s.
(ii) California Verbal Learning Test (CVLT I; Delis et al. 1987)
We (Alexander et al. 2003) administered the CVLT to a large group of frontal patients over many years, using the first version of the CVLT, using the standard administration.
Two measures are emphasized in this current paper to illustrate how different attentional processes can be necessary for successful memory performance. A measure of bias in recognition memory, the establishment of a criterion or threshold for saying ‘yes’ to words presented for recognition, is defined as the number of false positive responses (the number of times a subject says ‘yes’ to a non-target word). The measure of monitoring we use is ‘inconsistency’, defined as the number of times a word is recalled on one trial, then forgotten on the next, and so on.
(c) Feature integration test (FIT; Stuss et al. 1989, 2002b)
In each of the following tests of FIT, the subject was asked to respond as quickly as possible but not at the cost of making errors.
Simple RT. One stimulus consisting of a simple shape (e.g. circle) was repeated for 50 trials.
Single feature detection (easy choice RT). Four single geometrical shapes (e.g. circle, square, triangle and cross) were presented randomly. One shape was designated as a target, the other three were non-targets. The subject responded to the target with the dominant hand and to the non-target with the non-dominant hand.
Three feature detection (complex RT). The target was defined as a unique combination of a colour, shape and line orientation within the shape (e.g. a blue circle with horizontal lines inside the circle). The non-targets could share none, one or two features with the target.
Dependent measures were RT speed and the type and number of errors. In this current paper, we present only the results for the three feature detection test (complex RT). Error types analysed were false positives (non-target responded to as a target) and false negatives (target responded to as a non-target).
(d) ROBBIA (ROtman-Baycrest Battery to Investigate Attention; Alexander et al. 2005; Stuss et al. 2005; Picton et al. 2006, in press)
Several tests were developed as part of the ROtman-Baycrest Battery to Investigate Attention. Many of them evolved from the FIT.
Simple RT. For the simple RT, the capital letter ‘A’ was presented 50 times repeatedly and subjects responded by pressing button 1 as soon as the letter was presented.
Choice RT (single feature choice). The target was defined as one of the four letters (A, B, C and D) presented 25% of the time, the remaining letters being non-target feature distractors, each also presented with a probability of 25%. Responses were made to both targets and non-targets as in FIT; button 1 was pressed for the target and button 2 for the non-targets.
Few errors were made in the simple and choice RT tests. The dependent measurements were overall RT, as well as RT divided by interstimulus intervals (ISI). ISIs in the ROBBIA simple and choice RT tests were set at 3, 4, 5, 6 or 7 s, with each ISI occurring 10 times in a random order. The ISI was defined as the time from the offset of one stimulus (at the response) to the onset of the next (and is equivalent to response-to-stimulus interval).
Prepare RT. In this condition, choice RT (mentioned previously) with no warning stimulus was compared with two conditions with warning stimuli, 1 or 3 s prior to the trial.
Again, there were few errors in this test. Dependent measurements were RTs in each condition. The simple, choice and prepare data were presented in detail in Stuss et al. (2005).
Concentrate. Concentrate is a rapid serial response task consisting of five LEDs, each of which having a response button directly under the LED (Alexander et al. 2005). The LEDs would illuminate randomly. The subject's task was to press the response button directly under the LED when illuminated. Over an approximately 5 min period, 500 trials were presented continuously. Working memory demands are minimized, as the response demands are driven by the light illumination.
Dependent measurements in concentrate were the overall RT, as well as the number of errors, the latter divided by occurrence within each 100 trials.
Tap. The primary task in tap was to tap a response button at a rate of once every 1.5 s. There were two conditions, one requiring responding in time with a tone that regularly repeated and another demanding maintenance of the same regular response rhythm without any external stimulus.
The dependent measurement here was the intra-individual standard deviation, a measure of individual variability in performance, assessing the ability to maintain appropriate timing over time.
Suppress. Suppress assesses processes similar to the Stroop. Red or blue letters were presented at a rate of once every 3–4 s. For targets (25%), a red ‘X’ or a blue ‘O’, the subject pressed button 1; for all non-targets, button 2. The non-target ‘Stroop’ distractors (25%) were either a red ‘O’ or a blue ‘X’. Other non-targets (50%) were letters other than ‘X’ and ‘O’ in blue or red, excluding the potentially visually confusing letters C, D, G, Q, K and Y (Alexander et al. in press).
Dependent measurement was the number of false positive errors (the identification of any non-target as a target).
Nogo. In this test, we evaluated the ability to withhold responses to stimuli of varying probabilities of presentation (Picton et al. in press). Two conditions were administered; in each, four letters (A, B, C and D) were randomly presented with 25% probability. In the first condition, a response was required to the letter A, but no response for other letters (B, C and D). In the second half of the experiment, a response was required to the letters B, C and D but not to A.
The dependent measurement in nogo was the number of false alarms (or false positives).
One contribution of 14 to a Discussion Meeting Issue ‘Mental processes in the human brain’.
Endnotes
We believe there is evidence for four categories of frontal lobe functions, which supports the fractionation of frontal lobe functions: executive capacity (which we equate with attentional control), energization, behavioural self-regulation, and metacognition, which are related to different frontal anatomical regions (Stuss & Levine 2002; Stuss 2007; Stuss & Alexander in press). Only the first two [executive (within this, task setting and monitoring), and energization] are described in this current paper. Other investigators have also considered the frontal lobes to have multiple processes (Burgess et al. 2007; Robbins 2007).
The following number of frontal patients were assessed in each of the categories of tests: clinical neuropsychological tests, 33–56, depending on the test; FIT, 25; ROBBIA, 43. Overlap of patients was as follows: no overlap between FIT and ROBBIA; 3 patients were common to ROBBIA and clinical neuropsychological tests; 16 of the FIT patients were also assessed in the clinical neuropsychological tests.
References
- Alexander M.P. Chronic akinetic mutism after mesencephalic–diencephalic infarction: remediated with dopaminergic medications. Neurorehabil. Neural Repair. 2001;15:151–156. doi: 10.1177/154596830101500208. [DOI] [PubMed] [Google Scholar]
- Alexander M.P. Impairments of procedures for implementing complex language are due to disruption of frontal attention processes. J. Int. Neuropsychol. Soc. 2006;12:236–247. doi: 10.1017/S1355617706060309. [DOI] [PubMed] [Google Scholar]
- Alexander M.P, Stuss D.T, Fansabedian N. California verbal learning test: performance by patients with focal frontal and non-frontal lesions. Brain. 2003;126:1493–1503. doi: 10.1093/brain/awg128. doi:10.1093/brain/awg128 [DOI] [PubMed] [Google Scholar]
- Alexander M.P, Stuss D.T, Shallice T, Picton T.W, Gillingham S. Impaired concentration due to frontal lobe damage from two distinct lesion sites. Neurology. 2005;65:572–579. doi: 10.1212/01.wnl.0000172912.07640.92. doi:10.1212/01.wnl.0000172912.07640.92 [DOI] [PubMed] [Google Scholar]
- Alexander, M. P., Stuss, D. T., Picton, T., Shallice, T. & Gillingham, S. In press. Regional frontal injuries cause distinct forms of impaired attention to respond. Neurology [DOI] [PubMed]
- Basso G, Nichelli P, Wharton C.M, Peterson M, Grafman J. Distributed neural systems for temporal production: a functional MRI study. Brain Res. Bull. 2003;59:405–411. doi: 10.1016/s0361-9230(02)00941-3. doi:10.1016/S0361-9230(02)00941-3 [DOI] [PubMed] [Google Scholar]
- Borkowski J.G, Benton A.L, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. doi:10.1016/0028-3932(67)90015-2 [Google Scholar]
- Brass M, von Cramon D.Y. Selection for cognitive control: a functional magnetic resonance imaging study on the selection of task-relevant information. J. Neurosci. 2004;24:8847–8852. doi: 10.1523/JNEUROSCI.2513-04.2004. doi:10.1523/JNEUROSCI.2513-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T.S, Barch D.M, Cohen J.D. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol. Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. doi:10.1016/S0006-3223(99)00116-X [DOI] [PubMed] [Google Scholar]
- Brutkowski S. Functions of prefrontal cortex in animals. Physiol. Rev. 1965;45:721–746. doi: 10.1152/physrev.1965.45.4.721. [DOI] [PubMed] [Google Scholar]
- Burgess P.W, Shallice T. Fractionnement du syndrome frontal. Revue de Neuropsychologie. 1994;4:345–370. [Google Scholar]
- Burgess P.W, Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34:263–272. doi: 10.1016/0028-3932(95)00104-2. doi:10.1016/0028-3932(95)00104-2 [DOI] [PubMed] [Google Scholar]
- Burgess P.W, Gilbert S.J, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Phil. Trans. R. Soc. B. 2007;362:887–899. doi: 10.1098/rstb.2007.2095. doi:10.1098/rstb.2007.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.D, McClelland J.L, Dunbar K. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol. Rev. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. doi:10.1037/0033-295X.97.3.332 [DOI] [PubMed] [Google Scholar]
- Comalli P.E, Wapner S, Werner H. Interference effects of Stroop colour-word test in childhood, adulthood, and aging. J. Gen. Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- Coull J.T, Frackowiak R.S, Frith C.D. Monitoring for target objects: activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia. 1998;36:1325–1334. doi: 10.1016/s0028-3932(98)00035-9. doi:10.1016/S0028-3932(98)00035-9 [DOI] [PubMed] [Google Scholar]
- Delis D.C, Kramer J, Kaplan E, Ober B.A. Psychological Corporation; San Antonio, TX: 1987. California verbal learning test (cvlt) manual. [Google Scholar]
- Derfus J, Brass M, Neumann J, von Cramon D.Y. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum. Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. doi:10.1002/hbm.20127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell M, Vogt B.A. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. doi:10.1093/brain/118.1.279 [DOI] [PubMed] [Google Scholar]
- Diaz R, Robbins T.W, Roberts A.C. Dissociation in prefrontal cortex of affective attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. doi:10.1038/380069a0 [DOI] [PubMed] [Google Scholar]
- Drewe E.A. An experimental investigation of luria's theory on the effects of frontal lobe lesions in man. Neuropsychologia. 1975a;13:421–429. doi: 10.1016/0028-3932(75)90065-2. doi:10.1016/0028-3932(75)90065-2 [DOI] [PubMed] [Google Scholar]
- Drewe E.A. Go–no go learning after frontal lobe lesions in humans. Cortex. 1975b;11:8–16. doi: 10.1016/s0010-9452(75)80015-3. [DOI] [PubMed] [Google Scholar]
- Duncan J, Miller E.K. Cognitive focus through adaptive neural coding in the primate prefrontal cortex. In: Stuss D.T, Knight R.T, editors. Principles of frontal lobe function. Oxford University Press; New York, NY: 2002. pp. 278–291. [Google Scholar]
- Elsass P, Hartelius H. Reaction time and brain disease: relations to location, etiology and progression of cerebral dysfunction. Acta Neurol. Scand. 1985;71:11–19. doi: 10.1111/j.1600-0404.1985.tb03160.x. [DOI] [PubMed] [Google Scholar]
- Fletcher P.C, Henson R.N. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. doi:10.1093/brain/124.5.849 [DOI] [PubMed] [Google Scholar]
- Godefroy O, Lhullier C, Rousseaux M. Vigilance and effects of fatigability, practice and motivation on simple reaction time tests in patients with lesion of the frontal lobe. Neuropsychologia. 1994;32:983–990. doi: 10.1016/0028-3932(94)90047-7. doi:10.1016/0028-3932(94)90047-7 [DOI] [PubMed] [Google Scholar]
- Godefroy O, Cabaret M, Petit-Chenal V, Pruvo J.-P, Rousseaux M. Control functions of the frontal lobes. Modularity of the central-supervisory system? Cortex. 1999;35:1–20. doi: 10.1016/s0010-9452(08)70782-2. [DOI] [PubMed] [Google Scholar]
- Heilman K.M, Watson R.T. The neglect syndrome—a unilateral defect of the orienting response. In: Harnad S, Doty R.W, Jaynes J, Goldstein L, Krauthamer G, editors. Lateralization in the nervous system. Academic Press; New York, NY: 1977. pp. 285–302. [Google Scholar]
- Henson R.N.A, Shallice T, Dolan R.J. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999;122:1367–1381. doi: 10.1093/brain/122.7.1367. doi:10.1093/brain/122.7.1367 [DOI] [PubMed] [Google Scholar]
- Hockey G.R.J. Cognitive energetic control mechanisms in the management of work demands and psychological health. In: Baddeley A.D, Weiskrantz L, editors. Attention: selection, awareness and control. A tribute to Donald Broadbent. Clarendon Press; Oxford, UK: 1993. pp. 328–345. [Google Scholar]
- Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans A.C, Jones B.E. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J. Neurosci. 1997;17:4800–4808. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen S.D, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp. Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. doi:10.1007/BF00237911 [DOI] [PubMed] [Google Scholar]
- Knight R.T. Evoked potential studies of attention capacity in human frontal lobe lesions. In: Levin H, Eisenberg H, Benton F, editors. Frontal lobe function and dysfunction. Oxford University Press; New York, NY: 1991. pp. 139–153. [Google Scholar]
- Kornblum S, Stevens G.T, Whipple A, Requin J. The effects of irrelevant stimuli: 1. The time course of stimulus–stimulus and stimulus–response consistency effects with Stroop-like stimuli, Simon-like tasks, and their factorial combinations. J. Exp. Psychol. Hum. Percept. Perform. 1999;25:688–714. doi:10.1037/0096-1523.25.3.688 [Google Scholar]
- Leimkuhler M.E, Mesulam M.M. Reversible go–no go deficits in a case of frontal lobe tumor. Ann. Neurol. 1985;18:617–619. doi: 10.1002/ana.410180518. doi:10.1002/ana.410180518 [DOI] [PubMed] [Google Scholar]
- Lewis P.A, Miall R.C. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia. 2003;41:1583–1592. doi: 10.1016/s0028-3932(03)00118-0. doi:10.1016/S0028-3932(03)00118-0 [DOI] [PubMed] [Google Scholar]
- Luria A.R. Basic Books; New York, NY: 1966. Higher cortical functions in man. [Google Scholar]
- Luria A.R. Basic Books; New York, NY: 1973. The working brain: an introduction to neuropsychology. [Google Scholar]
- Luu P, Collins P, Tucker D.M. Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J. Exp. Psychol. Gen. 2000a;129:43–60. doi: 10.1037//0096-3445.129.1.43. doi:10.1037/0096-3445.129.1.43 [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker D.M. Medial frontal cortex in action monitoring. J. Neurosci. 2000b;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangels J.A, Ivry R.B, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Cogn. Brain Res. 1998;7:15–39. doi: 10.1016/s0926-6410(98)00005-6. doi:10.1016/S0926-6410(98)00005-6 [DOI] [PubMed] [Google Scholar]
- Mesulam M.-M. Davis; Philadelphia, PA: 1985. Principles of behavioral neurology. [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting: the role of the frontal lobes. Arch. Neurol. 1963;9:100–110. [Google Scholar]
- Niemi P, Näätänen R. Foreperiod and simple reaction time. Psychol. Bull. 1981;89:133–162. doi:10.1037/0033-2909.89.1.133 [Google Scholar]
- Norman D.A, Shallice T. Attention to action: willed and automatic control of behaviour. In: Davidson R.J, Shwartz G.E, Shapiro D, editors. Consciousness and self-regulation: advances in research and theory. Plenum; New York, NY: 1986. pp. 1–18. [Google Scholar]
- Pardo J.V, Fox P.T, Raichle M.E. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. doi:10.1038/349061a0 [DOI] [PubMed] [Google Scholar]
- Passingham R. Oxford University Press; Oxford, UK: 1993. The frontal lobes and voluntary action. [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. doi:10.1038/35077500 [DOI] [PubMed] [Google Scholar]
- Paus T, Zatorre R.J, Hofle N, Caramanos J.G, Petrides M, Evans A.C. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J. Cogn. Neurosci. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- Perret E. Left frontal lobe of man and suppression of habitual responses in verbal categorical behavior. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. doi:10.1016/0028-3932(74)90047-5 [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D.M. Comparative architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Elsevier; Amsterdam, The Netherlands: 1994. pp. 17–57. [Google Scholar]
- Picard N, Strick P.L. Motor areas of the medial wall: a review of their location and functional activation. Cereb. Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. doi:10.1093/cercor/6.3.342 [DOI] [PubMed] [Google Scholar]
- Picton T.W, Stuss D.T, Alexander M.P, Shallice T, Gillingham S. Keeping time: Effects of focal frontal lesions. Neuropsychologia. 2006;44:1195–1209. doi: 10.1016/j.neuropsychologia.2005.10.002. doi:10.1016/j.neuropsychologia.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Picton, T. W., Stuss, D. T., Alexander, M. P., Shallice, T., Binns, M. A. & Gillingham, S. In press. Effects of focal frontal lesions on response inhibition. Cereb. Cortex [DOI] [PubMed]
- Plum F, Posner J.B. 3rd edn. Davis; Philadelphia, PA: 1980. The diagnosis of stupor and coma. [Google Scholar]
- Posner M.I, Petersen S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. doi:10.1146/annurev.ne.13.030190.000325 [DOI] [PubMed] [Google Scholar]
- Richer F, Decary A, Lapierre M.-P, Rouleau I, Bouvier G, Saint-Hilaire J.-M. Target detection deficits in frontal lobectomy. Brain Cogn. 1993;21:203–211. doi: 10.1006/brcg.1993.1016. doi:10.1006/brcg.1993.1016 [DOI] [PubMed] [Google Scholar]
- Robbins T.W. Dissociating executive functions of the prefrontal cortex. Phil. Trans. R. Roc. B. 1996;351:1463–1470. doi: 10.1098/rstb.1996.0131. doi:10.1098/rstb.1996.0131 [DOI] [PubMed] [Google Scholar]
- Robbins T.W. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Phil. Trans. R. Soc. B. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. doi:10.1098/rstb.2007.2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert L, Grafman J. Sustained attention deficits in patients with right frontal lesions. Neuropsychologia. 1996;34:953–963. doi: 10.1016/0028-3932(96)00016-4. doi:10.1016/0028-3932(96)00016-4 [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Phil. Trans. R. Soc. B. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. doi:10.1098/rstb.1982.0082 [DOI] [PubMed] [Google Scholar]
- Shallice T. Fractionating the supervisory system. Brain Cogn. 2001;47:30. [Google Scholar]
- Shallice T. Fractionation of the supervisory system. In: Stuss D.T, Knight R.T, editors. Principles of frontal lobe function. Oxford University Press; New York, NY: 2002. pp. 261–277. [Google Scholar]
- Shallice T, Burgess P.W. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. doi:10.1093/brain/114.2.727 [DOI] [PubMed] [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. doi:10.1037/h0054651 [Google Scholar]
- Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14:S76–S84. doi: 10.1006/nimg.2001.0839. doi:10.1006/nimg.2001.0839 [DOI] [PubMed] [Google Scholar]
- Stuss D.T. Frontal lobes and attention: processes and networks, fractionation and integration. J. Int. Neuropsychol. Soc. 2006;12:261–271. doi: 10.1017/S1355617706060358. [DOI] [PubMed] [Google Scholar]
- Stuss D.T. New approaches to prefrontal lobe testing. In: Miller B, Cummings J, editors. The human frontal lobes: functions and disorders. 2nd edn. Guildford Press; New York, NY: 2007. pp. 292–305. [Google Scholar]
- Stuss, D. T. & Alexander, M. P. In press. Executive functions: is there a frontal lobe syndrome? In New encyclopedia of neuroscience
- Stuss D.T, Benson D.F. Neuropsychological studies of the frontal lobes. Psychol. Bull. 1984;95:3–28. doi:10.1037/0033-2909.95.1.3 [PubMed] [Google Scholar]
- Stuss D.T, Benson D.F. Raven Press; New York, NY: 1986. The frontal lobes. [Google Scholar]
- Stuss D.T, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu. Rev. Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. doi:10.1146/annurev.psych.53.100901.135220 [DOI] [PubMed] [Google Scholar]
- Stuss D.T, Stethem L.L, Hugenholtz H, Picton T.W, Pivik J, Richard M.T. Reaction time after head injury: fatigue, divided and focused attention and consistency of performance. J. Neurol. Neurosurg. Psychiatry. 1989;52:742–748. doi: 10.1136/jnnp.52.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T, Alexander M.P, Palumbo C.L, Buckle L, Sayer L, Pogue J. Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology. 1994;8:355–373. doi:10.1037/0894-4105.8.3.355 [Google Scholar]
- Stuss D.T, Shallice T, Alexander M.P, Picton T.W. A multidisciplinary approach to anterior attentional functions. Ann. N. Y. Acad. Sci. 1995;769:191–211. doi: 10.1111/j.1749-6632.1995.tb38140.x. doi:10.1111/j.1749-6632.1995.tb38140.x [DOI] [PubMed] [Google Scholar]
- Stuss D.T, Alexander M.P, Hamer L, Palumbo C, Dempster R, Binns M, Levine B, Izukawa D. The effects of focal anterior and posterior brain lesions on verbal fluency. J. Int. Neuropsychol. Soc. 1998;4:265–278. [PubMed] [Google Scholar]
- Stuss D.T, Toth J.P, Franchi D, Alexander M.P, Tipper S, Craik F.I.M. Dissociation of attentional processes in patients with focal frontal and posterior lesions. Neuropsychologia. 1999;37:1005–1027. doi: 10.1016/s0028-3932(98)00158-4. doi:10.1016/S0028-3932(98)00158-4 [DOI] [PubMed] [Google Scholar]
- Stuss D.T, Levine B, Alexander M.P, Hong J, Palumbo C, Hamer L, Murphy K.J, Izukawa D. Wisconsin card sorting test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. doi:10.1016/S0028-3932(99)00093-7 [DOI] [PubMed] [Google Scholar]
- Stuss D.T, Floden D, Alexander M.P, Levine B, Katz D. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia. 2001;39:771–786. doi: 10.1016/s0028-3932(01)00013-6. doi:10.1016/S0028-3932(01)00013-6 [DOI] [PubMed] [Google Scholar]
- Stuss D.T, Alexander M.P, Floden D.T, Binns M.A, Levine B, McIntosh A.R, Rajah M.N, Hevenor S.J. Fractionation and localization of distinct frontal lobe processes: evidence from focal lesions in humans. In: Stuss D.T, Knight R.T, editors. Principles of frontal lobe function. Oxford University Press; New York, NY: 2002a. pp. 392–407. [Google Scholar]
- Stuss D.T, Binns M.A, Murphy K.J, Alexander M.P. Dissociations within the anterior attentional system: effects of task complexity and irrelevant information on reaction time speed and accuracy. Neuropsychology. 2002b;16:500–513. doi: 10.1037//0894-4105.16.4.500. doi:10.1037/0894-4105.16.4.500 [DOI] [PubMed] [Google Scholar]
- Stuss D.T, Alexander M.P, Shallice T, Picton T.W, Binns M.A, MacDonald R, Borowiec A, Katz D. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. doi:10.1016/j.neuropsychologia.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what,” “when,” and “where” related information: an fMRI study. J. Cogn. Neurosci. 2005;17:981–993. doi: 10.1162/0898929054475226. doi:10.1162/0898929054475226 [DOI] [PubMed] [Google Scholar]
- Turner, M. S., Cipolotti, L., Yousry, T. & Shallice, T. In press. Qualitatively different memory impairments across frontal lobe subgroups. Neuropsychologia45, 1540–1552. (doi:10.1016/j.neuropsychologia.2006.11.013). [DOI] [PubMed]
- Vallesi A, Shallice T, Walsh V. Role of the prefrontal cortex in the foreperiod effect. TMS evidence for dual mechanisms in temporal preparation. Cereb. Cortex. 2007;17:466–474. doi: 10.1093/cercor/bhj163. doi:10.1093/cercor/bhj163 [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Heilman K.M. Response preparation and response inhibition after lesions of the medial frontal lobe. Arch. Neurol. 1987;44:1265–1271. doi: 10.1001/archneur.1987.00520240045010. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Phil. Trans. R. Soc. B. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. doi:10.1098/rstb.2007.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Baylis G.C. Effects of increased response dominance and contextual disintegration on the Stroop interference effect in older adults. Psychol. Aging. 1998;13:206–217. doi: 10.1037//0882-7974.13.2.206. doi:10.1037/0882-7974.13.2.206 [DOI] [PubMed] [Google Scholar]
- Wilkins A.J, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. doi:10.1016/0028-3932(87)90024-8 [DOI] [PubMed] [Google Scholar]