Abstract
The neuropsychological basis of attentional set-shifting, task-set switching and stop-signal inhibition is reviewed through comparative studies of humans and experimental animals. Using human functional neuroimaging, plus neuropsychological investigation of patients with frontal damage quantified by structural magnetic resonance imaging, and through parallels with effects of specific lesions of the prefrontal cortex (PFC) and striatum in rats and marmosets, it is possible to define both distinct and overlapping loci for tasks such as extra-dimensional shifting and reversal learning, stop-signal reaction time and task-set switching. Notably, most of the paradigms implicate a locus in the right PFC, specifically the right inferior frontal gyrus, possibly associated with processes of response inhibition. The neurochemical modulation of fronto-striatal circuitry in parallel with effects on task performance has been investigated using specific neuropharmacological agents in animals and by human psychopharmacological investigations, sometimes in conjunction with functional imaging. Evidence is provided for double dissociations of effects of manipulations of prefrontal cortical catecholamine and indoleamine (5-HT) systems that have considerable implications in the treatment of disorders such as Parkinson's disease, attention deficit/hyperactivity disorder and depression, as well as in theoretical notions of how ‘fronto-executive’ functions are subject to state-dependent influences, probably related to stress, arousal and motivation.
Keywords: prefrontal cortex, striatum, neurotransmitters, switching, inhibition
1. Introduction
About a decade ago, a Discussion Meeting of the Royal Society that was dedicated to the functions of the prefrontal cortex (PFC; Roberts et al. 1998a,b) helped to crystallize a number of issues that have since been the subject of intense theoretical and empirical effort. At that time, considerable attention was being paid to the concept that the PFC helped to mediate aspects of ‘working memory’ and its role in generating representations of the world, although the evidence from non-human primates (Goldman-Rakic 1998) did not always map convincingly onto the so-called executive aspects of human working memory (Baddeley & Della Salla 1998). Part of the reason for this mismatch arose from differences in the use of the term ‘working memory’. Goldman-Rakic emphasized the maintenance operation of working memory, i.e. the capacity to hold stimuli ‘on-line’ and protect them from disruption, whereas the human theories of working memory (e.g. Baddeley 1986) placed more weight on understanding how information stored in modality-specific short-term memory buffers was used and the coordination of these buffers by a hypothetical (and vaguely specified) ‘central executive’ (see also D'Esposito 2007). There was nevertheless a superficial coherence in terms of studies attempting to localize working memory functions in humans (Owen et al. 1990; Smith & Jonides 1995) and monkeys (Goldman-Rakic 1998).
One productive approach was to break down working memory functions into components such as those specified by the Baddeley model. This led to the suggestion that anatomically separate loci might mediate functions such as ‘holding stimuli online’ (ventrolateral PFC) and the manipulation of its contents using strategic encoding (dorsolateral PFC) (Owen et al. 1996; Petrides 1998; Robbins 1998; Bor et al. 2003). Further work has shown that the role of the PFC in response-selection processes in standard working memory paradigms may have been underestimated (e.g. Rowe et al. 2000): the role of the PFC in response selection had been a major theme of the early Shallice and Norman's model of PFC as a ‘supervisory attentional system’ (Shallice 1982).
Another consistent theme emerging about PFC function was the role of the ventromedial orbitofrontal cortex in emotional decision making, as determined especially by the study of single cases with damage including that region (Damasio 1998). This led to speculations about how these regions of the PFC, with their classical limbic connectivity, interacted with dorsolateral PFC regions in the control of cognition and behaviour. One promising basic neuroscience approach has been to track the impact of rewarding feedback in discrimination tasks in rhesus monkeys on single-cell activity throughout the PFC during the course of learning and performance (Hollerman et al. 2000; Miller & Cohen 2001). Another has been to postulate different forms of ‘marker’ that are monitored by the PFC to guide optimal performance (Damasio 1998; Shallice & Burgess 1998). A third, mainly employing functional brain imaging, has focused directly on the hypothesis that parts of the human medial and orbitofrontal cortex (OFC) mediate ‘reward’ or ‘goal’ representations (O'Doherty et al. 2001). In general, the OFC has been implicated in choice mechanisms that are recruited to deal with complex contingencies, as often occur, for example, in an economic context (Rogers et al. 1999a; McClure et al. 2004; Huettel et al. 2006; see also Dolan 2007).
This implication of the OFC in reward processes also had to be integrated with growing evidence of the importance of specified subcortical circuitry in the mediation of reward processes, notably dopamine-dependent functions of the nucleus accumbens (Robbins & Everitt 1992). This has led to a growing realization of the nodal position of the PFC in ‘loop’ circuitries involving connections between the OFC, other limbic structures, the nucleus accumbens, mediodorsal thalamus and ventral pallidum. Such neuroanatomical loops link other sectors of the PFC and functionally related regions of the striatum in a cascading series of serial as well as parallel circuitries (Alexander et al. 1986; Haber et al. 2000). The functioning of the cortico-striatal loops is also influenced by a number of ascending ‘neuromodulatory’ chemical neurotransmitter systems, notably the catecholamines (dopamine and noradrenaline), the indoleamine serotonin or 5-hydroxytryptamine (5-HT) and acetylcholine (Robbins 2000). These neurochemical systems, which are implicated in stress, arousal and mood as well as reward processes (see Robbins & Everitt 1992; Arnsten & Robbins 2002), may themselves, to some extent, be regulated by the descending influences of the PFC (e.g. Amat et al. 2005). In general, these cortico-striatal systems can be understood as incorporating mechanisms for the optimal selection of goals and responses, and for the optimal preparation of appropriate response outputs. Phasic activity in some of the neuromodulatory systems, especially the mesolimbic dopamine pathway, has been implicated in the mechanisms of learning, for example reflecting ‘error prediction signals’ (Schultz & Dickinson 2000). In addition, tonic levels of activity in the neuromodulatory systems can be understood as representing different states, in which various types of ‘executive operation’ are recruited and performed. Implementation of some tasks requiring executive control may be optimally performed in different states (e.g. of ‘arousal’, ‘fatigue’ or ‘mood’; Robbins 2000). Executive control refers to the collection of mechanisms that serve to optimize behavioural and cognitive output, and includes the regulation of input (e.g. over posterior cortical processing), output (e.g. via the basal ganglia and the associated cortico-striatal loops) and also the activity of the ascending neuromodulatory systems.
A particular function of the PFC in response selection becomes evident in fluctuating or ambiguous circumstances, for example in dual-task control, attentional conflict (e.g. Stroop interference) and changes in background distracting stimuli or instructions (e.g. contextual control; Cohen et al. 1998), as well as following changes in rewarding and error feedback (e.g. Wisconsin Card Sort Test, WCST). Most of these situations underline the principle that the supervisory attentional system of Shallice and Norman (Shallice 1982) becomes especially important in the selection of rapid responses to novel, often stressful situations, for example by adding more ‘weight’ or bias to particular representations. In neurobiological terms, this cognitive flexibility may correspond to the recruitment of the same sort of plastic mechanisms implicated in rapid learning itself, involving for example the phenomenon of long-term potentiation and the involvement of glutamate receptors, especially of the NMDA receptor subtype (Moghaddam 2004; Robbins & Murphy 2006). However, although the PFC has long been implicated in the mechanisms of cognitive flexibility, a conceptual basis for understanding this typically ‘executive’ function has been elusive or described only globally in terms of ‘behavioural inhibition’. The most potent way of effecting behavioural change is either to withdraw rewarding feedback for a particular response or shift it to another option. This feedback may be associated with particular stimuli or responses, or more usually with classes or categories of input and output, including perceptual dimensions and ‘task sets’ (i.e. specifications of particular stimulus–response (S–R) links). Moreover, the shifts in reinforcement contingencies may occur occasionally and encompass new learning (Slamecka 1968; Roberts et al. 1988), or may instead occur frequently for well-established responses or task sets (Monsell 2003). Thus, the subject may have to shift rapidly between responding to one of two established task sets such as naming digits and naming letters, when both are present in the array. Alternatively, the ‘task set’ may be to respond according to a choice reaction time procedure as rapidly as possible, but occasionally to cancel or countermand responding altogether, in the presence of a so-called ‘stop-signal’ (Logan & Cowan 1984).
In this paper, the neural substrates and their chemical neuromodulation of basic operations such as shifting and stopping are surveyed, drawing upon evidence from experimental animals and humans. It has become evident that, despite the considerable heuristic usefulness of the Norman and Shallice model, it is not feasible to make any more than a coarse mapping of their influential concepts of ‘attention to action’ onto the matrix of specific neural and neurochemical dissociations among different types of cognitive flexibility to be described below. The paper begins with the example of attentional set-shifting, which has been used, in both experimental animals and humans, to decompose the types of processes engaged by tests such as the WCST. This test is sensitive not only to deficits of cognitive rigidity in frontal patients, but also to a number of other disorders ranging from Parkinson's disease to schizophrenia. Two further paradigms used to model certain components of executive control processes in humans, task-set switching and stop-signal inhibition, will also be analysed. The structure of the paper will depend first on defining the types of operation that are measured in these paradigms and their neural correlates. These having been established, it is logical to examine in each case their neurochemical modulation by the chemically defined ascending systems. The relevance of these analyses to several neurological and neuropsychiatric disorders that exhibit substantial deficits in certain ones of these cognitive tests will then be made clear, an important link being that many of the medications employed in these disorders have selective actions on the chemical modulatory systems.
2. Attentional set-shifting
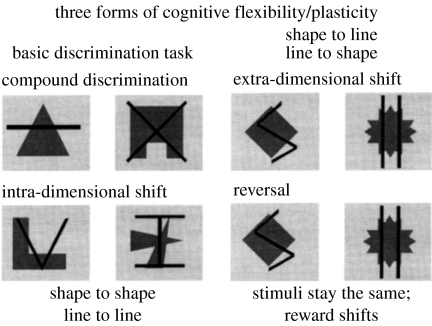
A major new finding presented at the Royal Society Discussion Meeting of 1996 was relevant to the issue of the role of the PFC in ‘cognitive flexibility’: two apparently similar forms of cognitive flexibility were mediated by very different regions of the PFC in the marmoset (Dias et al. 1996; Robbins 1998). Thus, when responding to complex (compound) stimuli was governed by a particular perceptual dimension (such as shapes, as distinct from an alternative dimension of superimposed lines; see figure 1), new learning was impaired in monkeys with lesions of the lateral PFC when the reinforcement contingencies were switched so as to render the previously irrelevant dimension relevant (figure 2). This shifting requirement is conceptually equivalent to the ‘category shift’ required in the WCST, as it involves a shift of responding that entails a switching of attention between two perceptual dimensions; hence the description ‘extra-dimensional shift’ (ed-shift). (An intra-dimensional shift (id-shift) occurs, by contrast, when new stimuli or exemplars are presented, but the subject has to continue to choose the same perceptual dimension (or ‘follow the same rule’) when responding to them. In this sense, each normal trial of the WCST (i.e. when there is no requirement to shift the rule) is akin to an id-shift.) A category shift in the WCST may also engage other processes besides switching; for example, the subject also has to identify the correct new category and resist the fact that previously it has been irrelevant (‘learned irrelevance’). They also have to realize that a shift is now necessary owing to the altered feedback. The precise nature of any failure to make the category shift can be analysed by further variations of the id-/ed-shift procedure, as implemented by Owen et al. (1993), in a study of frontal patients.
Figure 1.
Compound stimuli used in discrimination learning paradigm. The perceptual dimensions were shapes and lines. Exemplars of these dimensions could occur in combination with one another on successive trials, one exemplar of one dimension being correct. Three types of shift are shown: an intra-dimensional shift (ids) occurs when novel stimuli are used but the relevant stimulus dimension (i.e. shapes or lines) stays the same; an extra-dimensional shift (eds) occurs when an exemplar from the previously irrelevant dimension becomes correct. Reversal learning can occur at several stages, e.g. at the compound discrimination stage or after the id- or ed-shift. Here, the stimuli remain the same, but the exemplar that was previously correct is now incorrect and vice versa. See Dias et al. (1996, 1997) for further details.
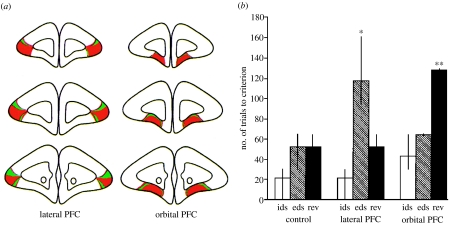
Figure 2.
(a) Representative coronal sections through the marmoset PFC showing the extent of lesions made to the lateral or orbitofrontal PFC, together with (b) doubly dissociable behavioural deficits on id-shifting, ed-shifting and reversal shifts (rev; *p<0.05, **p<0.01) (reproduced with permission from Dias et al. 1996, modified with permission from the publishers).
The lateral PFC-lesioned monkeys were not impaired when they were required merely to shift responding to the previously rewarded alternative (‘reversal learning’), suggesting that their deficit at the ed-shift stage was not simply a failure to detect the altered feedback. By contrast, lesions of the OFC produced exactly the opposite pattern: no deficit on the ed-shift, but impaired reversal learning. While these empirical data were clear and replicable (e.g. Dias et al. 1997), they provided several new challenges in terms of interpretation. For example, what was the theoretical significance of the findings, in particular the double dissociation, and were they of any significance for understanding the functions of the human PFC? Some advance has been made in addressing these issues. The most obvious point is the implication that both the lateral and OFC regions of the PFC are active during discrimination learning but have different functions in behavioural plasticity. The lateral PFC was implicated in the shifting of responding between abstract perceptual dimensions, whereas the OFC was necessary for the shifting of responding between different stimuli or objects with specific associations with reinforcement. This implies a hierarchical organization of function between lateral and OFC regions of the PFC, analogous to other proposed hierarchical relations between ventrolateral and dorsolateral PFC (Petrides 1998) and between the rostral PFC and other regions of PFC (Koechlin et al. 2003). The reason that this putative organization is suggested to be hierarchical, rather than just another example of functional specialization, is that discrimination learning is based on simple concrete features (e.g. triangle, curly) in the case of reversal learning, but entire, abstract dimensions (e.g. ‘shape’, ‘line’) in the case of ed-shifting. Moreover, an influential theory of discrimination learning (Sutherland & Mackintosh 1971) held that discrimination learning proceeds in a hierarchical fashion: first attention is attracted by feedback to a specific perceptual dimension; then, it must be identified which exemplar of the dimension is rewarded and which is punished prior to response selection. Presumably, these different stages of discrimination learning correspond to different functions mediated by the lateral PFC and the OFC.
The findings of Dias et al. (1996, 1997) also appear to show that, although both deficits can be classified as reflecting deficits in behavioural inhibition (owing to perseverative responding, either to previous exemplars or to dimensions in the face of non-reward), a process of response inhibition per se is not represented simply within a single PFC region. This had been previously mooted, for example, by Fuster's (1989) review, which suggested that the OFC had a function of behavioural inhibition. Rather, it appeared that the lateral PFC also performed inhibitory functions in response selection. Thus, response inhibitory functions are distributed widely within the PFC, analogous to Goldman-Rakic's view that working memory was organized on a modular basis within the PFC, subsuming processes of inhibition and selection, as well as holding stimuli online.
The second major question was whether the double dissociation of ed-shifting and reversal learning in the marmoset, a non-human primate, had any relevance for defining the functions of the human PFC. Although it might have been thought rather straightforward to test this relevance in humans, it has proved in fact to be more problematic than translating the basic findings to other species such as the rat. Birrell & Brown (2001) used an ingenious digging task for food rewards that required rats to discriminate between rough and smooth sand, or different smells associated with it. Rats with different lesions of PFC subregions were exposed to the same sequence of discrimination learning stages as the marmoset, including reversal learning, id-shifting and ed-shifting. A similar anatomical dissociation of effects of medial PFC and lateral portions of the OFC lesions was found on shifting and reversal, respectively (Brown & Bowman 2002). These data provide interesting pointers on homologies between the rodent and primate brain. In fact, there is now considerable evidence that lateral OFC lesions in the rat produce impairments in reversal learning in a number of sensory modalities (Schoenbaum et al. 2002; Chudasama & Robbins 2003). The evidence for neuroanatomical homologies between the primate and rodent brain suggests that the rat OFC is indeed related to the primate OFC (Preuss 1995; Brown & Bowman 2002); hence, their common involvement in reversal learning could be expected. The common involvement of the rat medial PFC and the primate lateral PFC in ed-shifting might also be expected on the grounds of the putative homology of these regions, although this is more controversial (Brown & Bowman 2002).
Is the same true of the human brain? One difficulty in investigating this possibility is that reversal learning for humans is generally a much easier task than the ed-shift. Using similar visual stimuli to those employed in the work with marmosets, it was nonetheless possible to show relatively selective deficits in reversal learning in patients with frontal-variant fronto-temporal dementia, for whom hypoperfusion initially occurs in the OFC (Rahman et al. 1999). Several other groups have also confirmed that the OFC mediates aspects of reversal learning (Fellows & Farrah 2003; O'Doherty et al. 2003; Hornak et al. 2004) on the basis of neuropsychological testing of patients or functional neuroimaging. However, none of these studies included a test of ed-shifting. We have been unable to pinpoint precisely those regions of the PFC that are especially implicated in the ed-shift from neuropsychological studies of cases with PFC damage. However, it is apparent that in two series of patients with damage to different regions of the PFC, arising from diverse aetiology and with largely lateral lesions that spared the OFC, the greatest deficit was in ed-shifting. Performance of the reversal stages of the tasks was not significantly affected (Owen et al. 1991).
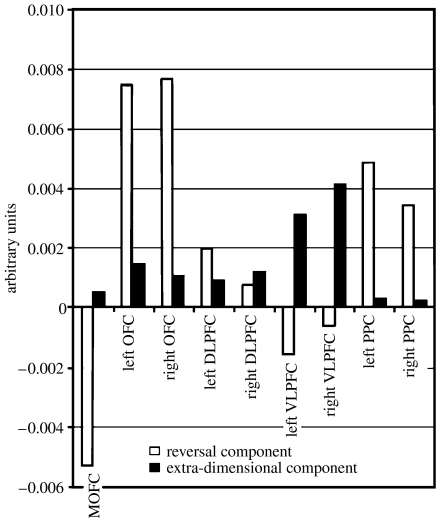
The other main technique that can be employed is that of functional neuroimaging. Our early attempt (Rogers et al. 2000) to study reversal learning and ed-shifting in a functional imaging context using H2O15 positron emission tomography (PET) was unsuccessful in showing activations in the OFC, presumably owing to limitations of the block design employed. Thus, reversal and shifts occur quite quickly and so any effects on cerebral activity may be diluted if it is averaged over many trials that include just one shift; an event-related design is thus to be preferred. However, there was activity shown in the ventromedial caudate nucleus for the contrast between an id-shift and reversal, suggesting that reversal is mediated in part by a cortico-striatal loop. This loop includes the OFC, given the anatomical connectivity existing between these regions, even if activation of the OFC itself was not evident. Moreover, a similar contrast between ed-shifting and id-shifting showed activity in the rostral and dorsolateral PFC. However, a recent elegant event-related functional magnetic resonance imaging (fMRI) study using methods that allowed resolution of activity within the OFC region has considerably clarified the situation: Hampshire & Owen (2006) had volunteers shift between attending to photographs of houses or faces when both were present within the same stimuli. They found that reversal was indeed associated with blood oxygen level-dependent (BOLD) signals in the OFC (as well as reductions in the activation of the medial PFC), whereas ed-shifting was associated most obviously with ventrolateral PFC activity (figure 3). Thus, it could be argued that there is considerable concordance between the findings for the marmoset and human functional neuroimaging. The lateral PFC lesion in the marmoset may correspond to the ventrolateral PFC highlighted in the Hampshire & Owen (2006) study. Nevertheless, it is significant that although the dorsolateral PFC was not specifically associated with responding at any one stage, it was active during most of the task, possibly reflecting an overall role in strategic processes contributing to problem solution.
Figure 3.
Results of an fMRI study on reversal learning, id-shifting and ed-shifting in human subjects. The regional cortical BOLD activations for contrasts involving reversal and ed-shifting are shown. Note the double dissociation between activations for reversal learning and ed-shifting. DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; PPC, posterior parietal cortex. Adapted with permission from Hampshire & Owen (2006) and publishers of Cerebral Cortex, Oxford University Press.
3. Effects of manipulating chemical neuromodulatory systems on attentional set-shifting and reversal learning
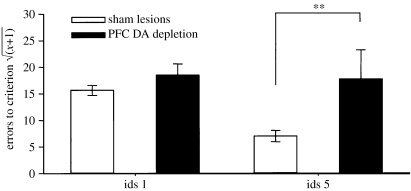
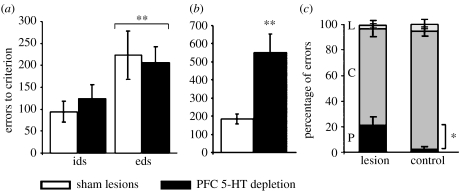
Hypoactivity of the mesocortical dopamine projection has been implicated not only in working memory dysfunction (Goldman-Rakic 1998), but also in clinical disorders such as schizophrenia and attention deficit/hyperactivity disorder (ADHD). Consequently, initial studies focused on the effects of profound (greater than 85%) dopamine depletion from the entire PFC in the marmoset and found surprisingly that, if anything, ed-shifting was not impaired but actually enhanced (Roberts et al. 1994). However, this was later found probably to result from a failure of the monkey to form stable attentional sets in the first place. This was shown by the fact that serial id-shifting, which normally leads to the establishment of an attentional ‘set’, was profoundly impaired (figure 4) and there were additional signs of attentional lability (Crofts et al. 2001). It is important to note that there were no other effects on discrimination or reversal learning (Roberts et al. 1994), even in the serial condition (Clarke et al. 2005). By contrast, selective 5-HT depletion had no effect on ed-shifting or serial id-shifting, but produced a large deficit in reversal learning (Clarke et al. 2004, 2005, 2007; see figure 5), largely due to perseverative responding to the previously rewarded object. Thus, these manipulations of two different monoamine pathways that innervate the PFC clearly have distinct effects on PFC-dependent mechanisms of cognitive flexibility. Although both systems innervate the entire PFC, they appear to have differential impact in distinct regions. Thus, for example, it is 5-HT depletion in the OFC that is implicated in reversal learning and we have not been able to discern so far any impact of 5-HT depletion on the performance of tasks (such as ed-shifting) dependent on lateral PFC regions in the marmoset.
Figure 4.
Effects of prefrontal dopamine depletion on the first and fifth stages of a serial id-shifting task, showing deficits in the lesioned group. *p<0.05, **p<0.01.
Figure 5.
Effects of selective prefrontal 5-HT (serotonin) depletion on id-shifting, ed-shifting and reversal learning; note the selective effect on the latter: (a) ed-shift, (b) reversal and (c) reversal error type. *p<0.05, **p<0.01. Adapted with permission from the Society for Neuroscience (© 2005) from Clarke et al. (2005).
There is some resonance of this work on reversal learning with what has been shown in human volunteers following transient depletion of central 5-HT by the tryptophan depletion technique. Park et al. (1994) first reported the effects on discrimination learning that were especially evident for reversal learning and Rogers et al. (1999b) also found that such depletion led to relatively selective effects on reversal learning in human subjects (but see also Talbot et al. 2006), with no effect on ed-shifting (Rogers et al. 1999b) or spatial working memory (Park et al. 1994). However, for mesocortical dopamine, the position is less clear owing to the difficulty in manipulating this system selectively in human subjects. However, studies on polymorphism for a gene controlling catechol-o-methyltransferase (COMT), which has been postulated to have a selective effect on PFC dopamine, have been shown to affect WCST performance, although the nature of the deficits are more in keeping with a difficulty in ed- than id-shifting (Mattay et al. 2003). Additional work in the rat with a pharmacological inhibitor of COMT, tolcapone, also showed improvements at the ed-stage in the rat version of the id-/ed-shift task, possibly as a consequence of enhanced PFC dopamine activity (Tunbridge et al. 2004). Thus, while the mesocortical dopamine projection has been implicated in both id- and ed-shifting, there is agreement of a lack of effect on reversal learning at the cortical level.
Recent work in the rat task has also shown an important role for PFC noradrenaline at the ed-shifting stage (Lapiz & Morilak 2005; D. S. Tait, V. J. Brown, A. Farovik, D. E. Theobald, J. W. Dalley & T. W. Robbins 2006, unpublished observations). This is consistent with data in humans in some respects, showing that both the noradrenergic agents, clonidine and idaxozan, produce detrimental effects at the ed stage, especially in combination (Middleton et al. 1999). It is intriguing that the three forms of shift described are each associated with a different monoamine operating probably in different sectors of the PFC; thus, reversal (5-HT), id-shifting (dopamine) and ed-shifting (noradrenaline) may be differentiated to some extent. The findings thus confirm the view that these ascending monoaminergic systems have distinct functions, and future work must be aimed at understanding the conditions or states under which they are active. One such state is stress, which is known to activate the monoaminergic systems (Arnsten & Robbins 2002). The finding by Liston et al. (2006) that chronic stress in rats that causes a retraction of dendritic arbours in the medial PFC, but not lateral OFC, selectively impairs attentional set-shifting but not reversal learning, is clearly a step in the direction of further analysis along these lines.
4. Attentional set-shifting: possible fronto-striatal substrates and clinical implications
Patients with basal ganglia diseases, such as early-in-the-course Huntington's and Parkinson's diseases, show impairments in ed-shifting, suggesting some mediation by striatal structures. In late Huntington's disease, the impairments are in simple reversal learning and patients rarely progress sufficiently to even attempt the ed-shift stage. This pattern of initial deficits in ed-shifting to reversal learning suggests a dorsal-to-ventral spread in pathology (Lange et al. 1995). Performance in the early stages of the id-/ed-shift task was remediated by l-Dopa medication in patients late-in-the-course with Parkinson's disease, although there was no conclusive evidence on whether ed-shifting was affected (Lange et al. 1992). In fact, subsequent work has largely cast doubt on the hypothesis that the ed-shift is dopamine (DA) dependent. Two studies of Parkinson's disease both on- and off-l-Dopa (Cools et al. 2001; Lewis et al. 2005) did not show a significant difference in the Parkinson's disease deficit at this stage, and studies of the effects of a D2 receptor antagonist (sulpiride) in normal volunteers showed only weak and inconsistent effects in normal subjects on a single (latency) measure (Mehta et al. 1999, 2004). Consequently, from human studies alone it is far from clear that dopamine, whether in the PFC or striatum, is implicated in the ed-shift.
This conclusion is supported by further studies in the marmoset in which dopamine was selectively and profoundly depleted in the caudate nucleus. Such depletion caused deficits in a spatial delayed response task indexing working memory and also produced reduced distractibility in the id–ed-shift task (Collins et al. 1998; Crofts et al. 2001). However, the striatal depletion had no effect on discrimination learning or reversal, id- or ed-shifting. The one deficit that was observed occurred at the end of the id-/ed-shift series, in which a novel shift was interpolated; namely, an ed-shift back to the previously reinforced dimension. Under these conditions a deficit was revealed, which had not been apparent in the parallel studies of PFC lesions (Dias et al. 1997). The finding has some theoretical significance as it suggests that the striatum and its dopaminergic innervation are important in the mediation of shifts between already established sets: the striatal dopamine-depleted animals were impaired when faced with an ambiguous choice of selecting one of the two options that had both previously been successful. In the case of the initial ed-shift of course, the animal is learning a new set of associations for a dimension that has never been reinforced.
These considerations suggest that the ed-shift, probably as distinct from reversal learning, does not depend so much upon striatal mechanisms and implicates, for example, PFC interactions with other cortical regions, especially in the parietal and temporal cortices (Rogers et al. 2000; Hampshire & Owen 2006). The deficits observed in ed-shifting in Parkinson's and Huntington's diseases may thus reflect the extra-striatal pathology, possibly in the PFC. On the other hand, reversal learning does implicate subcortical structures, including the striatum, based on several lines of evidence including effects of lesions in monkeys (Divac et al. 1967) and rats (Dunnett & Iversen 1981), as well as functional neuroimaging in humans. Indeed, there are indications that a probabilistic reversal task (where options are reinforced on a 80–20 or 70–30 basis, rather than 100–0) activates not only regions of the OFC, medial PFC and inferior frontal cortex, but also the ventral striatum (Cools et al. 2002). Furthermore, patients with Parkinson's disease are impaired on such a task following l-Dopa medication, which appears to alter signals related to the final response shift during reversal within the nucleus accumbens (Cools et al. 2007).
Other work from a slightly different perspective is consistent with the view of a separation of function between the PFC and striatum. Thus, Cools et al. (2004) found that switching responding between objects produced activations in fMRI for both the PFC and striatum in normal human volunteers, whereas activations following rule alternation were only seen in the PFC. A similar dissociation has been reported for patients with PFC and striatal lesions: the latter (though mainly comprising lesions of the putamen and not the caudate nucleus) were unimpaired in responding to higher-order ‘rules’ but exhibited problems alternating between objects (Cools et al. 2006). Overall, these latter studies are consistent with the view that the striatum exhibits control in the lower-order function of switching or shifting between objects, akin to reversal learning, rather than between more abstract rules, analogous to ed-shifting.
The modulatory role for dopamine in the nucleus accumbens of probabilistic reversal learning in humans contrasts with the role of OFC 5-HT in reversal learning in monkeys. However, the 5-HT system also evidently has a modulatory role. Evers et al. (2005) recently showed that a dorsomedial PFC locus was affected by tryptophan depletion during the performance of the probabilistic reversal task. Tryptophan depletion affected the BOLD signal specifically associated with negative feedback, a finding of relevance to our understanding of the cognitive deficits in depression, which is associated with disorders of 5-HT regulation. Depressed patients are especially sensitive to spurious feedback in the probabilistic reversal task, switching responding inappropriately (Murphy et al. 2002). The effects of low tryptophan depletion in the functional imaging task of normal volunteers contrasted with those of methylphenidate (a catecholaminergic agent) and sulpiride (a dopamine D2 receptor antagonist), both of which modulated the activity produced by a reversal shift in the inferior frontal gyrus (Clark et al. 2004). These data again suggest that these monoamine systems have distinct modulatory effects on task performance.
5. Task-set switching
One of the difficulties in interpreting changes in id- and ed-shifting, as well as reversal, is that they all depend on learning; the main measures are thus generally errors or trials to criterion. Although the paradigm includes internal controls for basic deficits in discrimination learning, it is possible that the task demands, for example, of attentional set-shifting, are conflated by the learning requirement. Set-shifting has recently been studied in other ways that allow a more analytic approach in a paradigm termed ‘task-set switching’ (Allport & Wyllie 1999; Monsell 2003). Formally, the human participant responds to a stimulus on each trial according to a well-established S–R rule specific to that task, but on different trials may have to respond according to a different rule defined by a different task. A task set is thus a set of processes linking sensory analysis, including identification and categorization, to particular responses and motor outputs; task sets frequently require reconfiguration if rules change. However, in the basic paradigm, the subject shifts and has to reconfigure between two well-established task sets. The critical issue is the processing cost of shifting from one task to another. This is frequently measured in an AABB design, which can isolate the latencies to repeat a task and to shift from one to the other; the difference between the two being called the ‘switch cost’. The fundamental finding is that shifting between the two tasks incurs a switch cost (in terms of errors as well as latencies). The major difference between the task-set and id-/ed-shifting paradigms is that task-set switching occurs between two well-established habits, such as naming digits or naming letters, and so no new contingency learning is required. Moreover, after minimal practice, reaction times remain stable over repeated testing. The switch cost may be reduced to some extent by preparation. Thus, if the interval between the response and stimulus (response–stimulus interval; RSI) is lengthened, then the switch cost is reduced as the subject presumably is able to reconfigure the tasks in advance. However, it is notable that there remains a ‘residual switch cost’ even under these conditions. Explanations of the residual switch cost are controversial and include the effects of persistent interference from previous task sets and a need to complete the reconfiguration of the task via an exogenous control process cued by the new task-set cue (i.e. a control process that is triggered by the external cues associated with each task set, see Monsell 2003). There are also a number of other variables that can affect performance on the task-set switching paradigm, including for example the congruence or S–R compatibility of the task sets and the amount of interference between the two task sets, e.g. if the cues for both tasks remain present in the array, thus causing inevitable interference through ‘crosstalk’ between the two.
6. Neural substrates of task-set switching
The neural substrates of task-set switching continue to provoke debate, with most of the evidence deriving from human functional neuroimaging or neuropsychological studies of groups with frontal brain damage or neurodegenerative diseases of the basal ganglia. A number of studies have suggested that left prefrontal cortical damage is especially important for task-set switching. Our own study (Rogers et al. 1998) showed that patients with left PFC damage had switch deficits but only in conditions in which there was interference between the two task sets. This was in agreement with the findings of Mecklinger et al. (1999) who found that patients with speech and language difficulties had the greatest switch deficits, implicating the left hemisphere, and also a single case described by Keele & Rafal (1999). Neuroimaging studies suggest that the maintenance and establishment of task set are functions mediated by the middle gyrus (MFG) of the left dorsolateral PFC (MacDonald et al. 2000; Garavan et al. 2002).
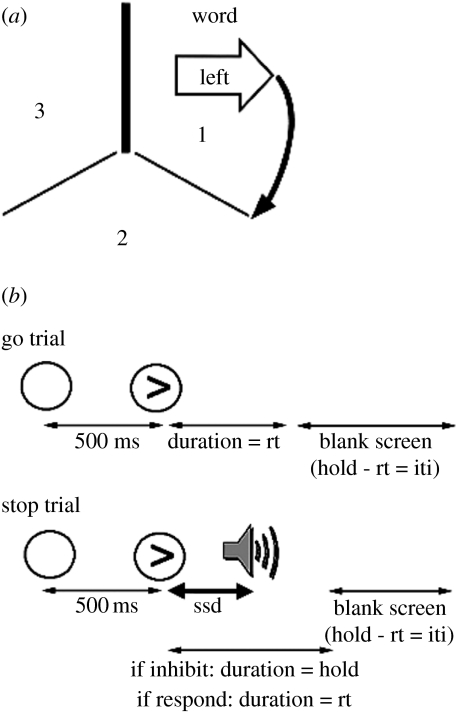
In a follow-up study to that of Rogers et al. (1998), we tested 36 patients with unilateral damage to either the right or left PFC, and used a novel structural imaging method to quantify the volume of damage to the defined regions of interest (ROI) on both sides: the superior frontal gyrus, middle frontal gyrus (MFG), inferior frontal gyrus (IFG), orbitofrontal and medial—see Aron et al. (2004a,b) for methods. A task-set shifting paradigm was used that included predictable shifts and a variation of the RSI (‘short’ and ‘long’) to allow different degrees of preparation for task-set reconfiguration. The tasks were (i) a word task in which stimuli were composed of a word (‘left’ or ‘right ’) inside a shape (left arrow, right arrow or rectangle) and (ii) an arrow task in which stimuli comprised either a left or right arrow shape surrounding a letter string (‘LEFT’, ‘RIGHT ’ or ‘XXX’; figure 6a). These three conditions of congruency, incongruency and neutrality also enabled the effects of congruency on task-set switching to be studied.
Figure 6.
Versions of tasks used in (a) task switching and (b) stop-signal inhibition paradigms. For task-set switching, the subject moves around the spatial array in positions 1, 2 and 3 on successive trials, cued to respond to the arrow or word. For stop-signal inhibition, on a proportion of trials, an auditory stimulus signals not to respond on that trial of a choice reaction time paradigm. rt, reaction time; ssd, stop-signal delay; iti, inter-trial interval. Modified with permission from the publishers of Brain. The Clarendon Press, Oxford University Press (Aron et al. 2004b) and the Society for Neuroscience © 2006 (Aron & Poldrack 2006).
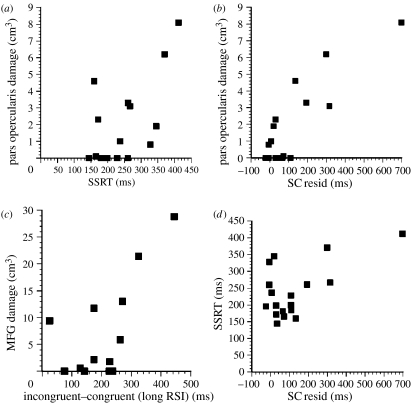
The behavioural results were complex but informative (figure 7). The left PFC group showed a general impairment in imposing the appropriate task set—as indicated by larger switch costs at both short and long RSIs. By contrast, the right PFC group had deficits in reaction time switch cost at the longer RSI. In addition, the right PFC group had difficulty in suppressing inappropriate responses or task sets, especially at short RSIs, as shown by a greater switch cost in errors for incongruent compared with congruent trials. Parametric correlational analysis of the structural imaging data with various parameters of task-set switching performance established that the only significant relationship to survive correction for multiple testing and also partial correlation was between right IFG damage and the residual switch cost (r=0.82, p<0.005; figure 7). The particular region implicated was the right pars opercularis (brain area (BA) 44). The left PFC group exhibited no reliable correlations for any of the switch cost measures, at any ROI. However, there were significant correlations for some of the congruency and interference measures; damage to the left MFG correlated with the difference between congruent and incongruent response latencies at the long RSI and with the difference between congruent and neutral conditions at short RSIs. Thus, the greater the MFG damage within the left PFC, the greater the tendency to activate the competing task and the incongruent response, even on no-switch trials (figure 7). This result suggests a basic deficit in endogenous task-set control (i.e. those aspects of task set that are controlled by ‘stimuli’ arising from internal processes such as response preparation).
Figure 7.
Main results from Aron et al. (2004a,b). (a) Relationship between stop-signal reaction time (SSRT) and volume of damage to the right pars opercularis. (b) Relationship between residual switch cost (SC resid) and volume of damage to the right pars opercularis. (c) Relationship between volume of damage to the medial gyrus of the left PFC and a measure of task congruency at long response–stimulus intervals. (d) Significant correlation between stop-signal reaction time performance and residual switch cost. Modified from Aron et al. (2004b), with permission from the publishers, The Clarendon Press (Oxford University Press).
To summarize the main components of task set, performance appears to be a product of an interaction between task-set inertia (the persistence of activation or inhibition from previous trials; Allport & Wyllie 1999), exogenous task-set activation and endogenous control. Exogenous task-set activation is cued by the stimulus and this is likely to happen inappropriately on switch trials. To overcome these biases, endogenous control is required, which biases attention to a particular S–R rule, along the lines originally suggested by Gilbert & Shallice (2002). On the other hand, endogenous control seems unable fully to overcome some forms of inappropriate responding, for example, caused by interference arising from incongruency.
The pattern of results described indicates that left PFC-lesioned patients did not have difficulty in suppressing the inappropriate response on switch trials at short RSIs. They showed a more general difficulty in imposing the appropriate task set at both short and long RSIs, consistent with a reduction of endogenous control and thus leading to an exaggerated influence of the exogenous cueing of task set. By contrast, the clear-cut deficits shown in the right PFC group strongly suggest that the right PFC has an important role in reactive inhibition, which becomes especially important under conditions of weak endogenous control. We know from functional neuroimaging studies that many cortical structures are active during complex situations such as task-set switching. Thus, the anterior cingulate cortex is likely to detect a conflict in task setting and the right IFG is likely to be recruited to inhibit the irrelevant response activation (Gehring & Knight 2000). In general, it is clear that several different regions of the PFC are implicated in task-set switching (Derrfuss et al. 2005), although we would argue for a specific role for the right IFG in response inhibition in certain circumstances. The earlier demonstrations (e.g. Dias et al. 1996) that behavioural inhibition is apparently a function of many prefrontal areas mean that we have to define in more detail what precisely these circumstances might be. A further attempt to do this is provided in §7, where inhibition has to be applied following response initiation. The conclusion that the right IFG is implicated in response inhibition is supported by several experiments using functional neuroimaging. Two particularly salient studies are by Konishi et al. (1999) and Swainson et al. (2000), both of which directly compared response inhibition with switching and found a locus in right IFG common to both. Swainson and colleagues' study is particularly convincing as the right IFG was implicated specifically in a Go–NoGo version of the task-set switching paradigm for the shift to stopping (‘suppression mode’).
Although the right IFG is activated by a large number of conditions (Duncan & Owen (2000) for a meta-analysis), there is a preponderance of studies that have specifically focused upon response inhibition (Garavan et al. 1999; De Zubicaray et al. 2000; Menon et al. 2001; Bunge et al. 2002; Garavan et al. 2002; Rubia et al. 2003) and also reversal or shifting (Dove et al. 2000; Nagahama et al. 2000; Monchi et al. 2001; Cools et al. 2002; Nakahara et al. 2002; Hampshire & Owen 2006). The latter two of these studies are of special interest, as they investigate the WCST and the ed-shift, respectively. While it is sometimes difficult to compare exact loci of activation sites across studies and tasks, it would appear that there is at least a rough association between the neuroanatomical areas subserving task-set switching and ed-shifting. A parsimonious account of this observation is that there are some shared processing resources between tasks requiring task-set switching and the ed-shift, presumably related to the shift or switch itself, and thereby contributory response selection, including inhibitory processes. Alternatively, given the prominence ascribed to an area somewhat posterior to the right IFG, the so-called ‘inferior frontal junction’ from meta-analyses of functional imaging data in related aspects of response control (Derrfuss et al. 2005), it may be that there is more differentiation of function within this region than this parsimonious view. In fact, careful scrutiny of the Hampshire and Owen activation suggests that the peaks were in BA 47 in the left hemisphere and the border of BA 47 and 45 on the right, which suggests caution in attributing too many functions to the pars opercularis in the right IFG. Nevertheless, the strength of the lesion data is that the precise regions within the right IFG are necessary for adequate task-set switching performance, thus demonstrating the causal importance of the right IFG, particularly the pars opercularis, in this situation.
Unlike ed-shifting, task-set switching appears to depend to a greater extent on basal ganglia mechanisms. Patients with Huntington's disease have profound problems in elementary aspects of task-set switching (Aron et al. 2003a–c), although the neuropathology of this disease may well encompass more than simply the basal ganglia. In Parkinson's disease, there are significant impairments in task-set switching under certain conditions (Cools et al. 2001). In particular, Parkinson patients were most impaired at short RSIs (Cools et al. 2003), but such deficits were remediated by l-Dopa, suggesting a dopaminergic modulation of striatal function. Mehta et al. (2004) also found recently that the dopamine D2 receptor antagonist sulpiride significantly increased switch cost in a standard task-set switching paradigm in human volunteers. This was striking, owing to the relative lack of effect of the drug on performance on an id-/ed-shifting task in the same subjects. Evidently, as well as the similarities described above in their requirements for response inhibition, there may be important differences between ed-shifting and task-set switching. The continuous nature of the latter task, and the need to shift rapidly between well-established motor sets, may make the latter task more dependent on basal ganglia functioning, whereas the ed-shift task has fewer motor components and may recruit additional associative and attentional mechanisms that are more dependent on posterior cortical structures (Rogers et al. 2000; Corbetta & Shulman 2002).
7. Stop-signal inhibition
The stop-signal inhibition paradigm (Logan & Cowan 1984) at first sight is unrelated to task-set switching and ed-shifting, although it also incorporates a major inhibition component. It is a sophisticated Go–NoGo task in which subjects are required to make speeded responses on ‘Go’ trials in choice reaction time procedure, but to inhibit responding on ‘NoGo’ trials; for example, to an auditory ‘beep’ that sounds on approximately 25% of the trials (figure 6b). This stop-signal is programmed to occur at different delays following the imperative signal, thus occurring at different times after the initiation of the response, and progressively taxing the ability of subjects to impose its suppression. A key parameter of the paradigm is the time it takes to inhibit a response, i.e. the stop-signal reaction time (SSRT), which can be computed from the response time distributions and is usually measured when the probability of a successful response inhibition is set at 0.5, taking into account also the variable delays after the imperative signal. The ‘race’ model explicitly assumes that there is a competition between hypothetically independent ‘go’ and ‘stop’ processes that determine performance (see Logan & Cowan (1984) and Aron et al. (2003a–c) for a more detailed account of the race model), although there may well be other ways of conceptualizing the component processes of this task. While the human version of SSRT invariably requires suppression of limb responses, it has also been employed in a saccadic variant in rhesus monkeys (Schall et al. 2002). These authors were able to show that temporally coincident with the suppression of responding by a stop-signal was an inhibition of neuronal firing in the vicinity of the animal's frontal eye fields, demonstrating in this preparation at least that psychological inhibition imparted by the SSRT task is translated into physiological inhibition.
8. Neural substrates of SSRT
Early primate studies showed that lesions of the inferior convexity, a likely homologue in macaques of the right IFG in humans, produced impairments in Go–NoGo performance (Iversen & Mishkin 1970). The likely role of the right IFG in response inhibition has already been mentioned, and several neuroimaging studies of Go–NoGo or SSRT performance in human subjects have confirmed this (Konishi et al. 1998; Garavan et al. 1999; Menon et al. 2001; Rubia et al. 2003; figure 8). We were also able to use the same group of unilateral frontal patients to compare their deficits in task-set switching (above) and SSRT. As before, we were able to relate the volume of damage in pre-defined ROI, including the right IFG, specifically to aspects of SSRT performance, notably the Go reaction time and the SSRT itself. We found that there was a significant slowing of SSRT in patients with right PFC but not left PFC damage. Furthermore, there was a highly significant correlation (r=0.83, p<0.005) between SSRT and volume of damage in the right IFG (figure 7). While there were weaker correlations between SSRT and damage to other sectors, they disappeared if account was taken of the fact that damage to some of these sectors was inevitably related to damage to the right IFG itself, because lesions typically extended across ROI. There was also a significant correlation (r=0.59, p<0.005) between the SSRT and the residual task switch cost in these same patients (figure 7), strongly supporting the hypothesis that the two tasks share some common processes, some of which are mediated by the right IFG region. There was no significant correlation between right IFG damage and Go reaction time (r=0.14), suggesting that the right IFG was specifically implicated in response inhibition processes.
Figure 8.
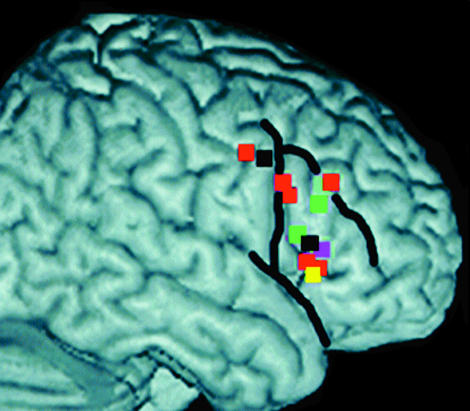
Talairach coordinates plotted from six neuroimaging studies of switching, sorting and reversing and boundaries of inferior frontal gyrus (for further details see Aron et al. 2004a,b). Adapted from Aron et al. (2004b), with permission from the publishers, The Clarendon Press (Oxford University Press).
A recent functional neuroimaging study (Aron & Poldrack 2006) has substantiated this conclusion, while going on to suggest distinct anatomical networks for the go and stop processes. Specifically, ‘Going’ significantly activated motor areas contralateral to the response hand including primary motor cortex, supplementary motor area (SMA), the putamen and the pallidum, whereas ‘Stopping’ significantly activated the right IFG, the pre-SMA, the globus pallidus and the right subthalamic nucleus (STN), a putative component of the ‘indirect’ pathway of the basal ganglia. Activation was significantly greater in the right IFG for subjects with faster SSRTs. These neuroimaging data have also been supported by a recent study showing that transcranial magnetic stimulation also impaired SSRT when applied over the right IFG region (Chambers et al. 2006). The activations of the right IFG and STN were correlated, consistent with the possibility of direct anatomical connections between the two areas (Aron et al. in press). This new study certainly suggests that the balance between ‘Going’ and ‘Stopping’ may be mediated by different portions of the fronto-striatal–pallidal systems.
However, it may be premature to assume that the STN itself is the key subcortical structure. Eagle et al. (in press) have found, using a version of the SSRT task for rats, that lesions to the STN globally disrupt performance, thus greatly complicating interpretation. By contrast, lesions of the medial striatum, but not the core region of the nucleus accumbens, do impact more selectively on SSRT performance (Eagle & Robbins 2003a,b). Of course, caution has to be exercised when extrapolating across species from rats to humans, but the structure of the basal ganglia has largely been conserved in evolutionary terms, making it likely that these areas are homologues of what is found in humans. The medial striatum is generally considered, for example, to be equivalent to the caudate nucleus. The situation is more complicated in the PFC; here, lesions of the OFC sector but not the infralimbic (ventromedial PFC) in rats selectively impair SSRT (Eagle & Robbins 2003b; Eagle et al. in press). It is not yet clear how these results relate to the focus in human studies on the right IFG.
9. Neurochemical modulation and clinical implications
The SSRT task has proven useful for measuring deficits in impulsivity in juvenile and adult ADHD (Solanto et al. 2001; Aron et al. 2003a–c). It is significant that the SSRT deficit in these conditions responds well to methylphenidate medication (Aron et al. 2003a–c), suggesting modulation by catecholamine systems, and is consistent with one model of subtypes of ADHD, suggesting a right frontal hypoplasia associated with problems in motor inhibition (Castellanos & Tannock 2002). These clinical observations raise several issues, including whether it is noradrenaline or DA (or both) that are responsible for the therapeutic effects of methylphenidate in ADHD and how they modulate the activity of the right IFG. A recent psychopharmacological study in human volunteers has shown that the relatively selective noradrenaline re-uptake blocker atomoxetine improves SSRT without affecting measures of attention and learning, whereas the selective serotonin re-uptake inhibitor, citalopram, affected learning but not attention or SSRT (Chamberlain et al. 2006). Other data are consistent with this selectivity; thus, both l-Dopa and tryptophan depletion (Clark et al. 2005) has relatively little effect on SSRT performance, suggesting that dopaminergic and serotoninergic influences on SSRT performance are only minor. The question of modulation of the right IFG can be addressed by pharmacological functional magnetic imaging studies. The results described above for the modulation of right IFG in probabilistic reversal learning may be salient. It is shown that methylphenidate (and also sulpiride) did modulate BOLD signals within the right inferior PFC (Clark et al. 2004), but that tryptophan depletion had no effect (Evers et al. 2005).
10. Conclusions
Studies of ed-shift, task-set switching and stop-signal inhibition have been reviewed. While these paradigms each have their distinctive features, they probably share some processes in common, related for example to response shifting and response inhibition in the context of behavioural change and cognitive plasticity. On the one hand, they appear to engage different networks within the cortico-striatal circuitry and probably have distinct interactions between the PFC and posterior cortical regions. On the other hand, they also appear to share some crucial neural substrates such as the inferior PFC, and this gives us potentially a powerful way of isolating executive functions thought to be mediated by the PFC. The evidence for this is based on the effects of precise lesions in monkeys and, in some cases, in rats on the performance of tasks that bear some clear relationship to some of those also used in humans (e.g. the id-/ed-shifting test). Lesions in humans are less readily quantified, but we (and others, e.g. Stuss et al. 1999; see also Stuss & Alexander 2007) have developed a method for this based on quantitative structural imaging. The crucial importance of such evidence from lesion and also transcranial magnetic stimulation studies is that it enables a determination to be made of the causal significance of an activation of a particular region in a functional imaging study. The advantage of the latter, of course, is that it is possible through neuroimaging to determine the entire neural network engaged by a particular task.
Although structures such as the right IFG within the inferior PFC have been implicated in the component process of many tasks (Duncan & Owen 2000), its role in tests requiring inhibition across a wide variety of tasks, including switching into a suppression mode (Swainson et al. 2000), is significant. This role may possibly extend to other situations such as dual-task interference (Herath et al. 2001), ‘memory inhibition’ (Anderson et al. 2004) and the modulation of anxious reactions (Bishop et al. 2004), through influences mediated to the hippocampus and amygdala, respectively. Indeed, the IFG is one of the most densely connected regions of the PFC (Miller & Cohen 2001) and is one of the last to develop in both ontogenetic and phylogenetic terms (Pandya & Barnes 1987).
Functions such as stopping, shifting and switching are influenced by internal state and are thus susceptible to modulation by ascending neurotransmitter systems mediating functions such as stress, arousal and motivation. We have explored the potentially distinct functions of the monoamine systems by means of selective neuropsychopharmacological manipulations of the dopamine, noradrenaline and serotonin systems in experimental animals and, where feasible, in humans. By using intra-cerebral treatments in animals or combining functional imaging and psychopharmacological investigations, we have shown how it is possible to gain some idea about how processing in different regions may be influenced by distinct neurochemical mechanisms. Such evidence of course is crucial for understanding how medications for conditions such as ADHD and depression actually work in neuropsychological terms.
Using these methods, we have gained some evidence for a separation of PFC regions in the mediation of id- and ed-shifting and reversal learning that holds across species. Even more surprising is that there is evidence for precise modulation of these functions by different monoamine systems projecting to the PFC in the marmoset, and also in humans (see Robbins & Roberts (in press) for a more detailed review of this evidence in infrahuman animals). It is also striking how performance on certain tasks, such as reversal learning, is so susceptible to manipulations of 5-HT, whereas other functions such as stop-signal inhibition are insensitive in our hands. By contrast, extrapolating across species, it appears that noradrenaline is implicated in both stop-signal inhibition and ed-shifting, with obvious implications for conditions such as ADHD. Furthermore, it is important to point out that neurotransmitter influences are determined by the neural context in which they occur; for example, there is evidence that striatal, but not PFC, DA modulates reversal learning.
The theoretical and adaptive significance of this complexity is still being addressed. The fact that the different neurotransmitter systems innervating the PFC and striatum not only interact with one another, but also exert specific functions, suggests that the functioning of the fronto-circuitry is state dependent, and that different aspects of executive control may need to be recruited to optimize behavioural and cognitive performance in those states. Understanding the nature of these states, by determining the precise circumstances in which these systems operate, should be a major goal of research in the next decade. Central to this effort will be the need to solve a modern version of an old conundrum: how basic functions mediated by the subcortical systems interact with higher cognitive processing? One distinct possibility is that it is achieved partly in a ‘top-down’ manner, i.e. those regions of the PFC that are engaged by particular task operations recruit activity in the neurochemically differentiated systems by means of descending projections to their sources in subcortical sites (e.g. Amat et al. 2005; Robbins 2005). Unravelling this form of ‘executive control’, with its implications for optimizing cognitive functioning and understanding of psychopathology and its treatment, is undoubtedly a challenge for the future.
Acknowledgments
I wish to thank A. Aron and A. Roberts for discussion and all of my other collaborators over the last decade who have contributed to the research reviewed here. This work was supported by programme grants from the Wellcome Trust. The Behavioural and Clinical Neuroscience Institute is supported by a joint award from the MRC and the Wellcome Trust.
Footnotes
One contribution of 14 to a Discussion Meeting Issue ‘Mental processes in the human brain’.
References
- Alexander G, DeLong M, Strick P.L. Parallel organisation of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. doi:10.1146/annurev.ne.09.030186.002041 [DOI] [PubMed] [Google Scholar]
- Allport A, Wyllie G. Task-switching: positive and negative priming of task-set. In: Humphreys G.W, Duncan J, Treisman A, editors. Attention, space and action: studies in cognitive neuroscience. Oxford University Press; Oxford, UK: 1999. pp. 273–296. [Google Scholar]
- Amat J, Baratta A, Paul E, Bland S.T, Watkins L.R, Maier S.F. The ventral medial prefrontal cortex determines how behavioral control over stress impacts behavior and dorsal raphé nucleus activity. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. doi:10.1038/nn1399 [DOI] [PubMed] [Google Scholar]
- Anderson M.C, Ochsner K.N, Kuhl B, Cooper J, Robertson E, Gabrieli S.W, Glover G.H, Gabrieli J.D.E. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. doi:10.1126/science.1089504 [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T, Robbins T.W. Neurochemical modulation of prefrontal cortical functions in humans and animals. In: Stuss D, Knight R, editors. The prefrontal cortex. Oxford University Press; New York, NY: 2002. pp. 51–84. [Google Scholar]
- Aron, A. R., Behrens, T. E., Frank, M. J., Smith, S. & Poldrack, R. A. In press. Triangulating a cognitive control network using diffusion-weighted MRI and functional MRI. J. Neurosci [DOI] [PMC free article] [PubMed]
- Aron A.R, Poldrack R.A. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. doi:10.1523/JNEUROSCI.4682-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R, Fletcher P.C, Bullmore E.T, Sahakian B.J, Robbins T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003a;6:115–116. doi: 10.1038/nn1003. doi:10.1038/nn1003 [DOI] [PubMed] [Google Scholar]
- Aron A, Watkins L, Sahakian B.J, Monsell S, Barker R.A, Robbins T.W. Task-set switching deficits in early-stage Huntington's disease: implications for basal ganglia function. J. Cogn. Neurosci. 2003b;15:629–642. doi: 10.1162/089892903322307357. doi:10.1162/jocn.2003.15.5.629 [DOI] [PubMed] [Google Scholar]
- Aron A, Dowson J, Sahakian B.J, Robbins T.W. Methylphenidate response inhibition in adults with attention-deficit/hyperactivity disorder. Biol. Psych. 2003c;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. doi:10.1016/S0006-3223(03)00609-7 [DOI] [PubMed] [Google Scholar]
- Aron A.R, Robbins T.W, Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004a;8:170–177. doi: 10.1016/j.tics.2004.02.010. doi:10.1016/j.tics.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Aron A.R, Monsell S, Sahakian B.J, Robbins T.W. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004b;127:1561–1573. doi: 10.1093/brain/awh169. doi:10.1093/brain/awh169 [DOI] [PubMed] [Google Scholar]
- Baddeley A.D. Clarendon Press; Oxford, UK: 1986. Working memory. [Google Scholar]
- Baddeley A, Della Salla S. Working memory and executive control. In: Roberts A.C, Robbins T.W, Weiskrantz L, editors. The prefrontal cortex: executive and cognitive functions. Oxford University Press; Oxford, UK: 1998. pp. 9–21. [Google Scholar]
- Birrell J.M, Brown V.J. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2001;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Lawrence A.D. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat. Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. doi:10.1038/nn1173 [DOI] [PubMed] [Google Scholar]
- Bor D, Duncan J, Owen A.M. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37:361–367. doi: 10.1016/s0896-6273(02)01171-6. doi:10.1016/S0896-6273(02)01171-6 [DOI] [PubMed] [Google Scholar]
- Brown V.J, Bowman E. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Bunge S.A, Dudukovic N.M, Thomason M.E, Vaidya M, Gabrieli J.D.E. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. doi:10.1016/S0896-6273(01)00583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat. Rev. Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chamberlain S.R, Muller U, Blackwell A.D, Clark L, Robbins T.W, Sahakian B.J. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.D, Bellgrove M.A, Stokes M.G, Henderson T.R, Garavan H, Robertson I.H, Morris A.P, Mattingley J.B. Executive ‘brake failure’ following deactivation of human frontal lobe. J. Cogn. Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins T.W. Dissociable contributions of the orbitofrontal and infralimbic cortex in pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J. Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Cools R, Evers L.E, van den Veen F, Jolles J, Sahakian B.J, Robbins T.W. Neurochemical modulation of prefrontal cortex function. FENS Abstr. 2004;2:A205.1. [Google Scholar]
- Clark L, Roiser J.P, Cools R, Rubinsztein D.C, Sahakian B.J, Robbins T.W. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology. 2005;182:570–578. doi: 10.1007/s00213-005-0104-6. doi:10.1007/s00213-005-0104-6 [DOI] [PubMed] [Google Scholar]
- Clarke H.F, Dalley J.W, Crofts H.S, Robbins T.W, Roberts A.C. Cognitive inflexibility following prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. doi:10.1126/science.1094987 [DOI] [PubMed] [Google Scholar]
- Clarke H.F, Walker S.C, Crofts H.S, Dalley J.W, Robbins T.W, Roberts A.C. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J. Neurosci. 2005;12:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. doi:10.1523/JNEUROSCI.3690-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H.F, Walker S.C, Dalley J.W, Robbins T.W, Roberts A.C. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb. Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. doi:10.1093/cercor/bhj120 [DOI] [PubMed] [Google Scholar]
- Cohen J.D, Braver T.S, O'Reilly R.C. A computational approach to prefrontal cortex, and schizophrenia: recent developments and current challenges. In: Roberts A.C, Robbins T.W, Weiskrantz L, editors. The prefrontal cortex: executive and cognitive functions. Oxford University Press; Oxford, UK: 1998. pp. 195–220. [Google Scholar]
- Collins P, Roberts A.C, Dias R, Everitt B.J, Robbins T.W. Perseveration and strategy in a novel spatial self-ordered sequencing task for non-human primates: effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J. Cogn. Neurosci. 1998;10:332–354. doi: 10.1162/089892998562771. doi:10.1162/089892998562771 [DOI] [PubMed] [Google Scholar]
- Cools R, Barker R, Sahakian B.J, Robbins T.W. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb. Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. doi:10.1093/cercor/11.12.1136 [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen A.M, Robbins T.W. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J. Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Barker R, Sahakian B.J, Robbins T.W. l-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. doi:10.1016/S0028-3932(03)00117-9 [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins T.W. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J. Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. doi:10.1523/JNEUROSCI.4312-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Lewis S.J, Clark L, Barker R.A, Robbins T.W. l-Dopa disrupts activity in the nucleus accumbens during reversal learning in Parkinson's disease. Neuropsychopharmacology. 2007;32:180–189. doi: 10.1038/sj.npp.1301153. doi:10.1038/sj.npp.1301153 [DOI] [PubMed] [Google Scholar]
- Cools R, Ivry R.B, D'Esposito M.D. The human striatum is necessary for responding to changes in stimulus relevance. J. Cog. Neurosci. 2006;18:1973–1983. doi: 10.1162/jocn.2006.18.12.1973. doi:10.1162/jocn.2006.18.12.1973 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:215–229. doi: 10.1038/nrn755. doi:10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Crofts H.S, Dalley J.W, Collins P, Van Denderen J.C.M, Everitt B.J, Robbins T.W, Roberts A.C. Differential effects of 6-OHDA lesions of the prefrontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb. Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. doi:10.1093/cercor/11.11.1015 [DOI] [PubMed] [Google Scholar]
- Damasio A.R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. In: Roberts A.C, Robbins T.W, Weiskrantz L, editors. The prefrontal cortex: executive and cognitive functions. Oxford University Press; Oxford, UK: 1998. pp. 36–50. [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Phil. Trans. R. Soc. B. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. doi:10.1098/rstb.2007.2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zubicaray G.I, Andrew C, Zelaya F.O, Williams S.C, Dumanoir C. Motor response suppression and the prepotent tendency to respond; a parametric fMRI study. Neuropsychologia. 2000;38:1280–1291. doi: 10.1016/s0028-3932(00)00033-6. doi:10.1016/S0028-3932(00)00033-6 [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon D.Y. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum. Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. doi:10.1002/hbm.20127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins T.W, Roberts A.C. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. doi:10.1038/380069a0 [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins T.W, Roberts A.C. Dissociable forms of inhibitory control within prefrontal cortex with an analogue of the Wisconsin card sort test: restriction to novel situations and independence from ’on-line’ processing. J. Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divac I, Rosvold H.E, Szwarcbart M.K. Behavioral effects of selective ablations of the caudate nucleus. J. Comp. Physiol. Psychol. 1967;63:184–190. doi: 10.1037/h0024348. doi:10.1037/h0024348 [DOI] [PubMed] [Google Scholar]
- Dolan R.J. The human amygdala and orbital prefrontal cortex in behavioural regulation. Phil. Trans. R. Soc. B. 2007;362:787–799. doi: 10.1098/rstb.2007.2088. doi:10.1098/rstb.2007.2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollman S, Schubert T, Wiggins C.J, von Cramon D.Y. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res. Cogn. Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. doi:10.1016/S0926-6410(99)00029-4 [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. doi:10.1016/S0166-2236(00)01633-7 [DOI] [PubMed] [Google Scholar]
- Dunnett S.B, Iversen S.D. Learning impairments following selective kainic acid-induced lesions within the neostriatum in rats. Behav. Brain Res. 1981;2:189–209. doi: 10.1016/0166-4328(81)90055-3. doi:10.1016/0166-4328(81)90055-3 [DOI] [PubMed] [Google Scholar]
- Eagle D.M, Robbins T.W. Inhibitory control in rats performing on the stop-signal reaction time task: effects of lesions of the medial striatum and d-amphetamine. Behav. Neurosci. 2003a;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. doi:10.1037/0735-7044.117.6.1302 [DOI] [PubMed] [Google Scholar]
- Eagle D.M, Robbins T.W. Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behav. Brain Res. 2003b;146:131–144. doi: 10.1016/j.bbr.2003.09.022. doi:10.1016/j.bbr.2003.09.022 [DOI] [PubMed] [Google Scholar]
- Eagle, D. M., Baunez, C., Hutcheson, D. M., Lehmann, O., Shah, A. P. & Robbins, T. W. In press. Stop-signal reaction time performance: role of prefrontal cortex and subthalamic nucleus. Cereb. Cortex [DOI] [PubMed]
- Evers E.A, Cools R, Clark L, van der Veen F.M, Jolles J, Sahakian B.J, Robbins T.W. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005;30:1138–1147. doi: 10.1038/sj.npp.1300663. doi:10.1038/sj.npp.1300663 [DOI] [PubMed] [Google Scholar]
- Fellows L.K, Farrah M.J. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. doi:10.1093/brain/awg180 [DOI] [PubMed] [Google Scholar]
- Fuster J.M. Raven; New York, NY: 1989. The prefrontal cortex: anatomy, physiology and neuropsychology of the frontal lobe. [Google Scholar]
- Garavan H, Ross T.J, Stein E.A. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc. Natl Acad. Sci. USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. doi:10.1073/pnas.96.14.8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross T.J, Murphy K, Roche R.A, Stein E.A. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. doi:10.1006/nimg.2002.1326 [DOI] [PubMed] [Google Scholar]
- Gehring W.J, Knight R.T. Prefrontal–cingulate interactions in action monitoring. Nat. Neurosci. 2000;3:516–520. doi: 10.1038/74899. doi:10.1038/74899 [DOI] [PubMed] [Google Scholar]
- Gilbert S.J, Shallice T. Task switching: a PDP model. Cogn. Psychol. 2002;44:297–337. doi: 10.1006/cogp.2001.0770. doi:10.1006/cogp.2001.0770 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. In: Roberts A.C, Robbins T.W, Weiskrantz L, editors. The prefrontal cortex: executive and cognitive functions. Oxford University Press; Oxford, UK: 1998. pp. 87–102. [DOI] [PubMed] [Google Scholar]
- Haber S.N, Fudge J.L, McFarland N.R. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Owen A.M. Fractionating attentional control using event-related fMRI. Cereb. Cortex. 2006;16:1279–1289. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Herath P, Klingberg T, Young J, Amutis K, Roland P. Neural correlates of dual task performance can be dissociated from those of divided attention: an fMRI study. Cereb. Cortex. 2001;11:796–805. doi: 10.1093/cercor/11.9.796. doi:10.1093/cercor/11.9.796 [DOI] [PubMed] [Google Scholar]
- Hollerman J.R, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Prog. Brain Res. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls E.T, Morris R.G, O'Doherty J, Bullock P.R, Polkey C.E. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J. Cogn. Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. doi:10.1162/089892904322926791 [DOI] [PubMed] [Google Scholar]
- Huettel S.A, Stowe C.J, Gordon E.M, Warner B.T, Platt M.L. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–775. doi: 10.1016/j.neuron.2006.01.024. doi:10.1016/j.neuron.2006.01.024 [DOI] [PubMed] [Google Scholar]
- Iversen S.D, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior frontal convexity. Exp. Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. doi:10.1007/BF00237911 [DOI] [PubMed] [Google Scholar]
- Keele S.W, Rafal R. Deficits in task set in patients with left prefrontal cortex lesions. In: Monsell S, Driver J, editors. Control of cognitive processes: attention and performance XVIII. MIT Press; Cambridge, MA: 1999. pp. 627–651. [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. doi:10.1126/science.1088545 [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakahama K, Uchida I, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related fMRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. doi:10.1093/brain/122.5.981 [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur. J. Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. doi:10.1046/j.1460-9568.1998.00167.x [DOI] [PubMed] [Google Scholar]
- Lange K, Robbins T.W, Marsden C.D, James M, Owen A, Paul G.M. l-Dopa withdrawal selectively impairs performance in tests of frontal lobe function in Parkinson's disease. Psychopharmacology. 1992;107:394–404. doi: 10.1007/BF02245167. doi:10.1007/BF02245167 [DOI] [PubMed] [Google Scholar]
- Lange K.W, Sahakian B.J, Quinn N.P, Marsden C.D, Robbins T.W. Comparison of executive and visuospatial function in Huntington's disease and dementia of the Alzheimer-type matched for degree of dementia. J. Neurol. Neurosurg. Psych. 1995;58:598–606. doi: 10.1136/jnnp.58.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz M.D.S, Morilak D.A. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2005;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. doi:10.1016/j.neuroscience.2005.09.031 [DOI] [PubMed] [Google Scholar]
- Lewis S.J.G, Slabosz A, Robbins T.W, Barker R.A, Owen A.M. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson's disease. Neuropsychologia. 2005;43:823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. doi:10.1016/j.neuropsychologia.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Liston C, Miller M.M, Goldwater D.S, Radley J.J, Rocher A.B, Hof P.R, Morrison J.H, McEwen B.S. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. doi:10.1523/JNEUROSCI.1184-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan G.D, Cowan W.B. On the ability to inhibit thought and action: a theory of an act of control. Psychol. Rev. 1984;91:295–327. doi: 10.1037/a0035230. doi:10.1037/0033-295X.91.3.295 [DOI] [PubMed] [Google Scholar]
- MacDonald A.W, Cohen J.D, Stenger V.A, Carter C.S. Dissociating the role of the dorsolateral prefrontal cortex and anterior cingulate cortex in cognitive control. Science. 2000;288:1235–1238. doi: 10.1126/science.288.5472.1835. doi:10.1126/science.288.5472.1835 [DOI] [PubMed] [Google Scholar]
- Mattay V.S, Goldberg T.E, Fera F, Hariri A.R, Tessitore R, Egan M.F, Kolachana B, Callicot J.H, Weinberger D.R. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc. Natl Acad. Sci. USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. doi:10.1073/pnas.0931309100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S, Laibson D.I, Loewenstein G, Cohen J.D. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. doi:10.1126/science.1100907 [DOI] [PubMed] [Google Scholar]
- Mecklinger A.D, von Cramon D.Y, Springer A, Matthes-von Cramon G. Executive control functions in task-switching: evidence from brain-injured patients. J. Clin. Exp. Neuropsychol. 1999;21:606–619. doi: 10.1076/jcen.21.5.606.873. [DOI] [PubMed] [Google Scholar]
- Mehta M.A, Sahakian B.J, McKenna P.J, Robbins T.W. Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson's disease. Psychopharmacology. 1999;146:162–174. doi: 10.1007/s002130051102. doi:10.1007/s002130051102 [DOI] [PubMed] [Google Scholar]
- Mehta M.A, Manes F.F, Magnolfi G, Sahakian B.J, Robbins T.W. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in healthy volunteers. Psychopharmacology. 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. doi:10.1007/s00213-004-1899-2 [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman N.E, White C.D, Glover G.H, Reiss A.L. Error-related brain activation during a Go/No Go response inhibition task. Hum. Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. doi:10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton H.C, Sharma A, Agouzoul D, Sahakian B.J, Robbins T.W. Idazoxan potentiates rather than antagonizes some of the cognitive effects of clonidine. Psychopharmacology. 1999;145:401–411. doi: 10.1007/s002130051074. doi:10.1007/s002130051074 [DOI] [PubMed] [Google Scholar]
- Miller E.K, Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology. 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. doi:10.1007/s00213-004-1792-z [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M.P, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the test identified by event-related functional magnetic resonance imaging. J. Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn. Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Murphy F.C, Michael A, Robbins T.W, Sahakian B.J. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol. Med. 2002;33:455–467. doi: 10.1017/s0033291702007018. doi:10.1017/S0033291702007018 [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi S, Fukuyama H, Shibasaki H. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb. Cortex. 2000;11:85–92. doi: 10.1093/cercor/11.1.85. doi:10.1093/cercor/11.1.85 [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hayashi T, Konishi S, Miyashita Y. Functional MRI of monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. doi:10.1126/science.1067653 [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach M.L, Rolls E.T, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001;4:95–102. doi: 10.1038/82959. doi:10.1038/82959 [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan R.J. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortex. J. Neurosci. 2003;23:7391–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A, Downes J.J, Sahakian B.J, Polkey C.E, Robbins T.W. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. doi:10.1016/0028-3932(90)90137-D [DOI] [PubMed] [Google Scholar]
- Owen A.M, Roberts A.C, Polkey C.E, Sahakian B.J, Robbins T.W. Extra-dimensional versus intradimensional set shifting performance following frontal lobe excision, temporal lobe excision or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. doi:10.1016/0028-3932(91)90063-E [DOI] [PubMed] [Google Scholar]
- Owen A.M, Roberts A.C, Hodges J.R, Summers B.A, Polkey C.E, Robbins T.W. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain. 1993;116:1159–1179. doi: 10.1093/brain/116.5.1159. doi:10.1093/brain/116.5.1159 [DOI] [PubMed] [Google Scholar]
- Owen A.M, Morris R.G, Sahakian B.J, Polkey C.E, Robbins T.W. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excision, temporal lobe excisions or amygdala-hippocampectomy in man. Brain. 1996;119:1597–1615. doi: 10.1093/brain/119.5.1597. doi:10.1093/brain/119.5.1597 [DOI] [PubMed] [Google Scholar]
- Pandya D.N, Barnes C.L. Architecture and connections of the frontal lobe. In: Perecman E, editor. The frontal lobes revisited. Lawrence Erlbaum; Hillsdale, NJ: 1987. pp. 41–68. [Google Scholar]
- Park S.B, Coull J.T, McShane R.H, Young A.H, Sahakian B.J, Robbins T.W, Cowen P.J. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology. 1994;33:575–588. doi: 10.1016/0028-3908(94)90089-2. doi:10.1016/0028-3908(94)90089-2 [DOI] [PubMed] [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. In: Roberts A.C, Robbins T.W, Weiskrantz L, editors. The prefrontal cortex: executive and cognitive functions. Oxford University Press; Oxford, UK: 1998. pp. 103–114. [Google Scholar]
- Preuss T.M. Do rats have a prefrontal cortex? The Rose–Woolsey–Akert Program reconsidered. J. Cogn. Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sahakian B.J, Hodges J.R, Rogers R.D, Robbins T.W. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain. 1999;122:1469–1493. doi: 10.1093/brain/122.8.1469. doi:10.1093/brain/122.8.1469 [DOI] [PubMed] [Google Scholar]
- Robbins T.W. Dissociating executive functions of the prefrontal cortex. In: Roberts A.C, Robbins T.W, Weiskrantz L, editors. The prefrontal cortex: executive and cognitive functions. Oxford University Press; Oxford, UK: 1998. pp. 117–130. [Google Scholar]
- Robbins T.W. Chemical neuromodulation of frontal-executive function in humans and other animals. Exp. Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. doi:10.1007/s002210000407 [DOI] [PubMed] [Google Scholar]
- Robbins T.W. Controlling stress: how the brain protects itself from depression. Nat. Neurosci. 2005;3:261–262. doi: 10.1038/nn0305-261. doi:10.1038/nn0305-261 [DOI] [PubMed] [Google Scholar]
- Robbins T.W, Everitt B.J. Functions of dopamine in the dorsal and ventral striatum. In: Robbins T.W, editor. Seminars in the neurosciences. Saunders; London, UK: 1992. pp. 119–127. [Google Scholar]
- Robbins T.W, Murphy E.R. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol. Sci. 2006;27:141–148. doi: 10.1016/j.tips.2006.01.009. doi:10.1016/j.tips.2006.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, T. W. & Roberts, A. C. In press. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb. Cortex [DOI] [PubMed]
- Roberts A, Robbins T.W, Everitt B.J. Extra- and Intra-dimensional shifts in man and marmoset. Q. J. Exp. Psychol. B. 1988;40:321–342. [PubMed] [Google Scholar]
- Roberts A.C, De Salvia M.A, Wilkinson L.S, Collins P, Muir J.L, Everitt B.J, Robbins T.W. 6-hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analogue of the Wisconsin card sorting test: possible interactions with subcortical dopamine. J. Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.C, Robbins T.W, Weiskrantz L. Oxford University Press; Oxford, UK: 1998a. The prefrontal cortex: executive and cognitive functions. [Google Scholar]
- Rogers R.D, Sahakian B.J, Hodges J.R, Polkey C.E, Kennard C, Robbins T.W. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson's disease. Brain. 1998b;121:815–842. doi: 10.1093/brain/121.5.815. doi:10.1093/brain/121.5.815 [DOI] [PubMed] [Google Scholar]
- Rogers R.D, Owen A.M, Middleton H.C, Williams E.J, Pickard J.D, Sahakian B.J, Robbins T.W. Choosing between small, likely rewards and large unlikely rewards activates inferior and orbital prefrontal cortex. J. Neurosci. 1999a;20:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R.D, et al. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology. 1999b;146:482–491. doi: 10.1007/pl00005494. doi:10.1007/PL00005494 [DOI] [PubMed] [Google Scholar]
- Rogers R.D, Andrews T.C, Grasby P.M, Brooks D, Robbins T.W. Contrasting cortical and sub-cortical PET activations produced by reversal learning and attentional-set shifting in humans. J. Cogn. Neurosci. 2000;12:142–162. doi: 10.1162/089892900561931. doi:10.1162/089892900561931 [DOI] [PubMed] [Google Scholar]
- Rowe J.B, Toni I, Josephs O, Frackowiak R.S.J, Passingham R.E. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. doi:10.1126/science.288.5471.1656 [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A.B, Brammer M.J, Taylor E. Right inferior cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. doi:10.1016/S1053-8119(03)00275-1 [DOI] [PubMed] [Google Scholar]
- Schall J.D, Stuphorn V, Brown J.W. Monitoring and control of action by the frontal lobes. Neuron. 2002;36:309–322. doi: 10.1016/s0896-6273(02)00964-9. doi:10.1016/S0896-6273(02)00964-9 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Ramus S. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odour discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. doi:10.1097/00001756-200205070-00030 [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. doi:10.1146/annurev.neuro.23.1.473 [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Phil. Trans. R. Soc. B. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. doi:10.1098/rstb.1982.0082 [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and the temporal organisation of behaviour. In: Roberts A.C, Robbins T.W, Weiskrantz L, editors. The prefrontal cortex: executive and cognitive functions. Oxford University Press; Oxford, UK: 1998. pp. 22–35. [Google Scholar]
- Slamecka N.J. A methodological analysis of shift paradigms in human discrimination learning. Psychol. Bull. 1968;69:423–438. doi: 10.1037/h0025762. doi:10.1037/h0025762 [DOI] [PubMed] [Google Scholar]
- Smith E.E, Jonides J. Working memory in humans: neuropsychological evidence. In: Gazzaniga M, editor. The cognitive neurosciences. MIT Press; Cambridge, MA: 1995. pp. 109–120. [Google Scholar]
- Solanto M, Abikoff H, Sonuga-Barke E, Schachar R, Logan G.D, Wigal T, Hectman L, Hinshaw S, Turkel E. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multi-modal treatment study of AD/HD. J. Abnorm. Child Psychol. 2001;29:215–228. doi: 10.1023/a:1010329714819. doi:10.1023/A:1010329714819 [DOI] [PubMed] [Google Scholar]
- Stuss D.T, Alexander M.P. Is there a dysexecutive syndrome? Phil. Trans. R. Soc. B. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. doi:10.1098/rstb.2007.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T, Toth J.P, Fianchi D, Alexander M.P, Tipper S, Craik F. Dissociation of attentional processes in patients with focal frontal and posterior lesions. Neuropsychologia. 1999;37:1005–1027. doi: 10.1016/s0028-3932(98)00158-4. doi:10.1016/S0028-3932(98)00158-4 [DOI] [PubMed] [Google Scholar]
- Sutherland N.S, Mackintosh N.J. Academic Press; New York, NY: 1971. Mechanisms of animal discrimination learning. [Google Scholar]
- Swainson R, Cunnington R, Jackson G.M, Rorden C, Peters A, Morris P.G, Jackson S.R. Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task-set shifting. J. Cogn. Neurosci. 2000;15:785–799. doi: 10.1162/089892903322370717. doi:10.1162/089892903322370717 [DOI] [PubMed] [Google Scholar]
- Talbot P.S, Watson D.R, Barrett S.L, Cooper S.J. Rapid tryptophan depletion improves decision-making cognition without affecting reversal learning or set-shifting. Neuropsychopharmacology. 2006;31:1519–1525. doi: 10.1038/sj.npp.1300980. doi:10.1038/sj.npp.1300980 [DOI] [PubMed] [Google Scholar]
- Tunbridge E.M, Bannerman D.M, Sharp T, Harrison P.J. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat. J. Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. doi:10.1523/JNEUROSCI.1124-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]