Abstract
The effects of nonglycated bovine serum albumin (BSA) and advanced glycosylation end products of BSA (AGE-BSA) on vascular responses of control and metabolic syndrome (MS) rats characterized by hypertriglyceridemia, hypertension, hyperinsulinemia, and insulin resistance were studied. Albumin and in vitro prepared AGE-BSA have vascular effects; however, recent studies indicate that some effects of in vitro prepared AGEs are due to the conditions in which they were generated. We produced AGEs by incubating glucose with BSA for 60 days under sterile conditions in darkness and at 37°C. To develop MS rats, male Wistar animals were given 30% sucrose in drinking water since weanling. Six month old animals were used. Blood pressure, insulin, triglycerides, and serum albumin were increased in MS rats. Contraction of aortic rings elicited with norepinephrine was stronger. There were no effects of nonglycated BSA or AGE-BSA on contractions in control or MS rats; however, both groups responded to L-NAME, an inhibitor of nitric oxide synthesis. Arterial relaxation induced using acetylcholine was smaller in MS rats. Nonglycated BSA and AGE-BSA significantly diminished relaxation in a 35% in the control group but the decrease was similar when using nonglycated BSA and AGE-BSA. This decrease was not present in the MS rats and was not due to increased RAGEs or altered biochemical characteristics of BSA. In conclusion, both BSA and AGE-BSA inhibit vascular relaxation in control artic rings. In MS rats the effect is lost possibly due to alterations in endothelial cells that are a consequence of the illness.
Keywords: AGE-BSA, BSA, metabolic syndrome, vascular relaxation
Introduction
Reducing sugars including glucose, fructose, trioses, and glyceraldehyde alter proteins by nonenzymatic glycosylation known as glycation or Maillard reaction. This reaction occurs in several steps, the initial ones being relatively fast and reversible, while the latter ones are slower and irreversible originating products known as advanced glycosylation end products (AGEs). AGEs were originally characterized by their yellow-brown fluorescent color and by their ability to cross-link with and between amino groups (Reynolds 1963). However, the term is now used for a broad range of advanced products of the Maillard reaction, including N-carboxymethyllysine (CML) and pyrroline (Reddy et al. 1995; Baynes and Thorpe 1999).
AGEs exert their actions by two different mechanisms: (1) modifying structural intra- and extracellular proteins and (2) binding to their receptors (RAGEs) that belong to the immunoglobulin family and are located in the plasma membranes of monocytes, macrophages, endothelial cells, and vascular smooth muscle cells (Brownlee 1995; Basta et al. 2002). When AGEs bind to their receptors they initiate second messenger cascades. They also generate reactive oxygen species which modulate cellular function and can induce inflammatory processes (Krieglstein and Granger 2001; Basta et al. 2002; Ramasamy et al. 2005).
Albumin is one of the main proteins undergoing glycation reactions due to its abundance in serum and to the fact that it can be glycated at multiple sites (Wautier and Guillausseau 1998). Both bovine serum albumin (BSA) and AGE-BSA have been reported to have vascular effects. BSA reacts with nitric oxide (NO) therefore modulating its biological actions and increasing endothelial permeability (Predescu et al. 2002). Some forms of AGEs also diminish vascular relaxation by decreasing the NO production of endothelial cells (Hogan et al. 1992; Xu et al. 2003). Nevertheless, there have been recent reports that other forms of in vitro prepared AGEs have different effects from the naturally produced AGEs (Valencia et al. 2004).
AGEs have been implicated in aging processes and in the development of chronic complications of diabetes such as renal and vascular damage and could also participate in complications of metabolic syndrome (Brownlee 1995; Wautier and Guillausseau 1998; Valencia et al. 2004). There is a significant association between a high incidence of cardiovascular events and endothelial damage in animal models and diabetic patients and one of the mechanisms underlying this association is the presence of AGEs in plasma (Tan et al. 2002).
A variant of fructose-induced hypertension by giving sucrose to rats has been developed in our institution. The fructose-fed rat becomes hypertensive, hypertriglyceridemic, hyperinsulinemic and has insulin resistance; it exhibits what is known as metabolic syndrome (MS) or “Syndrome X” (Hwang et al. 1987). Some of the characteristics of the model developed in our institution have already been described (Baños et al. 1997) and they coincide with those of fructose fed animals, i.e., our rats also develop hypertension, hypertriglyceridemia, hyperinsulinemia, and insulin resistance. They also show increased oxidative stress (Baños et al. 2005). Some of the characteristics of these animals are accentuated by age and have been associated with renal failure and cardiovascular diseases which are related to alterations in the structure and function of endothelial cells (Rubio et al. 2006). AGEs could play a role in the vascular damage found in MS rats. Therefore, the aim of the present paper was to determine the effect of nonglycated BSA and AGE-BSA on aortic rings from control and MS rats.
Results
Changes in body weight, arterial pressure, triglycerides, glucose, insulin, and serum albumin
Table I shows the values of body weight, arterial pressure, glucose, insulin, homeostasis model assessment (HOMA), triglycerides, and serum albumin for control and MS rats. Experimental animals developed metabolic syndrome characterized by arterial hypertension, hypertriglyceridemia, hyperinsulinemia, and insulin resistance. There was a statistically significant increase in the serum albumin values in MS rats. Although there was no significant change in body weight, the animals with MS clearly developed central adiposity.
Table I.
Clinical characteristics and biochemical parameters from control and MS rats
| Control | SM | |
|---|---|---|
| Body weight (g) | 558.3 ± 14.9 | 547.5 ± 38.1 |
| Arterial pressure (mm of Hg) | 101.9 ± 1.4 | 138.9 ± 0.8* |
| Triglycerides (mg/dL) | 55.6 ± 4.7 | 109.1 ± 12.8* |
| Glucose (mmol/L) | 5.9 ± 0.3 | 4.8 ± 0.7 |
| Insulin (μU/mL) | 6.5 ± 0.9 | 24.2 ± 5.7* |
| HOMA | 1.02 ± 0.5 | 5.02 ± 2.1* |
| Albumin (g/dL) | 3.3 ± 0.1 | 3.6 ± 0.1* |
Values are mean ± SEM, n = 8; *P < 0.01.
AGE-BSA preparation
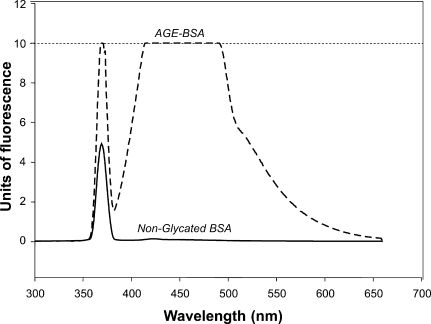
It was possible to produce AGE-BSA under the previously described condition. After incubation with glucose, the albumin solution took a yellow-brown color and showed the characteristic fluorescence spectrum of AGEs. Albumin incubated without glucose did not significantly change its appearance and did not have the characteristic fluorescence spectrum of AGEs (Figure 1).
Fig. 1.
Fluorescence spectrum of AGE-BSA and nonglycated BSA. Both solutions were tested at 2 mg/mL of protein. The peak of fluorescence is not present in the nonglycated BSA. The interrupted line represents the saturation level of the fluorescence detection by the equipment employed.
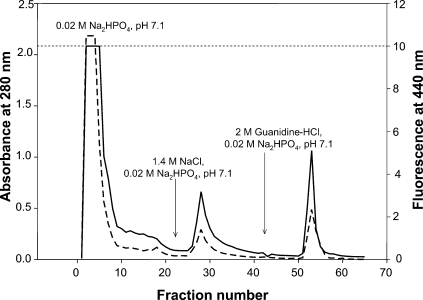
The purification of AGE-BSA on Affi-Gel-Blue is shown in Figure 2; three peaks were observed which corresponded to the highly glycated BSA (first one), the less glycated BSA moderately, and stronger bound to the chromatographic matrix (second and third peaks, respectively). The first peak was employed for subsequent analysis and tested for vasoreactivity.
Fig. 2.
Purification of AGE-BSA on Affi-Gel-Blue. Three peaks were observed: the first one corresponds to highly glycated BSA which did not bind to the matrix, the second one corresponds to the less glycated BSA which bound moderately, and the third peak corresponds to the stronger bound BSA. The first peak was the only one to be further analyzed. The arrows indicate the buffer used at each stage.
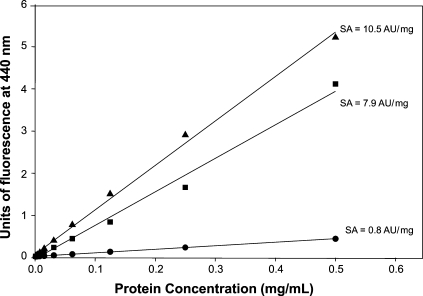
The AGE-BSA produced had a protein concentration of 53.8 mg/mL and a specific activity, before its purification in Affi-Gel Blue, of 7.9 AU/mg of protein and of 10.5 AU/mg of protein, after purification. Fluorescence was not significantly elevated when protein concentration of nonglycated BSA was increased (Figure 3). The value of specific activity was similar to that obtained by other authors using similar procedures (Verbeke et al. 1997).
Fig. 3.
Evaluation of the specific activity (SA) of AGE-BSA. The increase of specific activity obtained by the purification process on Affi-Gel-Blue is observed. Bold circles represent the nonglycated BSA. Bold squares represent the unpurified AGE-BSA and bold triangles represent the purified AGE-BSA. Specific activity increased in approximately 33% by the purification process.
Changes in mass and isoelectric point of BSA and glycated BSA
Table II shows the results of the analysis of the main glycation sites of the BSA structure. The analysis was done using the sequence of bovine serum albumin reported in the GenBank with number CAA76847 (gi:3336842) and the PyMOL v0.99 program (DeLano Scientific LLC, CA). The disposition of the lysine and arginine residues in the three-dimensional structure was evaluated. The analysis shows that a maximum of 52 residues from the 79 present in the molecule can be glycated. The rest of the residues are in the inside of the structure of BSA.
Table II.
Distribution of the amino acids lysine and arginine in the three-dimensional structure of BSA
| Amino acid position in the three-dimensional structure | |||
|---|---|---|---|
| Amino acid | Totally exposed | Partially exposed | Nonexposed |
| Lysine | 4,12,20,41,51,64,76, 116,127,132,136,180,187,211,224, 261,273,275,285,312,316,322,350,362,375,377,388,396, 465,474,499,504,520,523,524,535,537,556, 563,573. | 159,413,431,533. | 93,106,114,131, 204,221,232,239, 242,279,294,439, 544. |
| Arginine | 194,196, 198. | 143,185,409,412, 444. | 10,81,98,144,208, 217,256,335,336, 347,427,458,483, 484. |
| Total | 43 | 9 | 27 |
The amino acids localized in depression areas are considered as partially exposed.
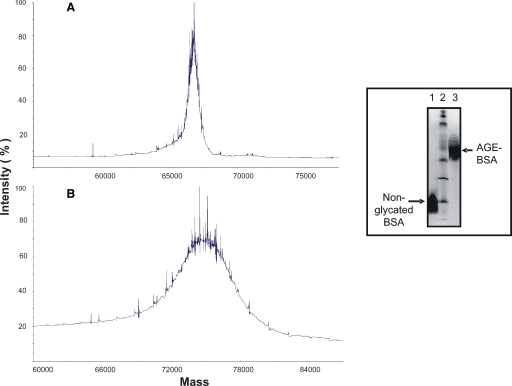
Figure 4 shows a molecular mass of 66,655.58 Da for nonglycated BSA (Figure 4A). After glycation, the molecular mass increased to 74,461.15 Da (Figure 4B). The net increase of 7,805.57 Da observed corresponds to the addition of 48 glucose molecules, considering that the molecular mass of glucose is 180.2 Da and that during each reaction of Amadori product, a water molecule is liberated (18 Da). The amount of glucose bound to BSA in our preparation was 48, very close to the 52 theoretically possible; therefore, we consider that our AGE-BSA is a highly glycated product.
Fig. 4.
Analysis of BSA and AGE-BSA by mass spectrometry. The mass increase of protein after glycation is observed. The mass of nonglycated BSA was 66,655.58 Da (A) and that of AGE-BSA was 74,461.15 Da (B). The net increase of mass was 7,805.57 Da, corresponding to the addition of 48 molecules of glucose to each BSA molecule. Inset: change of isoelectric point of BSA after glycation. Nonglycated BSA had a pI of 4.2 while AGE-BSA had a pI of 6.3. Line 2 is the broad range pI standard kit (pH 3–10).
Nonglycated BSA had an isoelectric point of 4.2. The glycation process changed the isoelectric point to 6.3 (Figure 4, inset).
Vascular responses
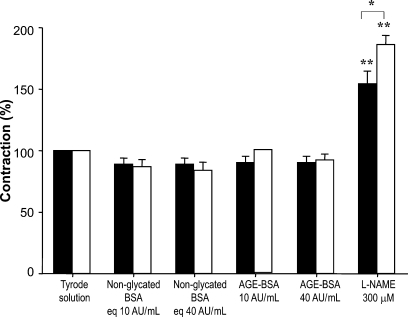
Norepinephrine-induced vasoconstriction was higher in MS rats than in control rats (Table III); nevertheless, vasoconstriction was not significantly modified in aortic rings from control or MS rats in the presence of nonglycated BSA or AGE-BSA. L-NAME significantly increased vascular contraction in both groups (154.41 ± 10.35% versus 186.18 ± 7.58%, respectively) since inhibition of NOS induces an imbalance in vasoconstriction and vasodilation which is greater in MS than in control rats (Figure 5).
Table III.
Values of contraction (g) and relaxation (%) of aortic rings in control and experimental rats
| Control | MS | |
|---|---|---|
| Vascular contraction | 1.5 ± 0.2 | 2.2 ± 0.2* |
| Vascular relaxation | 84.4 ± 2.6 | 64.9 ± 3.3* |
The results are expressed as relaxation percentage of the initial precontraction level with NE (1 μM). Final relaxation with Ach (1 × 10−6 M) is reported. Values are mean ± SEM, n = 8; *P < 0.001.
Fig. 5.
Effect of AGE-BSA on vascular contraction in aortic rings from control (solid bars) and MS rats (open bars). The contractions were induced by NE 1 μM and basal tension was normalized to 100% in control and MS rats. Tension values in grams are shown in Table III. Results are the means ± SEM of six independent experiments. *P < 0.05 between MS and control; **P < 0.001 between the Tyrode solution and L-NAME.
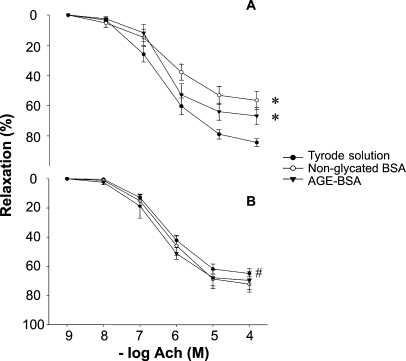
Basal relaxation was diminished in MS rats when compared to control rats (Table III). Figure 6 shows the effect of nonglycated BSA and AGE-BSA upon vascular relaxation in aortic rings from control and MS rats. In control rings nonglycated BSA and AGE-BSA at 40 AU/mL (3.8 mg/mL) significantly inhibited the endothelium-dependent vasorelaxation in a 35%. This effect was not present when a lower dose was used (10 AU/mL; 0.95 mg/mL). Fresh albumin, fatty acid free, diminished vasorelaxation in aortic rings from control rats in the same proportion as pre-incubated control BSA and AGE-BSA at a concentration of 3.8 mg/mL (data not shown).
Fig. 6.
Effect of nonglycated BSA and AGE-BSA at 40 AU/mL (3.8 mg/mL) on endothelium-dependent vasorelaxation in aortic rings from control (A) and MS rats (B). Results are expressed as relaxation percentage from the initial precontraction level with NE 1 μM. *P < 0.05 between the Tyrode solution and nonglycated BSA and AGE-BSA; #P < 0.05 between the Tyrode solution in MS and control rats.
In MS aortic rings vasorelaxation was not significantly affected by BSA or AGE-BSA at any of the doses tested.
Immunofluorescence of RAGEs
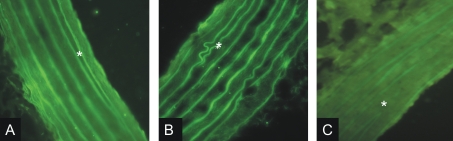
Results of expression of RAGE are shown in Figure 7. There is an increased RAGE immunoreactivity in aortas from MS rats when compared to control rats. In aortas from C rats fluorescence is observed mainly in the endothelium while in MS aortas both the endothelium and muscular fibers fluoresce. Damage due to the development of the metabolic syndrome can also be appreciated by the presence of buckles and a loss of continuity in elastic fibers.
Fig. 7.
Immunohistochemistry for RAGE in aortas of control (Panel A) and MS (Panel B) rats. Negative control (Panel C). Magnification 40×. Asterisk indicates internal elastic lamina. There is an increased RAGE immunoreactivity in MS rats.
Discussion
The aim of the present paper was to determine the effect of nonglycated BSA and AGE-BSA on vascular aortas from control and MS rats in which the condition is induced by a high ingestion of sucrose and where the structure and function of endothelial cells are altered. Both albumin and AGEs have been reported to have vascular effects (Vlassara et al. 1992). However, the vascular effects of AGEs might not be produced by all types of AGEs, since AGEs may have very different structures depending on the reactions generated during the in vitro glycation process of the protein by reducing sugars (Katchalsky and Sharon 1953). Some of the AGEs generated are capable of cross-linking to proteins and others have the capacity to interact with specific receptors (RAGEs) present in some cellular types (Valencia et al. 2004).
There have also been recent reports that some of the effects of in vitro prepared AGEs are due to metal ions which are contaminants commonly found in AGE preparations. Various metal ions have been shown to drive inflammatory responses via generation of oxidative stress (Valencia et al. 2004). The use of a chelator such as EDTA to facilitate the reaction could induce some of the effects reported to be produced by AGEs (Al-Abed et al. 1999).
Our results show that body weight was not significantly modified in MS rats even if the diet of the sucrose-fed rats was hypercaloric (Baños et al. 1997) (Table I). However, sucrose-fed animals showed increased central adiposity. Insulin was increased in MS rats and this increase accompanies an increase in blood pressure. Triglycerides were also elevated. Therefore the MS rats show many of the characteristics of metabolic syndrome. Serum albumin concentration was significantly higher in MS rats when compared to control rats. However, Rumble et al. (1997) reported that diabetic and hypertensive rats had lower serum albumin levels than the normotensive Sprague Dawley rats.
Vascular contraction to norepinephrine increased MS rats in this study. This has been previously described for this model (Baños et al. 1997) and additionally we have previously found that contraction to KCl in MS rats is also increased and that this is due to an elevated response to endothelin (Nava et al. 1999; Rubio et al. 2006). The increase in the circulating levels of albumin in the MS group may contribute to the increased vascular tone observed in this group. Indeed, an increase in circulating albumin levels has been observed in some forms of hypertensive pathology, due to the hemoconcentration induced by edema, which positively correlates with the hypertension levels (Allen and Patterson 1995). AGEs could also participate in the increase in vascular contraction in MS rats since they increase the production of endothelin-1 by endothelial cells (Tan et al. 2002). Our results show that vasoconstriction was not modified by nonglycated BSA or by AGE-BSA (Figure 5). This is in accordance with the results of Xu et al. (2003).
Vasorelaxation in MS rats was significantly diminished in comparison to control rats and could be attributed to an imbalance of constricting and relaxing factors as has been previously reported (Baños et al. 1997). Furthermore, as our model of MS rats is caused by the chronic ingestion of sucrose, vascular damage could be a probable consequence of AGEs increased production. AGEs deposit inside smooth muscle and endothelial cells as well as in extracellular matrix proteins increasing rigidity. Hogan et al. (1992) have reported that AGEs react with NO produced by endothelial cells. They also reported that AGEs reduce the antiproliferative effect of NO on aortic smooth muscle vascular and renal mesangial cells.
Insulin, which is also increased in our model, stimulates collagen synthesis in vascular smooth muscle cells (Ruiz Torres et al. 1998) and could contribute to diminished vasorelaxation in MS rats. Arterial endothelium-dependent relaxation is also diminished hypertensive rats (Ibarra et al. 1995; Küng and Lüscher 1995; Challah et al. 1997; Freitas et al. 2003; Shipley and Muller-Delp 2005). These authors have proposed that the reduced response to Ach could be a consequence of an impairment of either the generation (synthesis or release) of relaxant factors or the cellular response to them.
In our study, both nonglycated BSA and AGE-BSA decreased vasorelaxation in the same proportion in control rats. In arterial rings incubated in the presence of glycated and nonglycated BSA the response to acetylcholine was 35% reduced in comparison to control rings incubated in the absence of BSA (Figure 6A). The reduction in vascular relaxation in the presence of nonglycated BSA is in accordance with the report by Predescu et al. (2002). These authors suggested that BSA reacts with NO therefore modulating its biological actions and increasing endothelial permeability. Although in our experiments we also found a decrease by AGE-BSA, it seems to be due to BSA since there was not a significant difference in the reduction produced by AGE-BSA and by nonglycated BSA.
Previously, Xu et al. (2003) studied the vascular effects of AGEs produced in vitro testing vasoreactivity in rings of thoracic aorta from New Zealand white rabbits and in vitro production of NO in endothelial cells from human umbilical veins. These authors reported that there was not a significant effect of AGEs upon vasoconstriction induced by NE and our results are in accordance with this result. However, they found that AGEs diminished the NO production of isolated endothelial cells and that vasodilation was reduced in thoracic aortic rings while we found that AGEs did not modify vasodilation. Our data suggest that it is BSA that exerts the diminished vasorelaxing effect and not AGEs. In their study the authors produced AGEs incubating BSA with glucose 6-phosphate instead of glucose which is a faster reaction. Glucose 6-phosphate is a reducing sugar of great intracellular importance and can glycate easier than glucose since it is present in a higher proportion in the open-chain form (Hogan et al. 1992). Their reaction was facilitated by the use of EDTA which could induce the effect reported by them (Al-Abed et al. 1999). AGEs derived from a long period of incubation of BSA with glucose, as was done in this paper, conduces mainly to fluorescent cross-linking AGE production. Using specific antibodies, these authors found high levels of carboximetillysine (CML) in their preparation and identified CML as the AGE responsible of the NO synthesis inhibition. Therefore, the kind of AGEs used by them could explain the contradiction between their results and what we observed in the present paper. Furthermore, the difference of the species used for testing vasodilation in both papers (New Zealand rabbits and Wistar rats) might account for the contradicting results found.
The same authors (Xu et al. 2003) did not find the expected previously reported decrease in vascular relaxation when they added nonglycated BSA. The absence of a vascular effect of nonglycated BSA in the above-mentioned study is probably due to the very low doses assayed, in the order of μg/mL. It is known that albumin has a physiologic effect at higher concentration. Indeed, our own results show that the inhibitory effect of BSA or AGE-BSA was only observed when the protein concentration assayed was about of 3.81 mg/mL, but not when it was only of 0.95 mg/mL.
The response to nonglycated BSA and AGEs disappeared in the MS rats (Figure 6B). Alterations in the structure and function of the vascular structures caused by the metabolic syndrome could be the underlying cause of the lack of response in these animals. This physiological adaptation could be considered as a protector effect to counteract the increased basal vasoconstriction observed in metabolic syndrome rats, and must be better studied. We evaluated the presence of AGEs’ receptors (RAGEs) from aortas in MS rats to better explore the mechanism by which AGEs participate in this pathology. Many of the deleterious effects of AGEs are mediated by binding to their receptors which are present in various cellular types. In endothelial cells, the interaction AGE–RAGE modulates the expression of citocynes and adhesion molecules and increases the production of oxygen reactive species (ROS) generating oxidative stress (Ramasamy et al. 2005). Koyama et al. (2005) demonstrated that an alternative form of RAGE, the endogenous secretory RAGE (esRAGE), is associated with the metabolic syndrome or atherosclerosis in the absence of diabetes. Inflammation might be the factor linking the RAGE system with MS. Although a higher number of RAGEs were observed in the aortas from MS rats, AGE-BSA did not have any effect on arterial relaxation in this group. Moreover, the absence of differences in arterial relaxation induced by AGE-BSA or BSA in the control group suggests that the possible effect of AGE-BSA on vascular function in not mediated by RAGEs.
The exposition of the vascular tissue to AGE-BSA during the development of MS could also cause the loss of the relaxation capacity of aortas. The changes in the charge and composition of the BSA molecule produced by glycation increase its aggregation capacity. When the isoelectric point is closer to physiological pH, hydrophobic interactions are favored. These changes could increase the cross-linking activity of AGEs to proteins and the capacity to deposit in the extracellular matrix of the tissues. Correspondingly, we observed an increased aggregation capacity of AGE-BSA during alcohol precipitation or desalting. However, the loss of the relaxing capacity due to biochemical changes of AGE-BSA is not favored by our results since there were no differences in the relaxation in the presence of BSA or AGE-BSA.
We speculate that the cause could be the altered albumin internalization to endothelial cells and therefore a modified neutralization of NO. This should be further evaluated. The molecular structure and charge of albumin facilitate the cotransport of a number of hydrophobic molecules, enzymes, and hormones across the endothelium and the maintenance of vascular integrity and transvascular oncotic pressure gradient (Metha and Malik 2006). Endothelial permeability to plasma proteins and liquid is increased in inflammation, a condition manifested by protein-rich edema (Metha and Malik 2006). Inflammation is present in metabolic syndrome and therefore alterations in albumin transport across endothelial cells are likely to be present. Bevers et al. (2006) reported that in bEnd.3 endothelial cells, which only produce eNOS and have higher levels of NO than other endothelial cells, incubation with low albumin levels increased eNOs activity and the production of NO.
In conclusion both BSA and AGE-BSA inhibit vascular relaxation mediated by NO in control artic rings in the same proportion. This inhibitory effect is likely to be due to the structure of BSA and not to the fluorescent AGEs synthesized by the employed procedure. However, in MS rats this inhibitory vascular relaxation effect is lost, possibly due to alterations in endothelial cells that are a consequence of the illness.
Materials and methods
Animals and arterial pressure determination
Experiments in animals were approved by the Laboratory Animal Care Committee of our institution and were conducted in compliance with our institution's ethical guidelines for animal research.
Weanling male Wistar rats aged 25 days and weighing 50 ± 4 g were separated into two groups—group 1: control rats (C) were given tap water for drinking and group 2: MS rats were given 30% sucrose in drinking water during a 24-week period. All animals were fed Purina 5001 rat chow (Purina Mills Inc., Richmond, IN) ad libitum, which provides 14.63 kJ/g, with 23% protein, 12% fat, and 65% carbohydrate. Animals were kept at controlled temperature and a 12:12-h light-dark cycle.
Systolic arterial blood pressure was measured in conscious animals using the tail cuff method; the cuff was connected to a pneumatic pulse transducer (Narco Bio-Systems Inc., Healthdyne Co., Austin, TX) and a programmed electrosphyngomanometer. The mean of five independent determinations was calculated.
AGE-BSA preparation
AGE-BSA were prepared, purified, and characterized in the reproductive biology laboratory of Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” following the technique previously described (Makita et al. 1991, 1992).
AGE-BSA was prepared by incubating 5% of bovine serum albumin (BSA fraction V; Sigma Chemical, St. Louis, MO) in 200 mM of sodium phosphate buffer (pH 7.4) with 500 mM glucose for 60 days under sterile conditions, in darkness and at 37°C. After incubation, the product was reduced with 100 mM sodium borohydride (NaBH4, Sigma Chemical) for 30 min at room temperature to stabilize the Schiff bases formed (Eble et al. 1983; Fluckiger and Gallop 1984; Verbeke et al. 1997). Then, the unbound glucose and unreacted NaBH4 were removed by extensive dialysis against a 500 mM sodium phosphate buffer (pH 7.4). Less glycosylated BSA was removed from AGE-BSA by affinity chromatography on Affi-Gel Blue (Bio-Rad Laboratories, Richmond, CA) (Travis et al. 1976; Verbeke et al. 1997). The Affi-Gel matrix has a blue dye named Cibacron Blue F3GA, with natural affinity for native albumin. A column with 5 mL of matrix with a binding capacity of 170 mg of BSA was employed. 2 mL of AGE-BSA solution with a concentration of 53.8 mg/mL was applied in each purification process, using a flow rate of 1 mL/min. The chromatogram was performed evaluating a dilution 1/10 of each tube.
Protein concentration of AGE-BSA was evaluated by the Lowry method using BSA as standard (Lowry et al. 1951). Finally, AGE-specific fluorescence determination was performed by measuring emission at 440 nm upon excitation at 370 nm using an Aminco fluorescence spectrometer (Aminco-Bowman Series 2 SLM Instruments. Inc., Rochester, NY) (Monnier and Cerami 1981; Makita et al. 1992). Fluorescence value of AGE-BSA was measured at a protein concentration of 1 mg/mL and expressed in arbitrary units (AU). The specific activity (SA) was expressed as arbitrary units of fluorescence per mg of protein (AU/mg).
The control BSA sample (nonglycated BSA) was incubated under identical conditions but without glucose. AGE-BSA and its control (nonglycated BSA) were maintained at −70°C until used.
Mass increase analysis of glycated BSA
The mass change of BSA after glycation was analyzed by matrix-assisted laser desorption ionization mass spectrometry (MALDI-TOF) (Voyager DE-PRO from Applied Biosystems, CA), equipped with a nitrogen laser (337 nm), operating in a positive high-energy linear mode. This analysis was made in the Analysis and Molecular Diagnose Unit of the Insituto Nacional de Salud Pública de México.
Aliquots of 500 μL containing 10 mg/mL of BSA or AGE-BSA were diluted to 1.5 mL with deionized water and centrifuged in Amicon Ultracel-10 K (Millipore Corporation, MA) at 2,000 × g for 30 min to a final volume of 500 μL. Washing was repeated three times. A 10 μL aliquot was taken for MALDI-TOF analysis. The matrix solution employed for BSA analysis contained 10 mg/mL 3,5-dimethoxy-4-hydroxycinnamic acid (sinapinic acid) in 30% vol/vol acetonitrile in 0.1% vol/vol aqueous trifluoroacetic acid. For AGE-BSA the matrix solution contained 2,5-dihydroxybenzoic acid (gentisic acid, which is employed for analysis of glycoproteins) to 10 mg/mL in 50% vol/vol acetonitrile. A sample protein solution (1 μL) of a 1:1 vol/vol mixture of 1 mg/mL of total protein and the matrix solution were applied on a sample spot in a steel plate slide and dried in a warm air stream. Bovine's insulin (5,734.59 Da), E. coli's Thioredoxin (11,674.48 Da), and horse's Apomyoglobyn (16,952.56 Da) were used as standard for calibration.
Isoelectric point of glycated BSA
The change in the isoelectric point of BSA after glycation was analyzed by isoelectric focusing in polyacrylamide gels using a FastSystem (Pharmacia, Uppsala, Sweden). The bands were dyed with silver nitrate, and the isoelectric point was evaluated using the analytical unit of FastSystem employing a broad range standard kit (pH 3–10) (Amersham Biosciences, Buckinghamshire, United Kingdom) as a protein calibrator.
Blood sample collection and determination of glucose, insulin, triglycerides, and serum albumin
After overnight fasting (12 h), the animals were killed by decapitation and blood was collected. It was spun and the serum was separated by centrifugation at 600 × g during 15 min at room temperature and stored at −70°C until needed. Serum insulin was determined using a commercial radioimmunoassay (RIA) specific for rat (Linco Research, Inc., Missouri); its sensitivity was 0.1 ng/mL and intra- and interassay coefficients of variation were 5 and 10%, respectively. Glucose concentration was assayed using an enzymatic SERA-PAKR Plus from Bayer Corporation (Sées, France). Homeostasis model assessment (HOMA) was used as an index to measure the degree of insulin resistance and was calculated by the formula [insulin (μU/mL) × glucose (mmol/L)/22.5] (Matthews et al. 1985; Pickavance et al. 1999; Nandhini et al. 2005). Triglycerides (TGs) were determined by commercially available procedures (Randox, Laboratories LTD, Antrim, United Kingdom). Serum albumin was evaluated in both groups of animals using a kit commercialized by Hycel of Mexico which uses bromocresol green (IL-test Instrumentation Laboratory Company, Lexington, MA). Samples were read using an Ilab-600 automatic system.
Sample preparation and tension recording
The animals were killed by decapitation, and aortas were immediately dissected and placed in an oxygenated normal Tyrode solution (mM: 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 5 Hepes, and 5.5 glucose) pH 7.4. Arteries were carefully cleaned from connective and adipose tissue, taking care not to damage the endothelium. Tension measurements were made as previously described (Nava et al. 1999). Briefly, segments of about 2 to 3 mm long were cut and two 250-μm-diameter S-shaped silver wires (Medwire Corp, Mount Vernon, NY) were inserted into the lumen to measure tension developed transversely by rings of the vessel. One of the silver wires was fixed to the bottom of an in vitro chamber, and the other was attached to a tension transducer that was connected to a Grass polygraph model 79D. The chamber was filled with a Tyrode solution, and termorregulated and bubbled with carbogen (95% oxygen, 5% carbon dioxide). A basal passive tension of 2 g was applied after determination in preliminary tests that this was the optimal resting tension under our experimental conditions. Arteries were allowed to rest for 1 h and the solution was changed every 20 min. Contraction was induced twice by the addition of norepinephrine (NE) (1 μM). Arteries were washed by adding a fresh Tyrode solution to the chamber allowing the rings to return to their basal tension (2 g). The mean contraction value was considered as 100% of response. The vasodilator activity was studied by cumulative concentration–response curves to acetylcholine (Ach) (10−9−10−4 M) on precontracted aortic rings in the following experimental groups: (A) incubated only with the Tyrode solution, (B) incubated during 40 min with AGE-BSA at 10 AU/mL, (C) incubated with AGE-BSA at 40 AU/mL, (D) incubated with nonglycated BSA at a protein concentration equivalent to 10 UA/mL of AGE-BSA (0.95 mg/mL), (E) incubated with nonglycated BSA at a protein concentration equivalent to 40 AU/mL of AGE-BSA (3.81 mg/mL), and (F) incubated with L-NAME at 300 μM, in both control and MS rats. The percentage of the response in each experimental group was calculated in relation to the tension developed by the same ring during its basal contraction.
Immunohistochemistry of RAGEs
Aortic rings of both MS and C rats were quickly frozen in Tissue-Tek (Sakura Finetek USA, Inc., Torrance, CA). Sections were fixed with acetone and were blocked with PBS/Azide 0.02%/BSA 1% for 30 min.
Subsequently, sections were incubated during 2 h at room temperature with a rabbit polyclonal antibody against RAGE (1:50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Primary antibodies were detected by using goat anti-rabbit FITC (Jackson ImmunoResearch Laboratories Inc. West Grove, PA), at room temperature for 60 min. Negative controls were prepared by substituting the primary antibody with an irrelevant antibody. Staining was analyzed using fluorescence microscopy.
Statistical analysis
Results are expressed as mean ± standard errors of the mean (SEM) from 6 to 10 different artery preparations. The percentage of contraction in each experiment was calculated, and the mean was then determined. When applicable (comparisons between two values; MS and controls), statistical analysis was done by Student's t-test. Comparisons between groups were done by analysis of variance (ANOVA) or Anova on ranks followed by Student–Newman–Keuls or Dunn's tests, depending on whether the data were normally distributed or not, using the SigmaStat 2.0 program (Jandel Scientific, San Rafael, CA). Differences were considered statistically significant when P < 0.05.
Acknowledgments
The authors wish to thank Dr. Humberto Lanz Mendoza and Gerardo Hurtado Sil for mass analysis and Dr. Fernando Arteaga Cabello for isoelectric focusing analysis. The authors also want to thank Bertha Soto and Florencio Hernández for technical assistance.
Glossary
Abbreviations
- AGEs
advanced glycosylation end products
- BSA
bovine serum albumin
- CML
carboximetillysine
- esRAGE
endogenous secretory RAGE
- HOMA
homeostasis model assessment
- MS
metabolic syndrome
- NO
nitric oxide
- RAGEs
AGEs’ receptors
Conflict of interest statement
There are no conflicts of interest.
References
- Al-Abed Y, Kapurniotu A, Bucala R. Advanced glycation end products: Detection and reversal. Methods Enzymol. 1999;309:152–172. doi: 10.1016/s0076-6879(99)09013-8. [DOI] [PubMed] [Google Scholar]
- Allen MT, Patterson SM. Hemoconcentration and stress: A review of physiological mechanisms and relevance for cardiovascular disease risk. Biol Psychol. 1995;41(1):1–27. doi: 10.1016/0301-0511(95)05123-r. [DOI] [PubMed] [Google Scholar]
- Baños G, Carvajal K, Cardoso G, Zamora J, Franco M. Vascular reactivity and effect of serum in a rat model of hypertriglyceridemia and hypertension. Am J Hypertens. 1997;10:379–388. doi: 10.1016/s0895-7061(96)00400-1. [DOI] [PubMed] [Google Scholar]
- Baños G, Medina-Campos ON, Maldonado P, Zamora J, Pérez I, Pavón N, Pedraza-Chaverrí J. Antioxidant enzymes in hypertensive and hypertriglyceridemic rats: Effect of gender. Clin Exp Hypertens. 2005;1:45–57. doi: 10.1081/ceh-200044255. [DOI] [PubMed] [Google Scholar]
- Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, De Caterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: A mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes. 1999;48(1):1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Bevers LM, van Faassen EE, Vuong TD, Ni Z, Boer P, Koomans HA, Braam B, Vaziri ND, Joles JA. Low albumin levels increase endothelial NO production and decrease vascular NO sensitivity. Nephrol Dial Transplant. 2006;21:3443–3449. doi: 10.1093/ndt/gfl443. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- Challah M, Nadaud S, Philippe M, Battle T, Soubrier F, Corman B, Michel JB. Circulating and cellular markers of endothelial dysfunction with aging in rats. Am J Physiol. 1997;273:H1941–H1948. doi: 10.1152/ajpheart.1997.273.4.H1941. [DOI] [PubMed] [Google Scholar]
- Eble AS, Thorpe SR, Baynes JW. Nonenzymatic glucosylation and glucose-dependent cross-linking of protein. J Biol Chem. 1983;258(15):9406–9412. [PubMed] [Google Scholar]
- Fluckiger R, Gallop PM. Measurement of nonenzymatic protein glycosylation. Methods Enzymol. 1984;106:77–87. doi: 10.1016/0076-6879(84)06009-2. [DOI] [PubMed] [Google Scholar]
- Freitas MR, Schott C, Corriu C, Sassard J, Stoclet JC, Ramaroson A. Heterogeneity of endothelium-dependent vasorelaxation in conductance and resistance arteries from Lyon normotensive and hypertensive rats. J Hypertens. 2003;21:1505–1512. doi: 10.1097/00004872-200308000-00014. [DOI] [PubMed] [Google Scholar]
- Hogan M, Cerami A, Bucala R. Advanced glycosylation endproducts block the antiproliferative effect of nitric oxide. Role in the vascular and renal complications of diabetes mellitus. J Clin Invest. 1992;90:1110–1115. doi: 10.1172/JCI115928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang IS, Ho H, Hoffman G, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- Ibarra M, Meneses A, Ransanz V, Castillo C, Hong E. Changes in endothelium-dependent vascular responses associated with spontaneous hypertension and age in rats. Arch Med Res. 1995;26:S177–S183. [PubMed] [Google Scholar]
- Katchalsky A, Sharon N. Kinetics of aldose-amino acid interaction. Biochim Biophys Acta. 1953;10(2):290–301. doi: 10.1016/0006-3002(53)90252-2. [DOI] [PubMed] [Google Scholar]
- Koyama H, Shoji T, Yokoyama H, Motoyama K, Mori K, Fukumoto S, Emoto M, Shoji T, Tamei H, Matsuki H, et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:2587–2593. doi: 10.1161/01.ATV.0000190660.32863.cd. [DOI] [PubMed] [Google Scholar]
- Krieglstein CF, Granger DN. Adhesion molecules and their role in vascular disease. Am J Hypertens. 2001;14(6 Pt 2):44S–54S. doi: 10.1016/s0895-7061(01)02069-6. [DOI] [PubMed] [Google Scholar]
- Küng CF, Lüscher TF. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension. 1995;25:194–200. doi: 10.1161/01.hyp.25.2.194. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delanev V, Friedman EA, Cerami A, Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325:836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Makita Z, Vlassara H, Cerami A, Bucala R. Inmunochemical detection of advanced end products in vivo. J Biol Chem. 1992;267:5133–5138. [PubMed] [Google Scholar]
- Metha D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Cerami A. Nonenzymatic browning in vivo: Possible process for aging of long-lived proteins. Science. 1981;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Nandhini AT, Thirunavukkarasu V, Ravichandran MK, Anuradha CV. Effect of taurine on biomarkers of oxidative stress in tissues of fructose fed insulin-resistant rats. Singapore Med J. 2005;46(2):82–87. [PubMed] [Google Scholar]
- Nava P, Guarner V, Posadas R, Perez I, Baños G. Insulin-induced endothelin release and vasoactivity in hypertriglyceridemic and hypertensive rats. Am J Physiol. 1999;277(46):H399–H404. doi: 10.1152/ajpheart.1999.277.1.H399. [DOI] [PubMed] [Google Scholar]
- Pickavance LC, Tadayyon M, Widdowson PS, Buckingham RE, Wilding JP. Therapeutic index for rosiglitazone in dietary obese rats: Separation of efficacy and haemodilution. Br J Pharmacol. 1999;128(7):1570–1576. doi: 10.1038/sj.bjp.0702932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predescu D, Predescu S, Malik AB. Transport of nitrated albumin across continuous vascular endothelium. Proc Natl Acad Sci. 2002;99(21):13932–13937. doi: 10.1073/pnas.212253499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, Vannucci SJ, Shi Du Yan S, Herold K, Fang Yan S, Schmidt AM. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration and inflammation. Glycobiology. 2005;15(7):16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon (carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34(34):10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- Reynolds TM. Chemistry of nonenzymic browning: I. The Reaction between aldoses and amines. Adv Food Res. 1963;12:1–52. doi: 10.1016/s0065-2628(08)60005-1. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Baños G, Díaz E, Guarner V. Effect of age on insulin-induced endothelin release and vasoreactivity in hypertriglyceridemic and hypertensive rats. Exp Gerontol. 2006;41:282–288. doi: 10.1016/j.exger.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Ruiz Torres A, Melon J, Muñoz FJ. Insulin stimulates collagen síntesis in vascular smooth muscle cells from elderly patients. Gerontology. 1998;44:144–148. doi: 10.1159/000021998. [DOI] [PubMed] [Google Scholar]
- Rumble JR, Cooper ME, Soulis T, Cox A, Wu L, Youssef S, Jasik M, Jerumus G, Gilbert RE. Vascular hypertrophy in experimental diabetes. Role of advanced glycation end products. J Clin Invest. 1997;99:1016–1027. doi: 10.1172/JCI119229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res. 2005;66(2):374–383. doi: 10.1016/j.cardiores.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Tan KCB, Chow WS, Ai VHG, Metz C, Bucala R, Lam KSL. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care. 2002;25:1055–1059. doi: 10.2337/diacare.25.6.1055. [DOI] [PubMed] [Google Scholar]
- Travis J, Bowen J, Twksbury D, Jonson D, Pannell R. Isolation of albumin from whole human plasma and fractionation of albumin-depleted plasma. Biochem J. 1976;157:301–306. doi: 10.1042/bj1570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia JV, Mone M, Koehne C, Rediske J, Hughes TE. Binding receptor for advanced glycation end products (RAGE) ligands is not sufficient to induce inflammatory signals: Lack of activity of endotoxin-free albumin-derived advanced glycation end products. Diabetologia. 2004;47:844–852. doi: 10.1007/s00125-004-1392-9. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Perichon M, Borot-Laloi C, Schaeverbeke J, Bakala H. Accumulation of advanced glycosylation end products in the rat nephron: Link with circulating AGEs during aging. J Histochem Cytochem. 1997;45:1059–1068. doi: 10.1177/002215549704500804. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: A model for diabetic and aging complications. Proc Natl Acad Sci USA. 1992;89:12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wautier JL, Guillausseau PJ. Diabetes, advanced glycation endproducts and vascular disease. Vasc Med. 1998;3:131–137. doi: 10.1177/1358836X9800300207. [DOI] [PubMed] [Google Scholar]
- Xu B, Chibber R, Ruggerio D, Kohner E, Ritter J, Ferro A. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J. 2003;17(10):1289–1291. doi: 10.1096/fj.02-0490fje. [DOI] [PubMed] [Google Scholar]