Abstract
Guanylyl cyclase activating protein (GCAP-1), a Ca2+/Mg2+ sensor protein that accelerates retinal guanylyl cyclase (RetGC) in the light and decelerates it in the dark, is inactive in cation-free form. Binding of Mg2+ in EF-hands 2 and 3 was essential for RetGC activation in the conditions mimicking light adaptation. Mg2+ binding in EF-hand 2 affected the conformation of a neighboring non-metal binding domain, EF-hand-1, and increased GCAP-1 affinity for RetGC nearly 40-fold compared to the metal-free EF-hand 2. Mg2+ binding in EF-hand 3 increased GCAP-1 affinity for RetGC 5-fold and its maximal RetGC stimulation 2-fold. Mg2+ binding in EF-hand 4 affected neither GCAP-1 affinity for RetGC, nor RetGC activation. Inactivation of Ca2+ binding in EF-hand 4 was sufficient to render GCAP-1 a constitutive activator of RetGC, while the EF-hand 3 role in Ca2+-dependent deceleration of RetGC was likely to be through the neighboring EF-hand 4. Inactivation of Ca2+ binding in EF-hand 2 affected cooperativity of RetGC inhibition by Ca2+, but did not prevent the inhibition. We conclude that 1) Mg2+ binding in EF-hands 2 and 3, but not EF-hand 4, is essential for the ability of GCAP-1 to activate RetGC in the light; 2) Mg2+ or Ca2+ binding in EF-hand 3 and especially in EF-hand 2 is required for high-affinity interaction with the cyclase and affects the conformation of the neighboring EF-hand 1, a domain required for targeting RetGC; 3) RetGC inhibition is likely to be primarily caused by Ca2+ binding in EF-hand 4.
Calcium is the major regulator of the physiological responses in photoreceptor cells. Calcium enters outer segments (OS) of vertebrate photoreceptors through cGMP-gated Na+/Ca2+ channels in the OS plasma membrane and is continuously removed from the OS by a lightindependent Na+/K+, Ca2+ exchanger (for review, see (1–4). In the dark, cGMP keeps a small percentage of the Na+/Ca2+ channels open, and the hydrolysis of cGMP by a light-activated phosphodiesterase, PDE6, generates photoresponses in rods and cones. When light triggers cGMP hydrolysis, it also, through the closure of the channels, lowers the intracellular concentration of Ca2+ from near 250 nM in the dark to near 25 nM in the light (5–7). Guanylyl cyclase activating proteins (GCAPs) are Ca2+/Mg2+-sensor proteins that impart Ca2+ sensitivity to retinal guanylyl cyclase (RetGC), the enzyme that supplies the photoreceptor cell with cGMP (8–11). GCAPs become RetGC activators at low Ca2+ concentrations and inhibit it at high Ca2+, such that when the Ca2+ concentration drops upon the illumination, GCAPs activate RetGC to quickly restore the level of cGMP in photoreceptors and thus accelerate their recovery from excitation. Conversely, when RetGC produces enough cGMP to reopen the Na+/Ca2+ channels, Ca2+ re-enters the outer segments and the Ca2+-bound GCAPs decelerate cGMP synthesis.
GCAPs are recoverin-like neuronal calcium binding proteins, also referred to as Neuronal Calcium Sensors family, or NCS, a part of a larger superfamily of EF-hand Ca2+ binding proteins (12–16). Like all other members of that superfamily GCAPs have Ca2+-binding EF-hand domains of helix-loop-helix structure. The metalbinding loop in the NCS proteins is traditionally defined as 12 sequential amino acid residues, of which 6 residues provide the oxygen atoms required for the metal coordination, including the invariant first and the last coordinating residues, Asp and Glu, respectively. The N-terminal EF-hand domains in GCAP-1 and GCAP-2 lack some of the oxygen-containing groups required for coordinating the metal ion (17), but are instead required for their interaction with RetGC (18–20). We previously found that in the conditions that mimic light-adapted or dark-adapted photoreceptors, three other EF-hands in GCAP-1 are predominantly filled by either Mg2+ or Ca2+, respectively (21, 22).
There are multiple reports that substitutions of the first and the last coordinating amino acids inactivate EF-hands in NCS proteins (17, 22–25). Among these are the observations that inactivation of all three metal-binding EF-hands in GCAP-2 by substitution of the last Glu in EF-hand 2 and 3 with Gln and the first Asp in the EF-hand 4 with Asn make GCAP-2 a constitutive, insensitive to Ca2+, activator of RetGC (24). Similar effect was observed for GCAP-1, whose EF-hands were disabled by substitution of the last Glu in the Ca2+-binding loop with Asp (25). Those observations lead to the perception that metal-free GCAPs are the activators of RetGC and that GCAPs undergo transition from Ca2+-bound to metal-free form between dark and light. However, our recent study of metal-binding properties of GCAPs has also indicated that preventing Mg2+ binding in GCAP-1 hampers stimulation of RetGC-1 (22). We concluded from the previous study (22) that, since cation binding was not only required for inhibition of RetGC, but was also critical for RetGC activation under physiological conditions, the general view on the functioning of GCAPs as metal binding proteins in RetGC regulation needed to be revised (5–7, 26). Therefore, the functions of the specific EF-hands in GCAPs must be revisited to provide more adequate understanding of their role in RetGC regulation via Ca2+/Mg2+ exchange. The main purpose of this study was to determine the role of cation binding by EF-hands 2, 3 and 4 in GCAP-1 in creating its “activator” state, such as in the light-adapted photoreceptors.
EXPERIMENTAL PROCEDURES
Recombinant GCAP-1 and its mutants
All mutations were incorporated into bovine GCAP-1 cDNA by PCR using a “splicing by overlap extension” technique (27). Wild type GCAP-1 and its mutants used in this study also carried a D6S substitution that creates a recognition site for the yeast N-myristoyl transferase (28), and does not interfere with the RetGC regulation by the recombinant GCAP-1 (29, 30). GCAP-1 cDNA was expressed under control of the isopropyl-β-D-thiogalactopyranoside-regulated T7 promoter in a BL21(DE3)pLysS E. coli strain (Novagen/Calbiochem) harboring a pBB131 plasmid for a yeast N-myristoyl transferase expression as described (29, 30). Cells were grown in standard LB medium containing 40 µg/ml kanamycin and 100 µg/ml ampicillin. Free myristic acid was added from a concentrated ethanol solution to the suspension of bacterial cells to a final concentration of 100 µg/ml 20 min prior to the induction with 0.5 mM isopropyl-β-D-thiogalactopyranoside. Three hours after the induction, the bacterial pellet was harvested and the recombinant GCAPs were purified as described previously in detail (22). The concentration of GCAP-1 and its mutants was determined in 20 mM Na-phosphate buffer (pH 6.5) containing 6 M guanidine hydrochloride using extinction coefficients at 280 nm computed for the individual mutants from their amino acid composition (31) utilizing the ExPASY proteomic WWW server software from Swiss Institute of Bioinformatics (32).
Tryptophan fluorescence measurements
Fluorescence emission at 332 nm (excitation at 290 nm) was recorded at 23°C using 4 µM GCAP-1 in 0.6 ml of 100 mM MOPS/KOH, pH 7.2, 40 mM KCl and 1 mM EGTA. Small aliquots of concentrated MgCl2 solution were added to obtain the desired free Mg2+ concentrations. The free Ca2+ and Mg2+ concentrations in the solution were calculated according to the method of Brooks and Stoney (33), utilizing the algorithm of Marks and Maxfield (34). The data shown are representative from 3–4 independent experiments producing virtually identical results.
Guanylyl cyclase (GC) assay
Wild type RetGC-1 was expressed in HEK 293 human embryonic kidney cells as previously described (35). The assay mixture (25 µl) contained 30 mM MOPS/KOH, pH 7.2, 60 mM KCl, 4 mM NaCl, 1 mM DTT, 5 mM free Mg2+, 2 mM Ca/EGTA buffer, 0.3 mM ATP, 4 mM cGMP, 1 mM GTP, 1 µCi of [α-32P] GTP, 0.1 µCi of [8- 3H] cGMP, GCAP-1 and HEK 293 cell membranes. The reaction mixture was incubated for 40 min at 30°C, stopped by heating for 2.5 min at 95°C, and the aliquots were analyzed by TLC using fluorescent plastic-backed polyethylenimine cellulose plates (Merck) as described previously (9, 35). The data shown are representative from 3–5 independent experiments producing virtually identical results.
RESULTS
Mutations that inhibit cation binding in EF hands of GCAP-1 and may or may not result in constitutive activation of RetGC
In our previous study we found that disabling all three metal-binding EF-hands in GCAP-1 by mutations that non-selectively hampered both Ca2+ and Mg2+ binding failed to produce a constitutive, Ca2+-insensitive, activation of RetGC-1 (22). That was contrary to what one would expect if the apo form of the GCAP were the “activator” form and argued that the substitution of Ca2+ by Mg2+, rather than merely loss of Ca2+, was required to make GCAP-1 undergo the “inhibitor-to-activator” transition in the light. If this hypothesis were true, then in order to make GCAP-1 a constitutive activator of RetGC it should be made unable to bind Ca2+, yet still retain the ability to bind Mg2+ in its EF-hand(s). We found that the replacement of the last Glu in Ca2+-binding loops with Gln (Fig. 1) prevented binding of Ca2+, but had little effect on Mg2+-binding (22). Predictably, EF(2,3,4)− mutant generated by such a substitution in all three metal-binding EF-hands, GCAP-1(E75Q/E111Q/E155Q), activated RetGC-1 at low Ca2+ and continued to activate it even at high concentrations of Ca2+, which turn wild type GCAP-1 into a RetGC-1 inhibitor (Fig. 2). Contrary to that, a different EF(2,3,4)− mutant, GCAP-1(D64N/D100N/D102G/D144N/D148G), which binds neither Ca2+ nor Mg2+ (22), failed to activate RetGC within the same free Ca2+ range (Fig. 2). Evidently, in order for GCAP-1 to become a RetGC-1 activator under the conditions typical for light-adapted photoreceptors one or more of its EF-hands must be filled with Mg2+.
Fig. 1. EF-hands of bovine GCAP-1 and mutations used in this study.
Mutations introduced in EF-hands of GCAP-1: D64N, D100N/D102G, and D144N/D148G to disable both Ca2+ and Mg2+ binding to EF-hand 2, 3 and 4, respectively; E75Q, E111Q and E155Q to disable only Ca2+-binding to EF-hand 2, 3 and 4, respectively.
Fig. 2. The Ca2+ sensitivity of RetGC-1 regulation by wild type GCAP-1 and its EF(2,3,4)− mutants.
Recombinant RetGC-1 was assayed at various free Ca2+ concentrations in the presence of 5 µM wild type GCAP-1 (○), E75Q/E111Q/E155Q GCAP-1 (■), or D64N/D100N/D102G/D144N/D148G GCAP-1(▲), at saturating 5 mM free Mg2+. The data for wild type GCAP-1 were fitted by the equation, A = (Amax−Amin)/(1 + ([Ca]f/[Ca]1/2)n) + Amin, where A is the activity of RetGC-1, Amax and Amin is the maximal and minimal activity of RetGC in the assay, respectively, [Ca]1/2 is the free Ca2+ concentration required for half-maximal inhibition of RetGC-1 by GCAP, n is the cooperativity coefficient. For other conditions of the assay see EXPERIMENTAL PROCEDURES.
EF-hands 2 and 3 require Mg2+ binding for activation of RetGC under light-adapted conditions
To identify the EF hands that need to bind Mg2+ in order to maintain the activator state of GCAP-1, we disabled Mg2+ binding in the individual EF-hands by point mutations and tested their ability to activate RetGC-1 in the absence of Ca2+ in comparison with the wild type GCAP-1 or EF-hand mutants that retained Mg2+ binding (Fig. 1 and reference (22)).
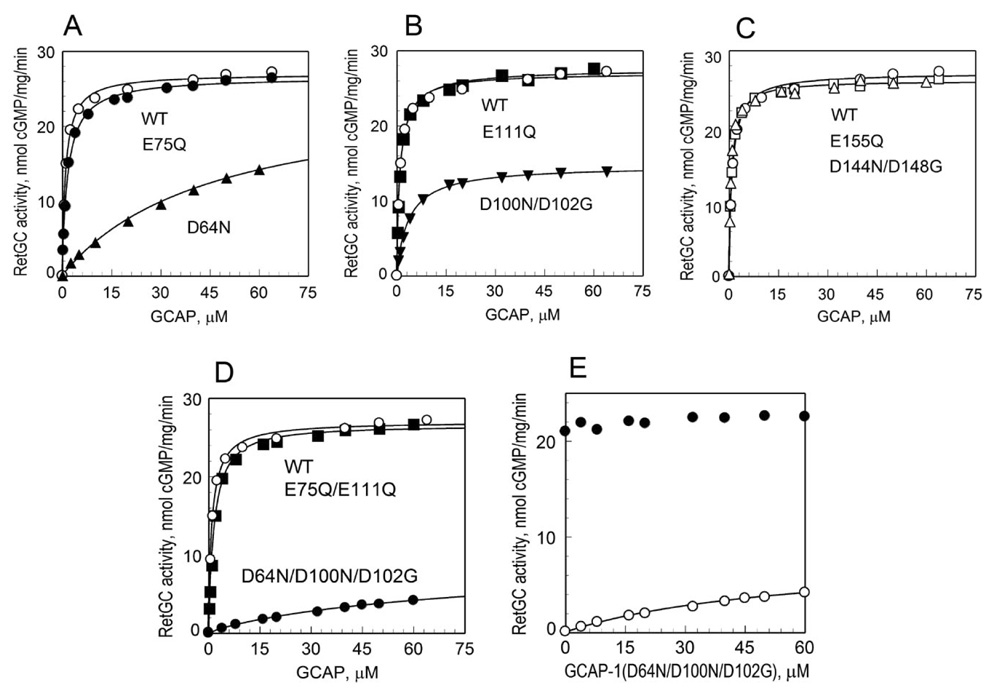
A substitution in EF-hand 2, D64N, that prevents both Ca2+ and Mg2+ binding (22) was compared to the wild type and to the E75Q substitution, which selectively preserves Mg2+ binding (22). While the GCAP-1(E75Q) showed dose dependency (K1/2) and the maximal activity of RetGC-1 stimulated by GCAP-1 (Amax) similar to the wild type, disabling of Mg2+ binding dramatically reduced the apparent affinity of GCAP-1(D64N) for RetGC-1, but had little effect on the Amax (Fig. 3A, Table 1).
Fig. 3. The effect of various substitutions in GCAP-1 EF-hands on RetGC-1 activation.
Mutations that either prevented or preserved Mg2+ binding were introduced in the individual EF-hands of GCAP-1 and the activity of RetGC-1 was measured in the presence of 1 mM EGTA, 5 mM free Mg2+, and increasing concentrations of GCAP-1 as described in EXPERIMENTAL PROCEDURES. A, Comparison of EF(2)− mutants: (○), wild type; (●), E75Q; (▲), D64N B, Comparison of EF(3)− mutants: (○), wild type; (■), E111Q; (▼), D100N/D102G C, Comparison of EF(4)− mutants: (○), wild type; (□), D144N/D148G; (△), E155Q. D, Comparison of EF(2,3)− mutants: (○), wild type; (■), E75Q/E111Q; (●), D64N/D100N/D102G. E, Competition between wild type GCAP-1 and its EF(2,3)− mutant, D64N/D100N/D102G. Increasing concentrations of the D64N/D100N/D102G mutant were added into GC assay mixture, alone (○) or in the presence of 3 µM wild type GCAP-1 (●). The assay mixture contained 1 mM EGTA and 5 mM free Mg2+. The data were fitted by the equation, A = Amax [GCAP]/(K1/2 + [GCAP]), where A is the activity of RetGC-1 in the assay, Amax is the maximal activity of RetGC, [GCAP] is the concentration of GCAP, K1/2 is the concentration of GCAP required for half-maximal activation of RetGC-1. The values of Amax and K1/2 are summarized in Table 1.
Table 1.
Activation of RetGC-1 by EF-hand mutants of GCAP-1
| Mutants | Amaxa nmol cGMP/mg/min | K1/2b µM | |
|---|---|---|---|
| Wild type | 24.1 ± 2.5 (N=3) | 1.17 ± 0.1 (N=3) | |
| EF(2)− | D64N | 25.6 ± 4.2 (N=3) | 58 ± 17 (N=3) |
| E75Q | 25.3 ± 2.5 (N=3) | 1.56 ± 1.5 (N=3) | |
| EF(3)− | D100N/D102G | 12.6 ± 2.4 (N=4) | 5.0 ± 1.5 (N=4) |
| E111Q | 26.8 ± 0.7 (N=3) | 1.05 ± 0.04 (N=3) | |
| EF(4)− | D144N/D148G | 25.6 ± 1.1 (N=3) | 1.3 ± 0.7 (N=3) |
| E155Q | 25 ± 0.3 (N=3) | 0.6 ± 0.1 (N=3) | |
| EF(2,3)− | D64N/D100N/D102G | 7.5 ± 0.6 (N=3) | 65 ± 22 (N=3) |
| E75Q/E111Q | 26.2 ± 1.9 (N=3) | 1.5 ± 0.25 (N=3) | |
Amax represents the maximal level of RetGC-1 activation by GCAP at low free Ca2+ in the presence of 5 mM free Mg2+ (mean ± S.D.)
K1/2 represents the concentration of GCAP required for half-maximal activation of RetGC-1 (mean ± S.D., N is the number of independent measurements)
Disabling of Mg2+-binding in EF-hand 3 by a double mutation, D100N/D102G (22), noticeably reduced both the apparent affinity of GCAP-1 for RetGC-1 and its maximal activation. Opposite to that, a substitution that preserves Mg2+ binding, E111Q, affected neither Amax nor K1/2 (Fig. 3B, Table 1).
Unlike EF-hands 2 or 3, disabling Mg2+-binding in EF-hand 4 by a double substitution, D144N/D148G (22), had no effect on Amax or K1/2 (Fig. 3C, Table 1). These data strongly indicate that among the three metal-binding EF-hands of GCAP-1 binding of Mg2+ in EF-hands 2 and 3 is important for RetGC-1 activation while Mg2+ in EF-hand 4 does not significantly contribute to creating the activator state of GCAP-1.
Indeed, simultaneous disabling of Mg2+-binding in both EF-hands 2 and 3 in GCAP-1(D64N/D100N/D102G) diminished its Amax and K1/2 even stronger than in the individually disabled EF-hands (Fig. 3D, Table 1). Unlike that, a different EF(2,3)− mutant, E75Q/E111Q, that retained Mg2+ binding in EF-hands 2 and 3 (22) demonstrated Amax and K1/2 similar to the wild type (Fig. 3D, Table 1). However, since the value for K1/2 was difficult to determine more accurately by on due to the low activity of the mutant, we additionally verified that the GCAP-1(D64N/D100N/D102G) lost its ability to interact with RetGC-1 in a competition experiment, where RetGC-1 was activated by 3 µM wild type GCAP-1 at low free [Ca2+] in the presence of increasing concentrations of the GCAP-1(D64N/D100N/D102G) (Fig. 3E). Even at 20-fold excess of the GCAP-1(D64N/D100N/D102G) over the wild type we find no evidence for its interference with the activation of RetGC-1. We therefore conclude that once GCAP-1 is lacking Mg2+ binding in both EF-hands 2 and 3, it becomes unable to properly interact with RetGC-1.
Mg2+ binding in EF-hand 2 affects conformation of EF-hand-like domain 1
Although both EF-hands 2 and 3 are involved in activation of RetGC-1 at low free Ca2+, it appeared that Mg2+ binding in the EF-hand 2 was the most critical for GCAP-1 affinity for the cyclase. There is a significant conformational difference between the Mg2+-bound and metal-free GCAP-1 (21, 22). In particular, binding of Mg2+ by EF-hands 2 and 3 causes structural changes in EF1, an EF-hand-like domain that cannot bind metal ion but is required for the interaction of GCAPs with RetGC (18–20). These changes can be monitored by a change in fluorescence of Trp21 located in the middle of the GCAP-1 EF1 domain (22). Since in addition to the Trp21 GCAP-1 contains two more Trp residues, Trp51 and Trp94, we replaced them with Phe and used the resultant GCAP-1(W51F/W94F) mutant (22) as a template for creating two other mutants, GCAP-1(D64N/W51F/W94F) and GCAP-1(E75Q/W51F/W94F), both with additional mutations affecting EF-hand 2. Each contained a single Trp21 residue, but only the latter mutant was able to bind Mg2+ in EF-hand 2. The GCAP-1(W51F/W94F) exhibited prominent Mg2+-dependent decrease in Trp21 fluorescence caused by conformational changes in the EF1 domain (Fig. 4 and Ref. (22)). Similar results were observed with the GCAP-1(E75Q/W51F/W94F), whose EF-hand 2 was modified by the E75Q mutation that preserved Mg2+-binding (Fig. 4). Contrary to that, disabling Mg2+-binding in EF-hand 2 by the D64N substitution in the GCAP-1(D64N/W51F/W94F) prevented the decrease in the Trp21 fluorescence. These results suggest that Mg2+ binding in EF-hand 2 creates proper conformation of the neighboring EF-hand-like domain, known to be required for GCAPs interaction with RetGC-1 (18, 19). The exact reason why Trp21 fluorescence does not stay completely flat, but slightly increases in the D64N mutant is unknown, but one possible explanation can be that when EF-hand 2 is in its apo (nonphysiological) form, cation binding to EF-hands 3 and/or 4, through conformational change in the rest of the molecule, affects the environment for Trp21 and thus produces the small increase in fluorescence. It is therefore only possible to see this effect when the EF-2 is inactivated and the rest of the Trp residues in the molecule are removed. In the presence of the functional EF-hand 2 the influence from the rest of the molecule on the environment of Trp21 is strongly opposed by the cation binding in the neighboring EF-hand 2 that results in decrease of fluorescence.
Fig. 4. Effect of disabling of Mg2+-binding in EF-hand 2 on the single Trp21 fluorescence in EF-hand 1.
Mutations, E75Q (▲), or D64N (●), were introduced to inactivate EF-hand 2 in a GCAP-1 mutant, W51F/W94F (□), that has a single remaining Trp21 residue (22). The fluorescence of the Trp21 was recorded as a function of Mg2+ in the presence of 1 mM EGTA.
Ca2+ binding and inhibition of RetGC
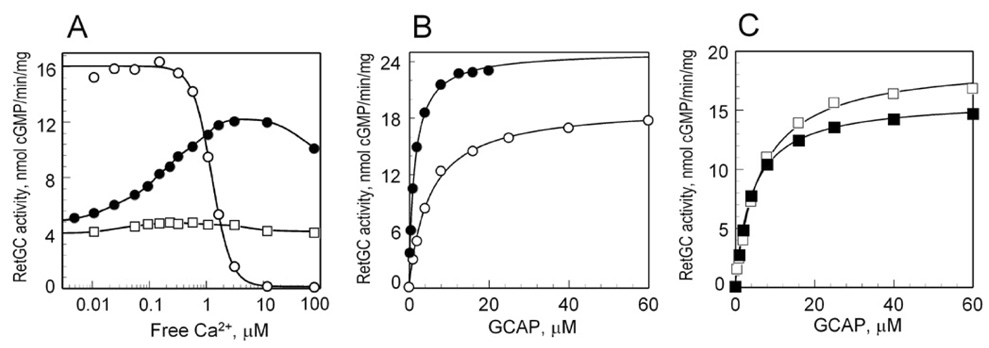
In photoreceptor cells, GCAP-1 undergoes transition between the Mg2+- and the Ca2+-bound forms as the concentration of intracellular Ca2+ changes between light and dark (21, 22), a process that turns GCAP-1 from RetGC activator into RetGC inhibitor. The replacement of Mg2+ by Ca2+ occurs in all three EF-hands (22), however, it is still unclear how individual EF-hands of GCAP-1 contribute to its transition to RetGC inhibitor form. Another group previously reported that disabling of Ca2+ binding in EF-hands 3 and 4 by substitution of the last Glu with Asp in the 12-amino acid Ca2+-binding loop converted GCAP-1 into a constitutive activator of RetGC (25). That mutant showed a similar to the wild type GCAP-1 dose dependency of RetGC stimulation at low free Ca2+ but did not inhibit cyclase at high free Ca2+ (25). However, the mutations used in ref. (25) do not disable binding of Mg2+ (22, 36–38). Therefore, to revisit this question, we used an EF(3,4)− mutant with EF-hands 3 and 4 disabled by different mutations, D100N/D102G and D144N/D148G, respectively. These mutations prevented both Mg2+ and Ca2+ binding to EF-hands 3 and 4, but preserved high-affinity Ca2+ and Mg2+ binding in EF-hand 2 (22). At low free Ca2+ the D100N/D102G/D144N/D148G EF(3,4)− mutant was able to activate RetGC-1, but much less efficiently than wild type GCAP-1 (Fig. 6 and Table 2). Surprisingly, the Ca2+ sensitivity of RetGC-1 regulation by this mutant was reversed: increase in Ca2+ concentrations further increased RetGC-1 stimulation (Fig. 5A), instead of inhibiting it. Such increase could only result from Ca2+ binding in EF-hand 2, because EF-hands 3 and 4 in this mutant do not bind Ca2+, even at the highest concentration used in the assay (22). Its apparent affinity for RetGC-1 was also reduced at low Ca2+ concentrations but increased with the rise of free Ca2+ in the assay (Table 2).
Fig. 6. Effect of individual EF-hands inactivation in GCAP-1 on Ca2+ sensitivity of RetGC-1 regulation.
RetGC activity was measured as a function of free Ca2+ concentrations at 5 mM free Mg2+ in the presence of 2 µM wild type GCAP-1, (○); E75Q, (●); E111Q, (■); E155Q, (□); D144N/D148G, (▼) or E111Q/D144N/D148G (△). The data for wild type GCAP-1 and E75Q mutant were fitted by the equation, A = (Amax−Amin)/(1 + ([Ca]f/[Ca]1/2)n) + Amin, where A is the activity of RetGC-1, Amax and Amin is the maximal and minimal activity of RetGC in the assay, respectively, [Ca]1/2 is the free Ca2+ concentration required for half-maximal inhibition of RetGC-1 by GCAP, n is the Hill coefficient. For other conditions of the assay see EXPERIMENTAL PROCEDURES.
Table 2.
Effect of Ca2+ binding in EF hand 2 on activation of RetGC-1 by EF(3,4)− mutant
| Mutantsa | Amaxb nmol cGMP/mg/min | K1/2c µM |
|---|---|---|
| GCAP-1 EF(3,4)− in EGTA | 16 ± 3.6 (N=4) | 4.4 ± 0.8 (N=4) |
| GCAP-1 EF(3,4)− at 10 µM [Ca]f | 22 ± 3.5 (N=3) | 1.2 ± 0.3 (N=3) |
| GCAP-1 E75Q/EF(3,4)− in EGTA | 18 ± 1.0 (N=3) | 6.3 ± 0.3 (N=3) |
| GCAP-1 E75Q/EF(3,4)− at 10 µM [Ca]f | 15 ± 1.0 (N=3) | 4.2 ± 0.3 (N=3) |
GCAP-1 EF(3,4)− and GCAP-1 E75Q/EF(3,4)− represent GCAP-1(D100N/D102G/D144N/D148G) and GCAP-1(E75Q/D100N/D102G/D144N/D148G), respectively.
Amax, the maximal level of RetGC-1 activation by GCAP at indicated free Ca2+ in the presence of 5 mM free Mg2+ (mean ± S.D., N is the number of independent measurements)
K1/2, the concentration of GCAP required for half-maximal activation of RetGC-1 (mean ± S.D., N is the number of independent measurements)
Fig. 5. Effect of EF-hand 2 transition between its Mg2+- and Ca2+-bound state on RetGC-1 activation.
A, Recombinant RetGC-1 was assayed for GC activity at 5 mM free Mg2+ and variable free Ca2+ concentrations in the presence of 2 µM GCAP-1: (○), wild type; (●), D100N/D102G/D144N/D148G; (□), E75Q/D100N/D102G/D144N/D148G. B, C, Dose-dependence of RetGC-1 activation by GCAP-1(D100N/D102G/D144N/D148G) (B) or GCAP-1(E75Q/D100N/D102G/D144N/D148G) (C) in the presence of 1 mM EGTA (open symbols) or 10 µM free Ca2+ (filled symbols). The concentration of free Mg2+ in the GC assay was 5 mM. The data were fitted as described in the legend to Fig. 3. The values of Amax and K1/2 are summarized in Table 2.
The EF(2,3,4)− mutant, E75Q/D100N/D102G/D144N/D148G, activated RetGC-1 in the absence of Ca2+ similarly to the GCAP-1 EF(3,4)− mutant, D100N/D102G/D144N/D148G, because the E75Q mutation preserves Mg2+-binding in EF-hand 2 (22). However it did not regulate RetGC-1 in a Ca2+-sensitive manner (Fig. 5A), because none of its EF-hands could now bind Ca2+ (22). The concentration of free Mg2+ in the assay was 5 mM, which saturates binding to EF-hand 2 in wild type GCAP-1 and in the EF(2,3,4)− mutant, E75Q/D100N/D102G/D144N/D148G (22). The transition of the EF-hand 2 from the Mg2+-bound to the Ca2+-bound form substantially increased the apparent affinity of the GCAP-1(D100N/D102G/D144N/D148G) for RetGC-1 (Table 2). The GCAP-1(E75Q/D100N/D102G/D144N/D148G) that could not exchange Mg2+ in EF-hand 2 for Ca2+ exhibited almost no change in its apparent affinity for RetGC-1 between the low and the high free Ca2+ (Fig. 5 and Table 2). Thus, in this artificial situation, when cation binding in EF-3 and EF-4 is completely disabled, the transition of EF-hand 2 from the Mg2+-bound to the Ca2+-bound form does not make GCAP-1 a RetGC-1 inhibitor and can even mimic RetGC-1 activator conformation.
Consistent with the earlier observations (25), this finding argues that EF-hand 2 is not essential for the Ca2+-dependent inhibition of RetGC-1 by GCAP-1. Indeed, inactivation of EF-hand 2 by a mutation, E75Q, did not make GCAP-1 a constitutive activator of RetGC-1 (Fig. 6 and Ref. (25)). However, unlike the total lack of effect in the ref. (25), we also found that disabling of Ca2+-binding in EF-hand 2 by the E75Q mutation in the conditions of saturation by Mg2+ to some extent affected both the cooperativity (n decreased from 2.34 ± 0.1 (N=5) in wild type to 1.7 ± 0.06 (N=3) in E75Q) and Ca2+-sensitivity of RetGC-1 regulation by GCAP-1 (Fig. 6). One possible explanation for the difference between the two observations is that the E75Q mutation in EF-hand 2 blocks Ca2+ binding more efficiently than the E75D, as we showed previously (22).
Contrary to the EF-hand 2, inactivation of both EF-hand 3 and 4 did make GCAP-1 a constitutive activator of RetGC-1, and so did the inactivation of the individual EF-hands 3 or 4 (Fig. 6 and Ref. (25)). Yet, at this point we cannot determine the direct contribution of EF-hand 3 in RetGC-1 inhibition by GCAP-1. Although our present findings do not exclude that this indeed maybe the case, we found that disabling of EF-hand 3 by various mutations always dramatically decreased the affinity of a wild-type EF-hand 4 for Ca2+ (22). In other words, we were unable to find a mutation that would disable Ca2+ binding in EF-hand 3 without also hampering Ca2+ binding in EF-hand 4 at the same time. In addition to that, the results shown in Fig. 6 can argue that Ca2+ binding in EF-hand 3 itself has relatively small additional effect when compared to the EF-hand 4. Hence, even in its Ca2+-bound form EF-hand 3 mostly preserves the activator form of the GCAP-1 rather than provides a conformational switch for RetGC inhibition. Therefore, it is more likely that the main role of EF-hand 3 in Ca2+-dependent inhibition of RetGC can be indirect, through regulation of Ca2+ binding to the neighboring EF-hand 4.
DISCUSSION
The role of EF-hands in RetGC regulation by GCAP-1
GCAP-1 contains four EF-hand structures of which three can bind either Ca2+ or Mg2+ under physiologically relevant conditions (Fig. 7A). We find in this study that both EF-hands 2 and 3 in GCAP-1 must be occupied by Mg2+ in order to maintain GCAP-1 in its RetGC activator state under the conditions that exist in photoreceptors in the light (Fig. 3, Table 1). The apo EF-hand 2 and 3 do not support the GCAP-1 conformation required for the interaction with the cyclase (Fig. 3). We consider one of the most important findings of this study that the EF-hand 2, whose role in RetGC regulation was previously deemed unclear (25), is in fact a crucial element in RetGC regulation by GCAP-1, and the cation binding in EF-hand 2 is required for high-affinity interaction with RetGC (Fig. 3 and 6 ;Table 1 and 2). Another EF-hand that cannot effectively maintain the activator conformation of GCAP-1 in its apo form is EF-hand 3. It also needs to be in a cation-bound form to enhance GCAP-1/RetGC-1 affinity, however, unlike EF-hand 2, the Mg2+ binding in EF-hand 3 is also important to keep the maximal level of RetGC stimulation (Fig. 3, Table 1).
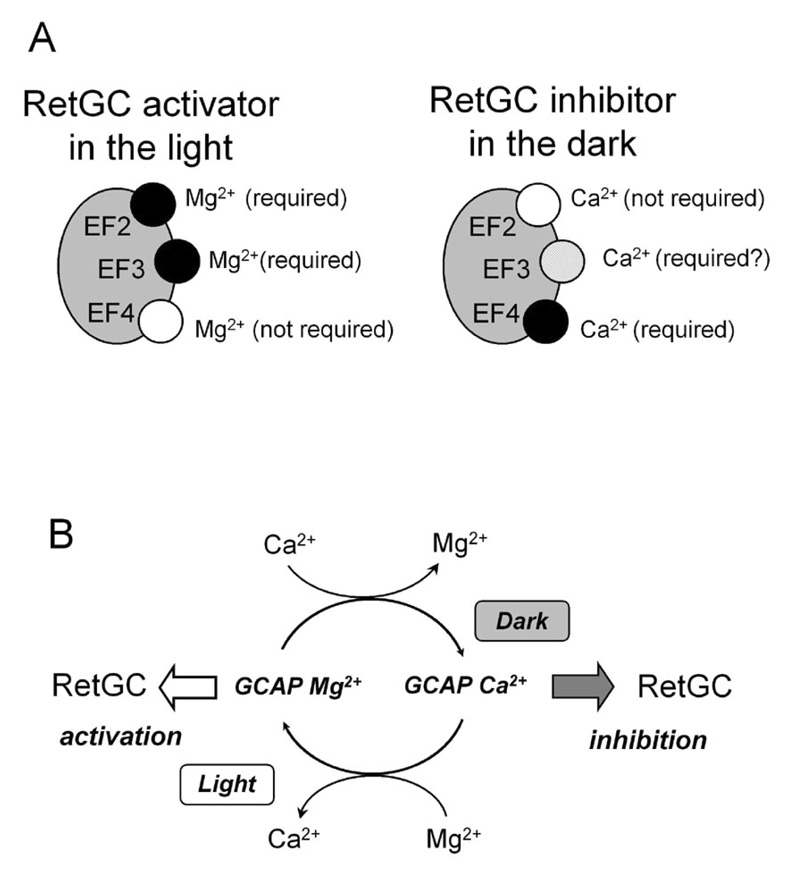
Fig. 7.
A, In light-adapted conditions, all three metal-binding EF-hands in GCAP-1 are occupied by Mg2+, however, only Mg2+ binding in EF-hands 2 and 3 is required for RetGC stimulation. The apo forms of these EF-hands do not create the proper conformation for GCAP-1. In the dark, all three EF-hands of GCAP-1 are predominantly occupied by Ca2+, however, the main requirement for converting GCAP-1 into the “RetGC inhibitor” is binding of Ca2+ in EF-hand 4. Ca2+ binding in EF-hands 2 and 3 is primarily required for GCAP-1 to preserve binding to RetGC and to facilitate the high-affinity binding of Ca2+ to EF-hand 4. B. Ca2+/Mg2+ exchange in EF-hands of GCAP-1 between light and dark provides functional switch between its “RetGC activator” and “RetGC inhibitor” states. Apo form of GCAP-1 has no function. Other explanations are in DISCUSSION.
Two sites for binding of GCAP to RetGC may be required to explain why prevention of Mg2+ binding in two different EF-hands can produce the differential decrease in Amax versus the increase of the apparent Km shown in Fig. 3 and Table 1 and Table 2. The properties of the mutants suggest that at least two types of interaction between RetGC-1 and GCAP-1 occur: a high-affinity “docking” of GCAP, supported by cation binding in the EF-hand 2 (and partially EF-hand 3), and binding at a secondary site in GCAP-1, regulated by the EF-hand 3. Hence, the lack of Mg2+ binding in EF-hand 2 drastically reduces the affinity for RetGC, while the lack of Mg2+ in EF-hand 3 produces smaller effect on the affinity, but substantially reduces the Amax of the cyclase.
Unlike other metal-binding EF-hands in GCAP-1, the EF-hand 4 does not contribute to the activation of the cyclase and is only required for switching RetGC back to the inhibited state upon increase in free Ca2+ in the dark. It is also important to note that both EF-hand 2 and EF-hand 3 retain the activator conformation of GCAP-1 when the inhibitory effect of Ca2+ binding EF-hand 4 is blocked (Fig. 6). Although such situation cannot be found in normal physiological condition, where only Mg2+-conformation exists in the light, and only Ca2+ conformation inhibiting RetGC via EF-hand 4 is present in the dark, under certain pathological conditions the ability of EF-hands 2 and 3 to efficiently maintain the active conformation of GCAP-1 even in Ca2+ bound form contributes to the abnormal activity of the cyclase in the dark found in association with congenital blindness (39–41). Apparently, blocking Mg2+ binding in EF-hand 3 can also create an artificial situation when the replacement of Mg2+ in the EF-hand 2 by Ca2+ mutant can partially compensate for the abnormal conformation of an apo form of EF-hand 3 (Fig. 5).
The role of cation binding in EF-hand 3 can be two-fold: i) to maintain the activator conformation of the GCAP-1 when filled with Mg2+; ii) to contribute to the inactivation of the cyclase when bound with Ca2+. The former role is supported by the results presented in this study, and the latter was also proposed previously by another group (25). While both roles are possible, we find that it is difficult to verify the direct contribution of the EF-hand 3 to inactivation of the cyclase, because a direct measurement of Ca2+ binding in EF-hand 4 showed that it was always suppressed when Ca2+ binding in the EF-hand 3 was affected (but not vice versa). Therefore, it is likely that the EF-hand 3 contributes to switching off the cyclase primarily through its indirect effect on the affinity of the EF-hand 4 for Ca2+, rather than in directly providing a conformational switch for RetGC inhibition. This would also suggest that Ca2+ binding in the EF-3/EF-4 globular domain occurs sequentially, first in EF-hand 3 and only after that in EF-hand 4.
The EF-hand domain 1, which cannot itself coordinate metal, has been shown to be crucial for GCAP-1 and GCAP-2 interaction with RetGC (18–20, 30, 42) and in some other NCS proteins for interaction with their targets (43–45). We found a striking effect of EF-hand 2 occupation by Mg2+ on the conformation of the EF-hand 1 domain, revealed by its fluorescence spectra (Fig. 4). It is therefore tempting to speculate that the EF-hand domain 1 is not only directly involved in high-affinity binding to RetGC, but also that the high-affinity binding is directly controlled via the conformation of its neighboring metal-binding EF-hand 2.
To summarize, based on this and the number of previous studies (19, 20, 30, 42), we can propose the following functions to the EF-hands in GCAP-1 (Fig. 7A):
EF-hand-like domain 1: No metal binding, contributes to the high-affinity interaction with RetGC.
EF-hand 2: Binding of Mg2+ in the light maintains the activator conformation of GCAP-1, controls the high-affinity binding of GCAP-1 to RetGC-1, presumably through involving EF-hand-like domain 1; it is not essential for the maximal level of stimulating activity of GCAP-1; replacement of Mg2+ by Ca2+ in this EF-hand has only small effect on inhibition of RetGC.
EF-hand 3: Binding of Mg2+ in the light maintains the optimal conformation of GCAP-1 for RetGC activation and, although to a lesser extent, contributes to the high-affinity GCAP/RetGC interaction; binding of Ca2+ fails to create the in inhibitory conformation of GCAP-1, but most likely contributes to the inactivation of the cyclase indirectly, by facilitating Ca2+-binding in the neighboring EF-hand 4;
EF-hand 4: Binding of Mg2+ in this EF-hand contributes to neither binding nor activation of RetGC in the light; binding of Ca2+ in this EF-hand provides a potent functional switch to turn the cyclase off.
Mg2+/Ca2+ cycle in GCAP-1 controls RetGC-1 regulation
Free Ca2+ concentrations in rods and cones change nearly 10-fold in response to illumination, in mammals - between 250 nM in the dark and 25 nM in the light (5–7). This provides a potent feedback for RetGC that accelerates the recovery of rods and cones (reviewed in (1, 4, 46)). Contrary to the previous view on GCAPs role in RetGC regulation via release and binding of Ca2+ to the apo form of GCAPs, we now argue that the actual process of switching the GCAP-1 between the “activator” and the “inhibitor” state is instead based on Mg2+/Ca2+ exchange, summarized in the schematics presented in Fig. 7B. The apo form of GCAP-1 is neither activator nor inhibitor of RetGC-1 and has no physiological role. Much like GDP/GTP exchange is required to regulate the conformation of a G-protein, the cation exchange in GCAP-1 is required to regulate RetGC-1. The fundamental differences from the GDP/GTP exchange in G-proteins is nevertheless apparent: no external receptor is required for GCAP-1 to release or bind the divalent cations, and this can be accomplished solely as a result of the free Ca2+ concentration change relative to the free Mg2+ concentrations in the dark versus light.
The critical difference between the activator and the inhibitor conformation of GCAP-1 must be very subtle compared, for example, with that of recoverin (49–51). This is not a difference between the apo protein and the metal bound form, but between the two metal-bound forms, Mg2+ versus Ca2+. Moreover, the EF-hands 1, 2 and 3 are even less likely to undergo major change between Mg2+ and Ca2+ form, since EF-2 and EF-3 are both capable of maintaining the high-affinity interaction with the cyclase in either Mg2+ or Ca2+ form. To date, only partial Ca2+-bound structure of two GCAPs was established (47, 48), but in order to properly understand the mechanism and the structural basis for the RetGC regulation, one would need to compare, potentially rather subtle, differences between Mg2+- and Ca2+-bound GCAP-1.
Footnotes
This work was supported by grant from the National Institutes of Health, EY11522 and by the Pennsylvania Lions Sight Conservation and Eye Research Foundation. A.M. Dizhoor is Martin and Florence Hafter Professor of Pharmacology.
The abbreviations used are: GCAP, guanylyl cyclase activating protein; RetGC, photoreceptor membrane guanylyl cyclase; EGTA, ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid; MOPS, 4-morpholinopropanesulfonic acid; OS-photoreceptor outer segments.
REFERENCES
- 1.Pugh EN, Jr, Nikonov S, Lamb TD. Curr. Opin. Neurobiol. 1999;9:410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]
- 2.Arshavsky VY, Lamb TD, Pugh EN., Jr Annu. Rev. Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 3.Olshevskaya EV, Ermilov AN, Dizhoor AM. Mol. Cell. Biochem. 2002;230:139–147. [PubMed] [Google Scholar]
- 4.Burns ME, Baylor DA. Annu. Rev. Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- 5.Olshevskaya EV, Calvert PD, Woodruff ML, Peshenko IV, Savchenko AB, Makino CL, Ho YS, Fain GL, Dizhoor AM. J. Neurosci. 2004;24:6078–6085. doi: 10.1523/JNEUROSCI.0963-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL. J. Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Nat. Genet. 2003;35:158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 8.Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 9.Dizhoor AM, Olshevskaya EV, Henzel WJ, Wong SC, Stults JT, Ankoudinova I, Hurley JB. J. Biol. Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- 10.Palczewski K, Subbaraya I, Gorczyca WA, Helekar BS, Ruiz CC, Ohguro H, Huang J, Zhao X, Crabb JW, Johnson RS, Walsh KA, Gray-Keller MP, Detwiller PB, Baehr W. Neuron. 1994;13:395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 11.Gorczyca WA, Gray-Keller MP, Detwiler PB, Palczewski K. Proc. Natl. Acad. Sci. U S A. 1994;91:4014–4018. doi: 10.1073/pnas.91.9.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palczewski K, Polans AS, Baehr W, Ames JB. Bioessays. 2000;22:337–350. doi: 10.1002/(SICI)1521-1878(200004)22:4<337::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Haeseleer F, Imanishi Y, Sokal I, Filipek S, Palczewski K. Biochem. Biophys. Res. Commun. 2002;290:615–623. doi: 10.1006/bbrc.2001.6228. [DOI] [PubMed] [Google Scholar]
- 14.Burgoyne RD, Weiss JL. Biochem. J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- 15.Lewit-Bentley A, Rety S. Curr. Opin. Struct. Biol. 2000;10:637–643. doi: 10.1016/s0959-440x(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 16.Burgoyne RD, O'Callaghan DW, Hasdemir B, Haynes LP, Tepikin AV. Trends Neurosci. 2004;27:203–209. doi: 10.1016/j.tins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Babu A, Su H, Ryu Y, Gulati J. J. Biol. Chem. 1992;267:15469–15474. [PubMed] [Google Scholar]
- 18.Ermilov AN, Olshevskaya EV, Dizhoor AM. J. Biol. Chem. 2001;276:48143–48148. doi: 10.1074/jbc.M107539200. [DOI] [PubMed] [Google Scholar]
- 19.Hwang JY, Schlesinger R, Koch KW. Eur. J. Biochem. 2004;271:3785–3793. doi: 10.1111/j.1432-1033.2004.04320.x. [DOI] [PubMed] [Google Scholar]
- 20.Sokal I, Li N, Klug CS, Filipek S, Hubbell WL, Baehr W, Palczewski K. J. Biol. Chem. 2001;276:43361–43373. doi: 10.1074/jbc.M103614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peshenko IV, Dizhoor AM. J. Biol. Chem. 2004;279:16903–16906. doi: 10.1074/jbc.C400065200. [DOI] [PubMed] [Google Scholar]
- 22.Peshenko IV, Dizhoor AM. J. Biol. Chem. 2006;281:23830–23841. doi: 10.1074/jbc.M600257200. [DOI] [PubMed] [Google Scholar]
- 23.Strynadka NC, James MN. Annu. Rev. Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- 24.Dizhoor AM, Hurley JB. J. Biol. Chem. 1996;271:19346–19350. doi: 10.1074/jbc.271.32.19346. [DOI] [PubMed] [Google Scholar]
- 25.Rudnicka-Nawrot M, Surgucheva I, Hulmes JD, Haeseleer F, Sokal I, Crabb JW, Baehr W, Palczewski K. Biochemistry. 1998;37:248–257. doi: 10.1021/bi972306x. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Nakatani K, Koutalos Y. J. Physiol. 2003;553:125–135. doi: 10.1113/jphysiol.2003.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton RM, Pease LR. McPherson MJ. Directed Mutagenesis: Practical Approach. 1991. pp. 217–250. [Google Scholar]
- 28.Duronio RJ, Rudnick DA, Adams SP, Towler DA, Gordon JI. J. Biol. Chem. 1991;266:10498–10504. [PubMed] [Google Scholar]
- 29.Dizhoor AM, Boikov SG, Olshevskaya EV. J. Biol. Chem. 1998;273:17311–17314. doi: 10.1074/jbc.273.28.17311. [DOI] [PubMed] [Google Scholar]
- 30.Krylov DM, Niemi GA, Dizhoor AM, Hurley JB. J. Biol. Chem. 1999;274:10833–10839. doi: 10.1074/jbc.274.16.10833. [DOI] [PubMed] [Google Scholar]
- 31.Gill SC, von Hippel PH. Anal. Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 32.Appel RD, Bairoch A, Hochstrasser DF. Trends Biochem. Sci. 1994;19:258–260. doi: 10.1016/0968-0004(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 33.Brooks SP, Storey KB. Anal. Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- 34.Marks PW, Maxfield FR. Anal. Biochem. 1991;193:61–71. doi: 10.1016/0003-2697(91)90044-t. [DOI] [PubMed] [Google Scholar]
- 35.Peshenko IV, Moiseyev GP, Olshevskaya EV, Dizhoor AM. Biochemistry. 2004;43:13796–13804. doi: 10.1021/bi048943m. [DOI] [PubMed] [Google Scholar]
- 36.Allouche D, Parello J, Sanejouand YH. J. Mol. Biol. 1999;285:857–873. doi: 10.1006/jmbi.1998.2329. [DOI] [PubMed] [Google Scholar]
- 37.Cates MS, Teodoro ML, Phillips GN., Jr Biophys. J. 2002;82:1133–1146. doi: 10.1016/S0006-3495(02)75472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Silva AC, Kendrick-Jones J, Reinach FC. J. Biol. Chem. 1995;270:6773–6778. doi: 10.1074/jbc.270.12.6773. [DOI] [PubMed] [Google Scholar]
- 39.Wilkie SE, Li Y, Deery EC, Newbold RJ, Garibaldi D, Bateman JB, Zhang H, Lin W, Zack DJ, Bhattacharya SS, Warren MJ, Hunt DM, Zhang K. Am. J. Hum. Genet. 2001;69:471–480. doi: 10.1086/323265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sokal I, Dupps WJ, Grassi MA, Brown J, Jr, Affatigato LM, Roychowdhury N, Yang L, Filipek S, Palczewski K, Stone EM, Baehr W. Invest. Ophthalmol. Vis. Sci. 2005;46:1124–1132. doi: 10.1167/iovs.04-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiguchi KM, Sokal I, Yang L, Roychowdhury N, Palczewski K, Berson EL, Dryja TP, Baehr W. Invest. Ophthalmol. Vis. Sci. 2004;45:3863–3870. doi: 10.1167/iovs.04-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otto-Bruc A, Buczylko J, Surgucheva I, Subbaraya I, Rudnicka-Nawrot M, Crabb JW, Arendt A, Hargrave PA, Baehr W, Palczewski K. Biochemistry. 1997;36:4295–4302. doi: 10.1021/bi963000d. [DOI] [PubMed] [Google Scholar]
- 43.Tachibanaki S, Nanda K, Sasaki K, Ozaki K, Kawamura S. J. Biol. Chem. 2000;275:3313–3319. doi: 10.1074/jbc.275.5.3313. [DOI] [PubMed] [Google Scholar]
- 44.Zhou W, Qian Y, Kunjilwar K, Pfaffinger PJ, Choe S. Neuron. 2004;41:573–586. doi: 10.1016/s0896-6273(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 45.Ames JB, Levay K, Wingard JN, Lusin JD, Slepak VZ. J. Biol. Chem. 2006;281:37237–37245. doi: 10.1074/jbc.M606913200. [DOI] [PubMed] [Google Scholar]
- 46.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Physiol. Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 47.Stephen R, Palczewski K, Sousa MC. J. Mol. Biol. 2006;359:266–275. doi: 10.1016/j.jmb.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ames JB, Dizhoor AM, Ikura M, Palczewski K, Stryer L. J. Biol. Chem. 1999;274:19329–19337. doi: 10.1074/jbc.274.27.19329. [DOI] [PubMed] [Google Scholar]
- 49.Flaherty KM, Zozulya S, Strayer L, McKay DB. Cell. 1993;75:709–716. doi: 10.1016/0092-8674(93)90491-8. [DOI] [PubMed] [Google Scholar]
- 50.Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Nature. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. Nature. 1995;376:444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]