Summary
The kidney is commonly affected in thrombotic thrombocytopenic purpura (TTP), a multi-system disorder with microvascular thrombosis of the capillaries and arterioles. Nevertheless, due to difference in its diagnostic criteria, the frequency and severity of renal dysfunction in TTP remains controversial. With the recent studies indicating that severe deficiency of a VWF cleaving protease, ADAMTS13, is the main cause of platelet thrombosis in TTP, it is now possible to define TTP at the molecular level. Among patients with acquired TTP due to inhibitory antibodies of ADAMTS13, renal dysfunction is usually mild; oliguria, fluid overload, hypertension, and need of dialysis support are infrequent. When any of these complications occur, one must re-examine the diagnosis of TTP and consider the possibility that the patient has another cause for these complications. In a patient with hereditary TTP, acute renal failure may ensue. However, the renal dysfunction is mostly reversible if the patients are promptly treated with plasma to replenish ADAMTS13. Patients with TTP, particularly of the hereditary type, may develop chronic renal failure. This complication may be a consequence of repeated insults by overt or subclinical microvascular thrombosis to the kidney, or it may have a separate cause. Therapy of hereditary TTP should aim not only to prevent acute exacerbations but also to minimize the risk of irreversible renal injury.
Keywords: Thrombotic thrombocytopenic purpura, ADAMTS13, von Willebrand factor, Thrombosis, Renal failure, Shear stress
Thrombotic thrombocytopenic purpura is a life-threatening, multisystem disease characterized in most cases by thrombocytopenia, microangiopathic hemolytic anemia and neurologic dysfunction such as mental changes or focal deficits1. These manifestations are believed to result from widespread thrombosis in the capillaries and arterioles of the brain and other organs. Thrombosis and consumption of platelets lead to ischemic injury of the affected organs and thrombocytopenia. Mechanical injury due to high shear stress created by microvascular thrombosis is believed to cause fragmentation of red blood cells and hemolysis.
It is widely recognized that TTP is commonly associated with renal abnormalities. However, the type and severity of renal abnormalities has been a subject of controversy. The first case of TTP described in 1924 by Moschcowitz had proteinuria with hyaline and granular cases in the urine, but had normal creatinine (1.1 mg/dL) and mildly elevated BUN (31.25 mg/dL)2. In subsequent years, as other disorders with thrombocytopenia, microangiopathic hemolysis and acute or chronic renal failure were increasingly recognized, TTP and renal failure became inextricably intertwined. Different schemes of classification have been developed to address this relation. In one scheme, patients with prominent neurologic abnormalities but no or mild renal function impairment are distinct from those presenting with profound renal failure. Thus, profound renal failure is considered to be uncharacteristic of TTP, and patients with anuria or oliguria are excluded from the diagnosis1. Furthermore, the renal criteria are inapplicable for the diagnosis of TTP if BUN is greater than 40 mg/dL or creatinine is greater than 3.0 mg/dL. In other schemes, TTP and HUS are distinguished based on the presence or absence of neurologic abnormalities, or they are not distinguished at all. One scheme includes renal dysfunction in scoring the severity of TTP3. In others , the patients are not distinguished according to the severity of renal dysfunction4;5. Because the etiology and pathophysiolgy of microvascular thrombosis were mostly unknown, it was impossible to decide which of these classifications was valid.
Advances in recent years have shown that microvascular thrombosis may occur via several distinct molecular mechanisms6–12. Consequently, it is now possible to propose a scheme of classifying thrombotic microangiopathy based on the underlying molecular defects and etiologies (Table 1). In this scheme, TTP is defined as a prothrombotic disorder due to severe deficiency of ADAMTS13. This deficiency may result from homozygous or compound heterozygous mutations of the ADAMTS13 gene, or, more commonly, due to inhibitory autoantibodies of the ADAMTS13 protein.
Table 1.
A pathophysiolgy- and etiology-based classification of thrombotic microangiopathy
| Clinical entity | Affected molecule | Cause | Etiology |
|---|---|---|---|
| TTP | ADAMTS13 | ADAMTS13 inhibitory antibody | Ticlopidine, HIV, or idiopathic (most cases) |
| ADAMTS13 mutations | Hereditary (autosomal recessive) | ||
| Atypical HUS | CFH | CFH mutation | Hereditary (autosomal dominant, variable penetrance) |
| CFH antibody | Acquired | ||
| MCP | MCP mutation | Hereditary (autosomal dominant, variable penetrance) | |
| IF | IF mutation | Hereditary (autosomal dominant, variable penetrance) | |
| BF | BF mutation (gain of function) | Hereditary (autosomal dominant) | |
| Unknown | Unknown | Unknown (50%–70% of atypical HUS) | |
| Secondary HUS | |||
| Stx-HUS | Shiga toxins | Bacterial infection | Stx+ E. coli or Sh. dysenteriae |
| TF-HUS | TF antigen | Bacterial infection | Bacterial neuraminidase (S. pneumoniae and other organisms) |
| Others | Unknown | Unknown | Lupus and related disorders, bone marrow/stem cell transplantation, neoplastic diseases, drugs, surgery, pregnancy (HELLP), pancreatitis, etc. |
| Other TMA* | |||
| PNH | CSRF | PIG-A | Somatic mutation |
| Tumor cell embolism | Unknown | Embolism of tumor cells | Metastasizing malignancies |
| Others | Unknown | Unknown | Unknown |
Not association with severe ADAMTS13 deficiency or renal abnormalities
Abbreviations. BF: complement B factor; CFH: complement factor H; CRSF: complement regulating surface proteins (e.g. CD55, CD59); IF: complement factor I; MCP: membrane cofactor protein; PNH: paroxysmal nocturnal hemoglobinuria; Stx: shiga toxins; TF: Thomsen–Friedenreich antigen; TMA: thrombotic microangiopathy
Idiopathic (atypical) HUS refers to the group of disorders that have evidence of renal failure without obvious causes or severe ADAMTS13 deficiency. These patients would have been classified under the diagnosis of TTP or TTP/HUS in some reports. Many of the cases in this group have heterozygous, homozygous, or compound heterozygous mutations of the proteins involved in regulating the activation of the complement system, such as complement factor H, complement factor I, membrane cofactor protein (CD46), and complement factor B. A defect in one of these proteins, transmitted as an autosomal dominant trait with variable penetrance and expression, may lead to uncontrolled complement activation, injuring the endothelial cells of the kidney and other organs. Complement dysregulation may also result from autoantibodies of complement factor H13. No defects are identified as yet in approximately 50%–70% of the cases of idiopathic HUS.
Secondary HUS includes the typical, diarrhea (+) HUS occurring after infection of E. coli O157:H7 or other shiga toxin producing microorganisms; and the HUS in association with Thomsen-Friedenreich (TF) antigen activation, following infection of Streptococcu pneumoniae or other neuraminidase-producing microorganisms. The category of secondary HUS also includes thrombotic microangiopathy in association with autoimmune connective tissue diseases, various drugs, bone marrow or stem cell transplantations, etc. The underlying molecular mechanisms remain mostly not understood in secondary group.
A third category of thrombotic microangiopathy, represented by the syndromes of diffuse microvascular thrombosis of paroxysmal nocturnal hemoglobinuria and tumor cell embolism that are neither due to severe ADAMTS13 deficiency nor associated with renal abnormalities. Not infrequently, there are cases of thrombotic microangiopathy whose molecular mechanisms or etiologies are unknown. In this scheme, there is no “TTP without severe ADAMTS13 deficiency”, because it is impossible to define such an entity.
With increasing use of ADAMTS13 assays in practice, it is now recognized that TTP is associated with a broader spectrum of severity than previously recognized and mentioned at the beginning of this article. A patient may be completely asymptomatic or present with isolated thrombocytopenia, focal neurologic deficit, or a constellation of thrombocytopenia, microangiopathic hemolysis, neurologic deficits (triad), renal abnormalities, and fever (pentad). Thus, the classic combination of microangiopathic hemolysis and thrombocytopenia is not essential for making the diagnosis of TTP.
In this article the author reviews the features of TTP in light of this new classification, with special emphasis on renal dysfunction in TTP.
ADAMTS13: structure and function
ADAMTS13 is a circulating metalloprotease of the “a disintegrin and metalloprotease with thrombospond type 1 motif” enzyme family14 that cleaves von Willebrand factor (VWF) at its Y1605-M1606 peptidyl bond in the central A2 domain15. As will be further elaborated later, the cleavage sites of VWF multimers are not accessible to ADAMTS13 unless shear stress or chaotropic reagents unfold its conformation16;17.
The ADAMTS13 gene contains 29 exons spanning approximately 37 kb on chromosome 9q347;18;19. It encodes a 4.7-kb transcript that is detectable in the liver, and a 2.4-kb transcript detectable in placenta, skeletal muscle, and certain tumor cell lines. In the liver, ADAMTS13 is expressed primarily in the vitamin A-enriched stellate cells20;21. Other studies suggest that ADAMTS13 may also be expressed in platelets, endothelial cells and renal podocytes22–25.
The full-length ADAMTS13 transcript encodes a precursor protein of 1427 amino acid residues. The sequence of ADAMTS13 exhibits a multi-domain structure that is common among proteases of the ADAMTS family and but also contains two unique CUB domains. The protein undergoes extensive glycosylation and other post-translation modifications before secretion. One modification, O-fucosylation of residues in the thrombospondin type 1 is believed to be essential for efficient secretion of the protease26.
ADAMTS13 is inactivated by disulfide bond-reducing agents, tetracyclines, or cation chelators such as phenanthroline and EGTA27. Although ADAMTS13 is stable in normal plasma, its activity may deteriorate rapidly in plasma samples of patients with liver diseases, disseminated intravascular coagulopathy or other pathological conditions28. This deterioration in vitro may contribute to inaccuracy of ADAMTS13 assays. In vitro, thrombin and plasmin may inactivate ADAMTS1329. It is speculated that process may be critical at sites of vessel injury for protecting VWF from cleavage. Thrombospondin may also protect VWF from cleavage by ADAMTS1330. Based on observations following infusion of normal plasma in patients with genetic deficiency of ADAMTS13, the elimination half-life of ADAMTS13 is approximately 2 days31.
Phylogenetically, ADAMTS13 diverts early from other members of the ADAMTS family of proteases14;32. In particular, ADAMTS13 contains an unusually short (41 amino acid residues) propeptide whose cleavage does not appear to be necessary for expression of proteolytic activity33. Enzymatic analysis of recombinant proteins expressed in mammalian cells in culture reveals that the VWF cleaving activity is markedly decreased34 but not abolished as previously reported35;36 when ADAMTS13 is truncated upstream of its spacer domain. The spacer domain is required for binding to a short fragment in the VWF A2 domain downstream of the cleavage site37 and also for recognition by TTP IgG34;38. One study also detected frequent interaction of TTP IgG with other domain fragments39; however, this observation remains controversial. The TSR 2–8 region is required for effective cleavage of VWF multimers but not of the VWF A2 fragment40.
How does severe ADAMTS13 deficiency lead to microvascular thrombosis?
VWF, the only known substrate of ADAMTS13, is secreted from vascular endothelial cells as disulfide bonded polymer of an enormous size (>20×106 Daltons) (Figure 1A). Endothelial VWF or the plasma large multimers derived from endothelial VWF after cleavage by ADAmts13 support platelet adhesion and aggregation at sites of vessel injury. High levels of shear stress promote platelet adhesion to VWF and its subsequent aggregation41. This shear responsiveness is critical for the unique capability of VWF to support platelet aggregation under high shear in the microvasculature. Studies using atomic force microscopy and fluorescent microscopy have demonstrated that shear stress causes conformational unfolding of VWF42;43. This conformational change provides a structural basis for understanding why shear stress enhances the adhesive activity of VWF44.
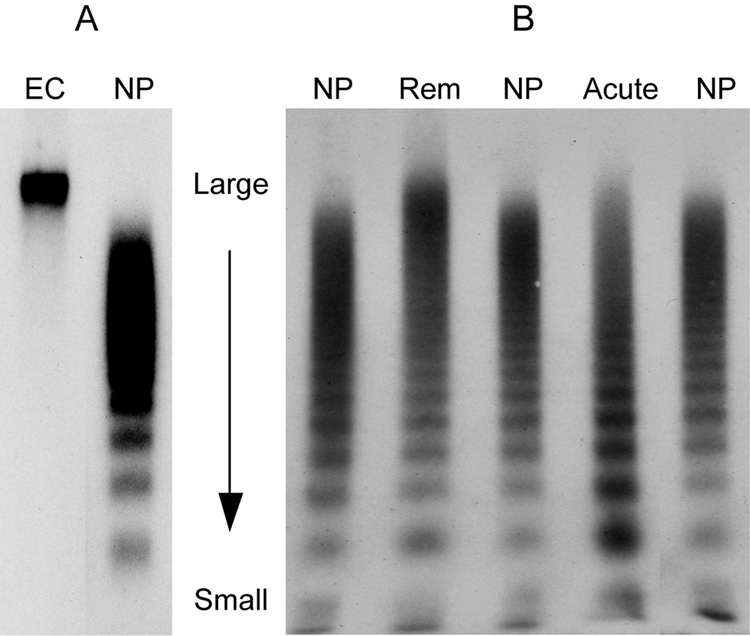
Figure 1.
SDS agarose gel electrophoresis comparing the multimeric compositions of endothelial and plasma VWF. VWF is visualized by probing with rabbit anti-VWF and 125I-labeled goat anti-rabbit IgG. A: endothelial secreted VWF consists of a single band that is much larger than the largest forms in plasma VWF multimers. B: The VWF compositions in the plasma samples from a patient of TTP during remission (Rem) and the same patient during acute relapse (Acute) are compared with those in three normal plasma samples (NP). Both plasma samples have ultra large multimers because their ADAMTS13 activity levels are decreased (15% and <10% respectively). In acute TTP, the ultra and large multimers are decreased because they are consumed in the process of VWF-platelet binding.
The conformational change induced by shear stress also makes VWF susceptible to cleavage by ADAMTS1316;45. In the circulation, ADAMTS13 cleaves VWF as soon as it is partially unfolded by shear stress, before the adhesive protein is fully activated to support platelet aggregation. This process maintains VWF in globular, inactive forms as its size become progressively smaller. In the absence of ADAMTS13, large VWF multimers becomes fully activated by shear stress, leading to VWF-platelet aggregation and microvascular thrombosis of TTP. The VWF-platelet binding explains why large multimers are decreased in TTP during periods of severe thrombocytopenia. It also explains why fragments of VWF produced by ADAMTS13 are present in normal plasma, and yet the activity of ADAMTS13 is undetectable in the plasma unless the VWF substrate is exposed to shear stress or chaotropic agents.
It is often stated that ultra large VWF multimers are uniquely hyperactive, and are responsible for inducing platelet aggregation in TTP. Empiric observations in patients with TTP do not support this view. In fact, ultra large multimers are present in many patients during remission (Figure 1B, Rem), when there is no evidence of active platelet aggregation and thrombosis, whereas during acute exacerbations, both the ultra large and large multimers are depleted from the circulation (Figure 1B, Acute). According to the scheme proposed here, the multimer pattern in plasma represents a snapshot of a dynamic equilibrium involving secretion of ultra large VWF from endothelial cells, shear-dependent cleavage of VWF by ADAMTS13, and consumption of the VWF multimers in microvascular thrombosis. In TTP, with progressively lower ADAMTS13 levels in the circulation, proteolysis of VWF becomes defective, resulting in the appearance of ultra large multimers in plasma. When ADAMTS13 becomes more severely deficient, the large and ultra large VWF are not immediately cleaved when they are unfolded by high shear stress in the circulation. These unfolded forms of VWF are depleted from the circulating plasma as they bind platelets and cause thrombosis in the microcirculation. Thus, depletion of large and ultra large multimers is observed in TTP where there is profound thrombocytopenia, whereas ultra large multimers are present during remission.
In vitro and ex vivo studies have shown that, under certain experimental conditions, VWF molecules may remain anchored to endothelial cells after secretion46–48. It is speculated that this anchoring phenomenon may contribute to or are essential for the development of microvascular thrombosis in TTP. Nevertheless, immnohistochemical studies shows that although the endothelial storage pool of VWF is decreased in TTP, the cell surface is not decorated by VWF fibers. Thus, further studies are needed to clarify whether VWF anchored to endothelial surface is mostly an extreme phenomenon occurring under highly perturbed conditions or indeed plays a role in the development of thrombosis in TTP.
Animal models of ADAMTS13 deficiency created by gene targeting techniques have uncovered additional complexity in the ADAMTS13 deficiency - microvascular thrombosis scheme48;49. In the original ADAMTS13 null mice, perturbation of endothelial cells with calcium ionophore A23187 results in more strings of platelets attached to VWF on endothelial surface48. Separately, infusion of epinephrine and collagen also causes more profound thrombocytopenia in the ADAMTS13 deficient mice 49. Nevertheless, the mice do not appear to suffer from the lack of ADAMTS13; their hemogram, fertility, and life span are not different from those of the wild type mice. When the ADAMTS13 null allele is transferred to mice of a different genetic background, the affected mice spontaneously develop systemic microvascular thrombosis similar to that observed in human TTP and die prematurely48. These observations suggest that in mice the propensity to develop thrombosis in the absence of ADAMTS13 is strongly affected by as yet unknown genetic traits. These traits may be relevant to the heterogeneous severity of TTP observed in patients.
Intriguingly, infusion of shiga toxins to susceptible ADAMTS13-deficient mice precipitates the manifestations of TTP, resulting in microvascular thrombosis, thrombocytopenia and death48. How shiga toxins aggravate microvascular thrombosis in the ADAMTS13 deficient mice and whether this process is relevant to human TTP or HUS remains unclear.
Incidence, etiologies and risk factors
At least 4 published studies have attempted to estimate the incidence of TTP50–53. In the Oklahoma registry, the only study in which the diagnosis of TTP is confirmed by ADAMTS13 deficiency, the incidence rate is 1.74 cases per million person-years. Female to male ratio is 2–3 to 1. The peak age is between 30 and 50 years. It is likely that the incidence rate will vary depending on the target population investigated.
An association between TTP and HIV infection remains controversial. Among the 27 cases in the Oklahoma registry, only one had HIV infection52. In contrast, at the author’s institution, more than 30% of the non-referral cases had HIV infection. Thus, in regions with low prevalence rates, the contribution HIV infection to the incidence of TTP may not be apparent. Patients with HIV infection not infrequently are complicated with Coombs-positive hemolytic anemia or other autoimmune disorders. It is conceivable that anti-ADAMTS13 antibodies may also result from the same process of humoral hyperactivity. Alternatively, HIV infected subjects may have higher risk of being exposed to unknown environmental or infectious factors that are involved in inducing the development of autoimmunity to ADAMTS13.
Ticlopidine may increase the risk of TTP by 200–500 folds54;55. In contrast, clopidogrel does not appear to increase the risk of TTP56. Similarly, other drugs commonly implicated in causing thrombotic microangiopathy, such as quinine, calcineurin inhibitors, and chemotherapeutic agents have not been associated with severe ADAMTS13 deficiency. Obesity may be a risk factor of TTP according to one study57. However, this associated has not been upheld in other studies.
A subset of patients has evidence of other autoimmune disease before or after their acute episodes of TTP. On the other hand, autoimmune connective tissue disorders and metastasizing neoplasm may cause thrombotic microangiopathy due to vasculitis, tumor cell embolism, or other as yet unknown mechanisms. Most of these conditions are not associated with severe ADAMTS13 deficiency.
The relation between TTP and pregnancy is complex. Overall, there is no definitive evidence that pregnancy increases the risk of de novo TTP. Among women in their productive age, TTP may occur coincidentally during pregnancy or the postpartum period. On the other hand, pregnancy decreases the level of ADAMTS13 by approximately 30%58. The protease activity decreases to lower levels if pregnancy is complicated with the HELLP syndrome. Thus, in women with pre-existing ADAMTS13 deficiency, pregnancy may induce exacerbation of TTP by suppressing the ADAMTS13 deficiency. In the postpartum period, recovery from immunotolerance of pregnancy may precipitate TTP by aggravating the pre-existing autoimmune response to ADAMTS13. Separately, the HELLP syndrome or atypical HUS occurring during pregnancy may be clinically difficult to distinguish from TTP.
Pathology
The hyaline thrombi of TTP are most frequently found in the arterioles and capillaries of heart, pancreas, spleen, kidney, adrenal glad, and brain. The thrombi are characteristically enriched in VWF and platelets and contain no or very little fibrin59;60. In sub-acute or chronic lesions, endothelial cells may proliferate to overlay the endoluminal thrombi, converting these lesions to subendothelial locations. Other secondary changes such as pre-stenotic aneurysmal dilatation and ischemic tissue necrosis may be noted. However, there is no or minimal infiltration of inflammatory cells at or near the affected vessels. Special staining for VWF and for CD61 (platelet glycoprotein IIIa)61 may help distinguish the VWF- and platelet-rich thrombi of TTP from the fibrin-rich thrombi of DIC, shiga toxin associated and other types of HUS62–66.
In the kidney, microthrombi typically affect one or a few segments of the glomeruli. Although the extent of glomerular thrombi varies widely among the patients, widespread glomerular or cortical necrosis and fibrosis are uncommon in TTP67.
Clinical manifestation
Acquired TTP The patients often present with weakness, pallor, petechiae, and headache, with or without somnolence or focal neurologic deficits. The course may be quickly complicated by seizures or coma if therapeutic intervention is delayed. Other manifestations of TTP include abdominal pain, nausea, vomiting, and even sudden death.
Laboratory tests often reveal severe thrombocytopenia (<20×109/L), at initial presentation. Because of increased awareness, relapsing TTP tends to be detected at an earlier stage with less profound thrombocytopenia. On the other hand, the platelet count may occasionally decrease precipitously to very low levels within 1–2 days of relapse, underscoring the unpredictability of the disease and importance of prompt therapeutic intervention.
Hemolytic anemia with elevated LDH and schistocytes on blood smears are present in most cases at their first presentation. Hemolytic anemia may occur days or months after the onset of thrombocytopenia. Before the onset of hemolysis, the patients may be mistaken to have ITP. Coagulation tests and fibrinogen levels are usually unremarkable. In severe cases, the fibrin degradation products may be elevated.
EKG often reveals non-specific ST-T changes or conduction distances. Liver function and electrolytes are not affected in most cases.
The conventional description of TTP presenting with marked thrombocytopenia, hemolytic anemia, and neurologic changes represents the more advanced stage of the disease. With the use of ADAMTS13 assays, a patient may be diagnosed at pre- or sub-clinical stages, before the onset of thrombocytopenia, microangiopathic hemolysis, neurological deficits, and/or fever.
Conventionally it is believed that TTP will deteriorate precipitously if the patient is not treated. While this is true for most cases, it is now also recognized that the course of the disease is more smoldering in a few cases.
In contrary to previous beliefs, neurological abnormalities are not pathognomonic of TTP; similar complications may affect 10%–30% of patients with typical or atypical HUS68–70.
Hereditary TTP Patients with hereditary TTP often have a history of hyperbilirubinemia requiring whole blood or exchange transfusion immediately after birth. Thrombocytopenia and hemolytic anemia may be noted. Strokes may occur at birth or during the neonatal period. After the neonatal period, the patient may present with thrombocytopenia and microangiopathic hemolysis weeks to years later. Exacerbations may be precipitated by fever, infections, diarrhea, trauma, surgery, or pregnancy. The frequency of exacerbation varies, ranging from days to years. Occasionally an asymptomatic patient may be diagnosed only during familial investigation of hereditary TTP. Despite an uneventful history, such patients are at risk of developing complications of TTP during periods of stresses from fever, infection, surgery, trauma, or pregnancy.
Laboratory diagnosis of TTP
Laboratory tests are essential for correct diagnosis of TTP. The tests can also provide invaluable information to assess the status of the disease during the course of treatment and remission. Ideally, laboratory analysis should include ADAMTS13 activity, inhibitors of ADAMTS13 or its binding IgG, SDS agarose for analysis of VWF multimers, and SDS PAGE for analysis of VWF proteolytic fragments. In most clinical settings, only ADAMTS13 activity and antibody assays are available with a reasonable turnaround time.
Several assays have been developed to determine ADAMTS13 activity in plasma28. In patients presenting with thrombocytopenia or declining platelet counts, the ADAMTS13 activity level is invariably very low (<5% or 10% depending on the assays used). On the other hand, a very low level of ADAMTS13 is not always associated with thrombocytopenia, because the process of VWF-platelet binding is likely to be affected by multiple factors such as the secretion of VWF from endothelial cells, the profile of shear stress in the microcirculation, the reactivity and availability of platelet receptors, and other as yet unknown mechanisms.
A multitude of factors may affect the reliability of ADAMTS13 assays, depending on their specific designs. The activity of the enzyme may decay in plasma samples of patients with liver disease, sepsis or disseminated intravascular coagulopathy28. Hemoglobin in plasma may interfere with certain assays71. Hyperbilirubinemia may affect the assays based on the measurement of fluorescent resonance energy transfer (FRET)72. Additionally, laboratory assays may occasionally produce incorrect results resulting from unknown causes73. Thus, interpretation of the ADAMTS13 results requires precaution and correlation with clinical findings. When there is any doubt, the assay should be repeated using a different methodology.
Management
Patients presenting with acute TTP require urgent therapy with plasma exchange74;75. Delay in instituting the therapy may increase the risk of serious complications or death. If plasma exchange is not immediately available, the patients should be treated with fresh frozen plasma76 until plasma exchange is instituted.
The treatment often includes anti-platelet agents and corticosteroids, although there is no clear evidence that usage of these modalities improve the outcome of patients treated with plasma exchange77. In refractory cases, vincristine, splenectomy, high dose immunoglobulin, cyclophophamide, and azathioprin have been used.
Plasma exchange is tapered once the platelet count is normalized for 2–3 days. Tapering may prevent relapses simply by extending the duration of plasma therapy until the ADAMTS13 inhibitor level recedes to lower levels. Tapering does not appear to affect the natural course of the disease.
The discovery of ADAMTS13 has generated new perspectives in the management of TTP. One is the use of rituximab, a chimeric monoclonal anti-CD20, to treat TTP78–82. By decreasing the levels of autoantibodies, rituximab may be an effective treatment for TTP. Its benefit may be quite impressive in some patients with persistent TTP due to low but persistent ADAMTS13 inhibitors. However, because of potential reporting bias, the exact response rate remains uncertain. The efficacy of rituximab will require further investigation in carefully designed studies before this treatment can be recommended for patients with acute TTP.
The other new perspective is that TTP may present with isolated thrombocytopenia or focal neurologic defects83;84. The diagnosis may be mistaken as idiopathic thrombocytopenic purpura, transient ischemic attacks, or strokes. Although such cases are probably infrequent, it is important to recognize them as the patients will respond to plasma exchange but not anticoagulation.
Because patients with hereditary TTP do not have inhibitors of ADAMTS13, they usually respond predictably to regular infusion of fresh frozen plasma at 10–15 mL/kg body weights. Typically, the treatment is given every 2–3 weeks. Chronic organ failure may become problematic if the patients are not adequately treated.
Course and outcome
The patients of acquired TTP often improve immediately after plasma exchange is initiated, with the platelet counts beginning to increase within a few days. The average number of plasma exchanges is approximately 15 sessions before remission is achieved75. Approximately half of the cases experience exacerbation after initial response85. Analysis of ADAMTS13 inhibitors shows that rising antibody levels are responsible for the exacerbation in most cases.
Relapse occurs in 30%–60% of the patients achieving remission. Relapse is quite common within the few weeks of remission86. However, it may also occur months or years after the initial episodes. In some cases, TTP may evolve to a persistent state requiring long-term plasma therapy78;79. Analysis of ADAMTS13 levels during remission shows that the ADAMTS13 activity remains decreased in most patients, suggesting that relapses result from exacerbation of the persistent autoimmunity in most cases.
The mortality rate of TTP is greater than 90% for untreated patients. For patients promptly treated with plasma exchange, this rate is decreased to 10%–20%85;87–89.
The current concepts in the pathophysiology and management of TTP are summarized in Table 2.
Table 2.
Current Concepts in the pathophysiology and management of TTP
| • | ADAMTS13 is a zinc metalloprotease critical for maintaining the balance between hemostasis and thrombosis in the microcirculation. Excessive cleavage of VWF by ADAMTS13 results in the bleeding diathesis of type 2A von Willebrand disease, whereas decreased cleavage of VWF in association with ADAMTS13 deficiency may cause microvascular thrombosis of TTP. |
| • | Acquired TTP is an autoimmune disorder with IgG inhibitors causing ADAMTS13 deficiency. Some cases have HIV infection or recent use of ticlopidine. Most cases are idiopathic. |
| • | In hereditary TTP, compound heterozygous or homozygous mutations of the ADAMTS13 gene causes severe ADAMTS13 deficiency. Hereditary TTP is uncommon but may cause serious complications if it is not recognized and managed appropriately. |
| • | The manifestations of TTP are more variable than previously recognized. TTP does not always present with thrombocytopenia and microangiopathic hemolysis. |
| • | With ADAMTS13 assays, it is now possible to diagnose TTP in patients with atypical features and distinguish the disease from other types of thrombotic microangiopathy. |
| • | Serial monitoring of the ADAMTS13 status may help assess the status of TTP during the course of its treatment and remission. |
| • | Plasma exchange remains the standard therapy of acquired TTP. However, it does not address the underlying autoimmune nature of the disease. |
| • | Discovery of ADAMTS13 autoantibodies contributes to the use of rituximab in patients with protracted TTP. |
Renal abnormalities in acquired TTP
Proteinuria and hematuria are common in TTP. In contrast, acute renal failure with marked azotemia, fluid overload, hypertension, and need of dialysis, is much less frequent, affecting 1 of 18, 3 of 31, 0 of 50, and 0 of 16 cases in 4 series of acquired TTP with severe ADAMTS13 deficiency85;87–89. The complications of severe renal failure are also not observed in our series of more than 35 non-referral cases of acquired TTP. Thus, in practice, a patient is unlikely to have TTP if the course is associated with one or more of features of profound renal failure. However, there are occasional exceptions.
The renal manifestations of hematuria, proteinuria, and mild impairment of the clearance function in TTP are consistent with the focal and segmental distribution of microthrombi most commonly observed at pathological examination described earlier. Why does severe renal failure develop in some cases? To explore this question, the author has reviewed his series of more than 150 cases of acquired autoimmune TTP, and find two patients, both in the referral group, that had complications of acute renal failure requiring dialysis (Table 3). One case was a 31-year-old male presenting with TTP and acute renal failure 4 years after undergoing total colectomy for ulcerative colitis90. The patient was later found to have anti-glomerular basement membrane nephropathy when he had recurrence of acute renal failure without thrombocytopenia or microangiopathic hemolysis. Interestingly, a previous study has noted that anti-glomerular basement membrane nephropathy is frequently complicated with histopathological evidence of thrombotic microangiopathy91. A recent report has described a case with presumed TTP and the renal limited variant of Goodpasture’s syndrome. Nevertheless, the diagnosis of TTP was not confirmed with ADAMTS13 analysis in that case92.
Table 3.
Patients with acute renal failure and severe acquired ADAMTS13 deficiency
| Authors | Age, sex | Clinical features | ADAMTS13 analysis | Treatment | Outcome |
|---|---|---|---|---|---|
| Ahmed, et al90 | 31 yrs, M | TTP occurring 4 years after total colectomy for ulcerative colitis; achieved sustained remission after 4 weeks of plasma exchange and other therapies. The course was complicated by ARF requiring dialysis on day 12. | ADAMTS13 activity <10% IgG inhibited ADAMTS13 | Plasma exchange and dialysis | Recurrence of ARF11months after the episode of TTP, not accompanied by thrombotic microangiopathy. ADAMTS13 was only slightly decreased (58%). Laboratory studies revealed anti-glomerular basemembrane nephropathy. |
| Pham, et al93 | 57 yrs, M | Anuria and renal failure immediately after renal allograft transplantation for polycystic kidney disease | ADAMTS13 activity <10% IgG inhibited ADAMTS13 | Dialysis and plasma exchange | Gradual recovery of renal function after 10 sessions of plasma exchange. Normal ADAMTS13 and negative inhibitor 3 months from the episode. |
| Hunt, et al94 | 1.5 yrs, M | E. coli O157 associated gastroenteritis, followed by seizures, fluctuating levels of consciousness, and anuria. Other features: yponatremia and elevated transaminases | ADAMTS13 <1% Inhibitors detected by plasma mixing study | Dialysis x10 days and infusion of fresh frozen plasma x7 days | Normal renal function by day 18 Normal ADAMTS13 at age 5 |
| Veyradier, et al95 | 4 yrs, F | Hemolysis, thrombocytopenia, and acute renal dysfunction after 2 days of bloody diarrhea due to E. coli O157:H7 infection (positive IgM antibody) | ADAMTS13 activity <5% ADAMTS13 inhibitors not detectable by plasma mixing study | Plasma exchange | Recovery in 15 days Normal renal function and normal ADAMTS13 activity after a 3- month remission |
The other case of TTP requiring dialysis in our series was a 57-year-old male that developed the complication of renal failure immediately after receiving a cadaveric renal allograft for polycystic kidney disease93. Post-operatively the patient remained anuric, accompanied by thrombocytopenia and microangiopathic hemolysis. He achieved remission after cyclosporine was discontinued and daily plasma exchange was instituted. Laboratory analysis revealed that his ADAMTS13 activity was below the level of detection, and his IgG inhibited the ADAMTS13 activity in normal plasma. Three months later, the patient had normal renal function and ADAMTS13 activity level and had no detectable inhibitors of the protease.
In the literature, transient ADAMTS13 inhibitors have also been described in two cases of thrombotic microangiopathy in association with E coli O157 infection94;95. One of these two cases required dialysis for severe renal failure with anuria and hyponatremia. Both cases recovered their renal functions and had normal ADAMTS13 activity levels at follow-up investigation, suggesting that both had self-limited autoimmune reaction to ADAMTS13.
Overall, current evidence indicates that renal function impairment is mild and transient in most cases of acquired TTP. Renal failure may become more apparent when the course of TTP is protracted. Severe renal dysfunction occurs mostly in association with other causes of renal failure. The occurrence of ADAMTS13 inhibitors in a patient with renal allograft, a patient with anti-glomerular basement membrane nephropathy and two patients with E. coli O157 infection is intriguing. The implications of these cases remain unclear.
Renal abnormalities in hereditary TTP
Of the more than 75 cases of severe hereditary ADAMTS13 deficiency reported in the literature7;73;96–116, nine (11%) had episodes of acute renal failure7;96;101;114–116. The episodes of acute renal failure often but not invariably recurred in the same patients during exacerbation of TTP. Improvement following plasma infusion or exchange was observed in all but one case, who died after cholecystectomy due to exacerbation of TTP complicated by renal failure and gastrointestinal bleeding111. There is insufficient data to determine how frequent residual renal abnormalities persist after the acute episodes.
Nine of the reported cases of hereditary TTP developed chronic renal failure between age 5 – 44 years (Table 4)7;98;103;107;110;112;114;115. Five of these patients required dialysis. Three cases had episodes of acute renal function impairment before the onset of chronic renal failure. One patient received a renal graft at 15 years of age for chronic renal failure112. However the graft soon failed due to disease recurrence.
Table 4.
Chronic renal failure in hereditary TTP
| Case | Authors | Sex | Renal function | Age, yrs | Plasma therapy |
|---|---|---|---|---|---|
| 1 | te Loo, et al98 | M | Mild elevation of creatinine during acute exacerbation. Creatinine gradually increased to 130 μmol/L | 5 | FFP only during exacerbations. Renal function improved but did not normalize after institution of maintenance plasma therapy every 2 weeks |
| 2 | Levy, et al | M | Renal dysfunction with elevated creatinine noted | 16 | Chronic thrombocytopenia Infrequent plasma therapy only during episodes of exacerbations. |
| 3 | Assink, et al103 | M | Proteinuria, hematuria, GFR 60 mL/min/1.73 m2 | 11.5 | Regular plasma infusion since age 7 years |
| 4 | Matsumoto, et al107 | M | Dialysis | 23 | 160 mL every 2–4 weeks since age 8 years |
| 5 | Matsumoto, et al107 | F | Renal function deteriorated during treatment with low doses of FFP. | 21 | FFP 80 mL every 3 weeks since age 4 years. Renal function stabilized after plasma therapy was gradually increased since age 21 years to 500 mL every 2 weeks |
| 6 | Snider, et al110 | F | End stage renal failure requiring dialysis | 19 | Plasma infusion, then plasma exchange, every 2–4 weeks since age 2 years. Stable without plasma therapy after splenectomy and maintenance hemodialysis at age 19 years. |
| 7 | Veyradier, et al112 | M | End-stage renal failure and transplantation. Returned to dialysis due to disease recurrence. | 15 | No maintenance plasma therapy mentioned before renal failure. |
| 8 | Noris, et al114 | F | Acute renal failure during episodes of exacerbation. Dialysis since age 44. Died at age 55 of a stroke. S890I mutation in complement factor H. | 44 | FFP only for exacerbations. |
| 9 | Donadelli, et al115 | M | Renal insufficiency during acute episode. Dialysis since age 32. | 32 | FFP only for exacerbations. |
The risk of chronic renal failure does not appear to be associated with any particular ADAMTS13 genotypes. In two siblings, one developed chronic renal failure due to a concurrent mutation in complement factor H, demonstrating that other genetic traits may contribute to the risk of renal failure complicating the course of TTP114. In two cases, the renal function continued to deteriorate while the patients were treated with small amount (80–160 mL) of fresh frozen plasma every 2–4 weeks. One case ended up requiring dialysis. In the other case, the renal function stabilized after the treatment was increased to 400 mL fresh frozen plasma every two weeks. Intriguingly, one case continued to have frequent exacerbations of TTP and developed end stage renal disease despite maintenance plasma therapy. Unexpectedly, the course of TTP stabilized after the patient underwent splenectomy and was started on hemodialysis. It is doubtful that the experience in this case is applicable to other cases of hereditary TTP.
There are various reasons why patients with hereditary TTP were not treated with maintenance plasma infusion. In some cases, the diagnosis was not recognized until it was too late. In other cases, the patients or their parents were unwilling to follow physicians’ recommendations. In a few cases, maintenance plasma therapy was not deemed medically indicated. Because of concerns of adverse reactions and life quality, clinicians are understandably reluctant to commit a child to long-term plasma therapy unless are clear indications of benefit.
Nevertheless, this review reveals that renal failure is not an uncommon complication of hereditary TTP. Thus, until more definitive data become available, it is prudent to recommend preventive plasma therapy for a patient in whom the hereditary TTP causes chronic proteinuria, hematuria, or progressive decline of the renal clearance function. The amount and frequency of plasma infusion should be sufficient to prevent acute exacerbations, stabilize renal function, and minimize the severity of hematuria and proteinuria. Investigations for other causes may also be indicated if the course of renal failure is unexpectedly swift or severe.
Why is hereditary TTP more prone to be complicated with acute and chronic renal failure than acquired TTP? In a recent investigation, glomerular podocytes are found to express ADAMTS1325. Conceivably, this locally derived ADAMTS13 may contribute to the cleavage of VWF in the glomeruli of normal subjects or of patients with acquired TTP. Patients with hereditary TTP may be more likely to have extensive glomerular thrombosis and renal failure because they lack this protective effect of local ADAMTS13. The characteristic features of renal failure in TTP are summarized in Table 5.
Table 5.
Characteristic features of renal failure in TTP
| • | Hematuria and proteinuria are common in patients with acquired TTP during acute exacerbation. |
| • | Renal function impairment is mild and reversible in most cases of acquired TTP. Chronic renal failure may occur if the course of TTP is protracted. |
| • | Acute renal failure occurs in very few patients with acquired ADAMTS13 deficiency. Concurrent disorders may cause or contribute to the severity of renal failure |
| • | Acute and chronic renal failures are not infrequent in patients with hereditary TTP, affecting approximately 10% of the reported cases. |
| • | A concurrent factor H mutation has been detected in a patient whose hereditary TTP was complicated with acute and chronic renal failures. |
| • | Expression of ADAMTS13 in glomerular endothelial cells and podocytes may contribute to the cleavage of VWF locally in the glomeruli of patients with acquired TTP, minimizing the severity of their renal thrombosis and functional impairment. On the other hand, deficient ADAMTS13 expression in podocytes may account for the higher risk of renal failure in patients with hereditary TTP. |
| • | Urinalysis and renal function should be monitored in patients with TTP during remission. To minimize the risk of chronic injury to the kidney and other vital organs, maintenance plasma therapy should be considered for most patients with hereditary TTP, particularly if the patient has developed acute renal failure during exacerbations, evidence of progressive decline of the glomerular filtration rate, or has chronic hematuria and/or proteinuria. |
| • | The dose and frequency of maintenance plasma therapy should be sufficient to prevent acute exacerbations, stabilize the renal function, and minimize the severity of hematuria and proteinuria. |
| • | Investigation for other causes should be pursued if the course of renal deterioration is unexpectedly swift or unresponsive to plasma therapy. |
Conclusion
Recent advances have delineated the molecular and genetic defects underlying many cases of thrombotic microangiopathy. The new knowledge has provided a molecular framework for classifying this hitherto confounding syndrome. Specifically, the discovery of ADAMTS13 has provided a scientific basis for distinguishing TTP from other types of thrombotic microangiopathy, leading to improvement in the diagnosis and management of the disease. Further studies are needed to identify genes that may affect the phenotypic severity of TTP and to improve the accuracy and reliability of laboratory assays of ADAMTS13. Renal failure is an infrequent but serious complication of TTP that occurs more frequently in patients with the hereditary type. The long-term management of TTP, particularly of the hereditary type, should be tailored to prevent acute exacerbations and reduce the risk of chronic renal damage. Future development of protein, gene or cell-based ADAMTS13 replacement therapy may reduce the risk of adverse reactions commonly associated with long-term plasma therapy.
Acknowledgment
The author’s works cited in this article are supported in part by a grant (R01 HL62136) from the Heart, Lung and Blood Institute of the National Institute of Health.
References
- 1.Bukowski RM. Thrombotic thrombocytopenic purpura. A review. Prog Hemost Thromb. 1982;6:287–337. [PubMed] [Google Scholar]
- 2.Moschcowitz E. An acute febrile pleiochromic anemia with hyaline thrombosis of the terminal arterioles and capillaries: an undescribed disease. Proc.N.Y.Pathol.Soc. 1924;24:21–24. [PubMed] [Google Scholar]
- 3.Rose M, Eldor A. High incidence of relapses in thrombotic thrombocytopenic purpura. Clinical study of 38 patients. Am.J.Med. 1987;83:437–444. doi: 10.1016/0002-9343(87)90753-4. [DOI] [PubMed] [Google Scholar]
- 4.Remuzzi G. HUS and TTP: variable expression of a single entity. Kidney Int. 1987;32:292–308. doi: 10.1038/ki.1987.206. [DOI] [PubMed] [Google Scholar]
- 5.Remuzzi G, Galbusera M, Noris M, et al. von Willebrand factor cleaving protease (ADAMTS13) is deficient in recurrent and familial thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Blood. 2002;100:778–785. doi: 10.1182/blood-2001-12-0166. [DOI] [PubMed] [Google Scholar]
- 6.Warwicker P, Goodship TH, Donne RL, et al. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 1998;53:836–844. doi: 10.1111/j.1523-1755.1998.00824.x. [DOI] [PubMed] [Google Scholar]
- 7.Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 8.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 9.Caprioli J, Noris M, Brioschi S, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavanagh D, Richards A, Fremeaux-Bacchi V, et al. Screening for complement system abnormalities in patients with atypical hemolytic uremic syndrome. Clin.J.Am.Soc.Nephrol. 2007;2:591–596. doi: 10.2215/CJN.03270906. [DOI] [PubMed] [Google Scholar]
- 11.Copelovitch L, Kaplan BS. Streptococcus pneumoniae-associated hemolytic uremic syndrome. Pediatr.Nephrol. 2007 doi: 10.1007/s00467-007-0518-y. [PMID: 17564729] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caprioli J, Remuzzi G. Complement hyperactivation may cause atypical haemolytic uraemic syndrome-- gain-of-function mutations in factor B. Nephrol.Dial.Transplant. 2007 doi: 10.1093/ndt/gfm193. [DOI] [PubMed] [Google Scholar]
- 13.Jozsi M, Strobel S, Dahse HM, et al. Anti-factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood. 2007;22:2452–2454. doi: 10.1182/blood-2007-02-071472. [DOI] [PubMed] [Google Scholar]
- 14.Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int.J.Biochem.Cell Biol. 2004;36:981–985. doi: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Dent JA, Berkowitz SD, Ware J, Kasper CK, Ruggeri ZM. Identification of a cleavage site directing the immunochemical detection of molecular abnormalities in type IIA von Willebrand factor. Proc.Natl.Acad.Sci.U.S.A. 1990;87:6306–6310. doi: 10.1073/pnas.87.16.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai HM, Sussman II, Nagel RL. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83:2171–2179. [PubMed] [Google Scholar]
- 17.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 18.Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J.Biol.Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 19.Soejima K, Mimura N, Hirashima M, et al. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J.Biochem.(Tokyo) 2001;130:475–480. doi: 10.1093/oxfordjournals.jbchem.a003009. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W, Inada M, Lee TP, et al. ADAMTS13 is expressed in hepatic stellate cells. Lab Invest. 2005;85:780–788. doi: 10.1038/labinvest.3700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uemura M, Tatsumi K, Matsumoto M, et al. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922–924. doi: 10.1182/blood-2005-01-0152. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, Murata M, Matsubara Y, et al. Detection of von Willebrand factor-cleaving protease (ADAMTS-13) in human platelets. Biochem.Biophys.Res.Commun. 2004;313:212–216. doi: 10.1016/j.bbrc.2003.11.111. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Choi H, Bernardo A, et al. Platelet-derived VWF-cleaving metalloprotease ADAMTS-13. J.Thromb.Haemost. 2005;3:2536–2544. doi: 10.1111/j.1538-7836.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 24.Turner N, Nolasco L, Tao Z, Dong JF, Moake J. Human endothelial cells synthesize and release ADAMTS-13. J.Thromb.Haemost. 2006;4:1396–1404. doi: 10.1111/j.1538-7836.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 25.Manea M, Kristoffersson A, Schneppenheim R, et al. Podocytes express ADAMTS13 in normal renal cortex and in patients with thrombotic thrombocytopenic purpura. Br.J.Haematol. 2007;138:651–662. doi: 10.1111/j.1365-2141.2007.06694.x. [DOI] [PubMed] [Google Scholar]
- 26.Ricketts LM, Dlugosz M, Luther KB, Haltiwanger RS, Majerus EM. O-fucosylation is required for ADAMTS13 secretion. J.Biol.Chem. 2007;282:17014–17023. doi: 10.1074/jbc.M700317200. [DOI] [PubMed] [Google Scholar]
- 27.Tsai HM, Sussman II, Ginsburg D, et al. Proteolytic cleavage of recombinant type 2A von Willebrand factor mutants R834W and R834Q: inhibition by doxycycline and by monoclonal antibody VP-1. Blood. 1997;89:1954–1962. [PubMed] [Google Scholar]
- 28.Tsai HM. Measurement of ADAMTS13. International Rev Thromb. 2006;1:40–48. [PMC free article] [PubMed] [Google Scholar]
- 29.Crawley JT. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. 2005;105:1085–1093. doi: 10.1182/blood-2004-03-1101. [DOI] [PubMed] [Google Scholar]
- 30.Bonnefoy A, Daenens K, Feys HB, et al. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107:955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furlan M, Robles R, Morselli B, Sandoz P, Lammle B. Recovery and half-life of von Willebrand factor-cleaving protease after plasma therapy in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 1999;81:8–13. [PubMed] [Google Scholar]
- 32.Nicholson AC, Malik SB, Logsdon JM, Jr, Van Meir EG. Functional evolution of ADAMTS genes: evidence from analyses of phylogeny and gene organization. BMC.Evol.Biol. 2005;5:11–23. doi: 10.1186/1471-2148-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majerus EM, Zheng X, Tuley EA, Sadler JE. Cleavage of the ADAMTS13 propeptide is not required for protease activity. J.Biol.Chem. 2003;278:46643–46648. doi: 10.1074/jbc.M309872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou W, Dong L, Ginsburg D, Bouhassira EE, Tsai HM. Enzymatically active ADAMTS13 variants are not inhibited by anti-ADAMTS13 autoantibodies: a novel therapeutic strategy? J Biol.Chem. 2005;280:39934–39941. doi: 10.1074/jbc.M504919200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J.Biol.Chem. 2003;278:30136–30141. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soejima K, Matsumoto M, Kokame K, et al. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood. 2003;102:3232–3237. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]
- 37.Gao W, Anderson PJ, Majerus EM, Tuley EA, Sadler JE. Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc.Natl.Acad.Sci.U.S.A. 2006;103:19099–19104. doi: 10.1073/pnas.0607264104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luken BM, Turenhout EA, Hulstein JJ, et al. The spacer domain of ADAMTS13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb.Haemost. 2005;93:267–274. doi: 10.1160/TH04-05-0301. [DOI] [PubMed] [Google Scholar]
- 39.Klaus C, Plaimauer B, Studt JD, et al. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 2004;103:4514–4519. doi: 10.1182/blood-2003-12-4165. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Bouhassira EE, Tsai HM. An IAP retrotransposon in the mouse ADAMTS13 gene creates ADAMTS13 variant proteins that are less effective in cleaving von Willebrand factor multimers. Blood. 2007;110:886–893. doi: 10.1182/blood-2007-01-070953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss HJ, Turitto VT, Baumgartner HR. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. I. Shear rate--dependent decrease of adhesion in von Willebrand's disease and the Bernard-Soulier syndrome. J.Lab Clin.Med. 1978;92:750–764. [PubMed] [Google Scholar]
- 42.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, et al. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 43.Schneider SW, Nuschele S, Wixforth A, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. PNAS. 2007;104:7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai HM. Shear stress and von Willebrand factor in health and disease. Semin.Thromb.Hemost. 2003;29:479–488. doi: 10.1055/s-2003-44556. [DOI] [PubMed] [Google Scholar]
- 45.Donadelli R, Orje JN, Capoferri C, Remuzzi G, Ruggeri ZM. Size regulation of von Willebrand factor-mediated platelet thrombi by ADAMTS13 in flowing blood. Blood. 2006;107:1943–1950. doi: 10.1182/blood-2005-07-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chauhan AK, Motto DG, Lamb CB, et al. Systemic antithrombotic effects of ADAMTS13. J.Exp.Med. 2006;203:767–776. doi: 10.1084/jem.20051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 48.Motto DG, Chauhan AK, Zhu G, et al. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J.Clin.Invest. 2005;115:2752–2761. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banno F, Kokame K, Okuda T, et al. Complete deficiency in ADAMTS13 is prothrombotic, but it alone is not sufficient to cause thrombotic thrombocytopenic purpura. Blood. 2006;107:3161–3166. doi: 10.1182/blood-2005-07-2765. [DOI] [PubMed] [Google Scholar]
- 50.Torok TJ, Holman RC, Chorba TL. Increasing mortality from thrombotic thrombocytopenic purpura in the United States--analysis of national mortality data, 1968–1991. Am.J.Hematol. 1995;50:84–90. doi: 10.1002/ajh.2830500203. [DOI] [PubMed] [Google Scholar]
- 51.Miller DP, Kaye JA, Shea K, et al. Incidence of thrombotic thrombocytopenic purpura/hemolytic uremic syndrome. Epidemiology. 2004;15:208–215. doi: 10.1097/01.ede.0000113273.14807.53. [DOI] [PubMed] [Google Scholar]
- 52.Terrell DR, Williams LA, Vesely SK, et al. The incidence of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS-13 deficiency. J.Thromb.Haemost. 2005;3:1432–1436. doi: 10.1111/j.1538-7836.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- 53.Schech SD, Brinker A, Shatin D, Burgess M. New-onset and idiopathic thrombotic thrombocytopenic purpura: incidence, diagnostic validity, and potential risk factors. Am.J.Hematol. 2006;81:657–663. doi: 10.1002/ajh.20669. [DOI] [PubMed] [Google Scholar]
- 54.Bennett CL, Davidson CJ, Raisch DW, et al. Thrombotic thrombocytopenic purpura associated with ticlopidine in the setting of coronary artery stents and stroke prevention. Arch.Intern.Med. 1999;159:2524–2528. doi: 10.1001/archinte.159.21.2524. [DOI] [PubMed] [Google Scholar]
- 55.Tsai HM, Rice L, Sarode R, Chow TW, Moake JL. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern.Med. 2000;132:794–799. doi: 10.7326/0003-4819-132-10-200005160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bennett CL, Kim B, Zakarija A, et al. Two mechanistic pathways for thienopyridine-associated thrombotic thrombocytopenic purpura: a report from the SERF-TTP Research Group and the RADAR Project. J.Am.Coll.Cardiol. 2007;50:1138–1143. doi: 10.1016/j.jacc.2007.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicol KK, Shelton BJ, Knovich MA, Owen J. Overweight individuals are at increased risk for thrombotic thrombocytopenic purpura. Am.J.Hematol. 2003;74:170–174. doi: 10.1002/ajh.10418. [DOI] [PubMed] [Google Scholar]
- 58.Lattuada A, Rossi E, Calzarossa C, Candolfi R, Mannucci PM. Mild to moderate reduction of a von Willebrand factor cleaving protease (ADAMTS-13) in pregnant women with HELLP microangiopathic syndrome. Haematologica. 2003;88:1029–1034. [PubMed] [Google Scholar]
- 59.Asada Y, Sumiyoshi A, Hayashi T, Suzumiya J, Kaketani K. Immunohistochemistry of vascular lesion in thrombotic thrombocytopenic pupura, with special reference to factor VIII related antigen. Thromb Res. 1985;38:467–479. doi: 10.1016/0049-3848(85)90180-x. [DOI] [PubMed] [Google Scholar]
- 60.Tsai HM, Chandler WL, Sarode R, et al. von Willebrand factor and von Willebrand factor-cleaving metalloprotease activity in Escherichia coli O157:H7-associated hemolytic uremic syndrome. Pediatr.Res. 2001;49:653–659. doi: 10.1203/00006450-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Burke AP, Mont E, Kolodgie F, Virmani R. Thrombotic thrombocytopenic purpura causing rapid unexpected death: value of CD61 immunohistochemical staining in diagnosis. Cardiovasc.Pathol. 2005;14:150–155. doi: 10.1016/j.carpath.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Tsai HM, Tarr PI. Rebuttal to: von Willebrand factor-cleaving protease in childhood diarrhea-associated haemolytic uraemic syndrome. Thromb.Haemost. 2002;87:548–550. [PubMed] [Google Scholar]
- 63.Matsumae T, Takebayashi S, Naito S. The clinico-pathological characteristics and outcome in hemolytic-uremic syndrome of adults. Clin.Nephrol. 1996;45:153–162. [PubMed] [Google Scholar]
- 64.Taylor CM, Chua C, Howie AJ, Risdon RA. Clinico-pathological findings in diarrhoea-negative haemolytic uraemic syndrome. Pediatr.Nephrol. 2004;19:419–425. doi: 10.1007/s00467-003-1385-9. [DOI] [PubMed] [Google Scholar]
- 65.Tinaztepe K, Akkok N, Tinaztepe B. Hemolytic-uremic syndrome (HUS): a clinicopathological study of 15 cases. Turk.J Pediatr. 1993;35:23–36. [PubMed] [Google Scholar]
- 66.Loirat C, Sonsino E, Varga MA, et al. Hemolytic-uremic syndrome: an analysis of the natural history and prognostic features. Acta Paediatr.Scand. 1984;73:505–514. doi: 10.1111/j.1651-2227.1984.tb09962.x. [DOI] [PubMed] [Google Scholar]
- 67.Hosler GA, Cusumano AM, Hutchins GM. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome are distinct pathologic entities. A review of 56 autopsy cases. Arch.Pathol.Lab Med. 2003;127:834–839. doi: 10.5858/2003-127-834-TTPAHU. [DOI] [PubMed] [Google Scholar]
- 68.Bale JF, Jr, Brasher C, Siegler RL. CNS manifestations of the hemolytic-uremic syndrome. Relationship to metabolic alterations and prognosis. Am.J Dis.Child. 1980;134:869–872. doi: 10.1001/archpedi.1980.02130210053014. [DOI] [PubMed] [Google Scholar]
- 69.Cimolai N, Morrison BJ, Carter JE. Risk factors for the central nervous system manifestations of gastroenteritis-associated hemolytic-uremic syndrome. Pediatrics. 1992;90:616–621. [PubMed] [Google Scholar]
- 70.Tapper D, Tarr P, Avner E, Brandt J, Waldhausen J. Lessons learned in the management of hemolytic uremic syndrome in children. J.Pediatr.Surg. 1995;30:158–163. doi: 10.1016/0022-3468(95)90554-5. [DOI] [PubMed] [Google Scholar]
- 71.Studt JD, Hovinga JA, Antoine G, et al. Fatal congenital thrombotic thrombocytopenic purpura with apparent ADAMTS-13 inhibitor: in-vitro inhibition of ADAMTS-13 activity by hemoglobin. Blood. 2005;105:542–544. doi: 10.1182/blood-2004-06-2096. [DOI] [PubMed] [Google Scholar]
- 72.Meyer SC, Sulzer I, Lammle B, Kremer Hovinga JA. Hyperbilirubinemia interferes with ADAMTS-13 activity measurement by FRETS-VWF73 assay: diagnostic relevance in patients suffering from acute thrombotic microangiopathies. J.Thromb.Haemost. 2007;5:866–867. doi: 10.1111/j.1538-7836.2007.02438.x. [DOI] [PubMed] [Google Scholar]
- 73.Savasan S, Lee SK, Ginsburg D, Tsai HM. ADAMTS13 gene mutation in congenital thrombotic thrombocytopenic purpura with previously reported normal VWF cleaving protease activity. Blood. 2003;101:4449–4451. doi: 10.1182/blood-2002-12-3796. [DOI] [PubMed] [Google Scholar]
- 74.Bukowski RM, King JW, Hewlett JS. Plasmapheresis in the treatment of thrombotic thrombocytopenic purpura. Blood. 1977;50:413–417. [PubMed] [Google Scholar]
- 75.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N.Engl.J Med. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 76.Byrnes JJ, Lian EC. Recent therapeutic advances in thrombotic thrombocytopenic purpura. Semin.Thromb Hemost. 1979;5:199–215. doi: 10.1055/s-0028-1087153. [DOI] [PubMed] [Google Scholar]
- 77.Allford SL, Hunt BJ, Rose P, Machin SJ. Guidelines on the diagnosis and management of the thrombotic microangiopathic haemolytic anaemias. Br.J.Haematol. 2003;120:556–573. doi: 10.1046/j.1365-2141.2003.04049.x. [DOI] [PubMed] [Google Scholar]
- 78.Yomtovian R, Niklinski W, Silver B, Sarode R, Tsai HM. Rituximab for chronic recurring thrombotic thrombocytopenic purpura: a case report and review of the literature. Br.J.Haematol. 2004;124:787–795. doi: 10.1111/j.1365-2141.2004.04836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gutterman LA, Kloster B, Tsai HM. Rituximab therapy for refractory thrombotic thrombocytopenic purpura. Blood Cells Mol.Dis. 2002;28:385–391. doi: 10.1006/bcmd.2002.0522. [DOI] [PubMed] [Google Scholar]
- 80.Fakhouri F, Vernant JP, Veyradier A, et al. Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13-deficient thrombotic thrombocytopenic purpura: a study of 11 cases. Blood. 2005;106:1932–1937. doi: 10.1182/blood-2005-03-0848. [DOI] [PubMed] [Google Scholar]
- 81.Scully M, Cohen H, Cavenagh J, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS-13. Br.J.Haematol. 2007;136:451–461. doi: 10.1111/j.1365-2141.2006.06448.x. [DOI] [PubMed] [Google Scholar]
- 82.Schleinitz N, Ebbo M, Mazodier K, et al. Rituximab as preventive therapy of a clinical relapse in TTP with ADAMTS13 inhibitor. Am.J.Hematol. 2007;82:417–418. doi: 10.1002/ajh.20764. [DOI] [PubMed] [Google Scholar]
- 83.Downes KA, Yomtovian R, Tsai HM, et al. Relapsed thrombotic thrombocytopenic purpura presenting as an acute cerebrovascular accident. J Clin.Apheresis. 2004;19:86–89. doi: 10.1002/jca.20007. [DOI] [PubMed] [Google Scholar]
- 84.Tsai HM, Shulman K. Rituximab induces remission of cerebral ischemia caused by thrombotic thrombocytopenic purpura. Eur.J.Haematol. 2003;70:183–185. doi: 10.1034/j.1600-0609.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 85.Vesely SK, George JN, Lammle B, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102:60–68. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 86.Bell WR. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome relapse: frequency, pathogenesis, and meaning. Semin.Hematol. 1997;34:134–139. [PubMed] [Google Scholar]
- 87.Raife T, Atkinson B, Montgomery R, Vesely S, Friedman K. Severe deficiency of VWF-cleaving protease (ADAMTS13) activity defines a distinct population of thrombotic microangiopathy patients. Transfusion. 2004;44:146–150. doi: 10.1111/j.1537-2995.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 88.Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043–4049. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coppo P, Bengoufa D, Veyradier A, et al. Severe ADAMTS13 deficiency in adult idiopathic thrombotic microangiopathies defines a subset of patients characterized by various autoimmune manifestations, lower platelet count, and mild renal involvement. Medicine (Baltimore) 2004;83:233–244. doi: 10.1097/01.md.0000133622.03370.07. [DOI] [PubMed] [Google Scholar]
- 90.Ahmed S, Siddiqui AK, Chandrasekaran V. Correlation of thrombotic thrombocytopenic purpura disease activity with von Willibrand factor-cleaving protease level in ulcerative colitis. Am.J Med. 2004;116:786–787. doi: 10.1016/j.amjmed.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 91.Stave GM, Croker BP. Thrombotic microangiopathy in anti-glomerular basement membrane glomerulonephritis. Arch.Pathol.Lab Med. 1984;108:747–751. [PubMed] [Google Scholar]
- 92.Terryn W, Benoit D, Loo AV, et al. Goodpasture's syndrome associated with autoimmune thrombotic thrombocytopenic purpura an unusual case. Nephrol.Dial.Transplant. 2007 doi: 10.1093/ndt/gfm483. [PMID: 17640937] [DOI] [PubMed] [Google Scholar]
- 93.Pham PT, Danovitch GM, Wilkinson AH, et al. Inhibitors of ADAMTS13: a potential factor in the cause of thrombotic microangiopathy in a renal allograft recipient. Transplantation. 2002;74:1077–1080. doi: 10.1097/00007890-200210270-00003. [DOI] [PubMed] [Google Scholar]
- 94.Hunt BJ, Lammle B, Nevard CH, Haycock GB, Furlan M. von Willebrand factor-cleaving protease in childhood diarrhoea-associated haemolytic uraemic syndrome. Thromb Haemost. 2001;85:975–978. [PubMed] [Google Scholar]
- 95.Veyradier A, Brivet F, Wolf M, et al. Total deficiency of specific von Willebrand factor-cleaving protease and recovery following plasma therapy in one patient with hemolytic-uremic syndrome. Hematol.J. 2001;2:352–354. doi: 10.1038/sj.thj.6200126. [DOI] [PubMed] [Google Scholar]
- 96.Furlan M, Robles R, Solenthaler M, et al. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood. 1997;89:3097–3103. [PubMed] [Google Scholar]
- 97.Allford SL, Harrison P, Lawrie AS, et al. Von Willebrand factor--cleaving protease activity in congenital thrombotic thrombocytopenic purpura. Br.J.Haematol. 2000;111:1215–1222. doi: 10.1046/j.1365-2141.2000.02503.x. [DOI] [PubMed] [Google Scholar]
- 98.te Loo DM, Levtchenko E, Furlan M, Roosendaal GP, van den Heuvel LP. Autosomal recessive inheritance of von Willebrand factor-cleaving protease deficiency. Pediatr.Nephrol. 2000;14:762–765. doi: 10.1007/pl00013432. [DOI] [PubMed] [Google Scholar]
- 99.Barbot J, Costa E, Guerra M, et al. Ten years of prophylactic treatment with fresh-frozen plasma in a child with chronic relapsing thrombotic thrombocytopenic purpura as a result of a congenital deficiency of von Willebrand factor-cleaving protease. Br.J.Haematol. 2001;113:649–651. doi: 10.1046/j.1365-2141.2001.02808.x. [DOI] [PubMed] [Google Scholar]
- 100.Kokame K, Matsumoto M, Soejima K, et al. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc.Natl.Acad Sci U.S.A. 2002;99:11902–11907. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sasahara Y, Kumaki S, Ohashi Y, et al. Deficient activity of von Willebrand factor-cleaving protease in patients with Upshaw-Schulman syndrome. Int.J.Hematol. 2001;74:109–114. doi: 10.1007/BF02982559. [DOI] [PubMed] [Google Scholar]
- 102.Antoine G, Zimmermann K, Plaimauer B, et al. ADAMTS13 gene defects in two brothers with constitutional thrombotic thrombocytopenic purpura and normalization of von Willebrand factor-cleaving protease activity by recombinant human ADAMTS13. Br J Haematol. 2003;120:821–824. doi: 10.1046/j.1365-2141.2003.04183.x. [DOI] [PubMed] [Google Scholar]
- 103.Assink K, Schiphorst R, Allford S, et al. Mutation analysis and clinical implications of von Willebrand factor-cleaving protease deficiency. Kidney Int. 2003;63:1995–1999. doi: 10.1046/j.1523-1755.63.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 104.Bestetti G, Stellari A, Lattuada A, et al. ADAMTS 13 genotype and vWF protease activity in an Italian family with TTP. Thromb.Haemost. 2003;90:955–956. doi: 10.1160/TH03-03-0150. [DOI] [PubMed] [Google Scholar]
- 105.Jubinsky PT, Moraille R, Tsai HM. Thrombotic thrombocytopenic purpura in a newborn. J.Perinatol. 2003;23:85–87. doi: 10.1038/sj.jp.7210853. [DOI] [PubMed] [Google Scholar]
- 106.Schneppenheim R, Budde U, Oyen F, et al. von Willebrand factor cleaving protease and ADAMTS13 mutations in childhood TTP. Blood. 2003;101:1845–1850. doi: 10.1182/blood-2002-08-2399. [DOI] [PubMed] [Google Scholar]
- 107.Matsumoto M, Kokame K, Soejima K, et al. Molecular characterization of ADAMTS13 gene mutations in Japanese patients with Upshaw-Schulman syndrome. Blood. 2004;103:1305–1310. doi: 10.1182/blood-2003-06-1796. [DOI] [PubMed] [Google Scholar]
- 108.Pimanda JE, Maekawa A, Wind T, et al. Congenital thrombotic thrombocytopenic purpura in association with a mutation in the second CUB domain of ADAMTS13. Blood. 2004;103:627–629. doi: 10.1182/blood-2003-04-1346. [DOI] [PubMed] [Google Scholar]
- 109.Schiff DE, Roberts WD, Willert J, Tsai HM. Thrombocytopenia and severe hyperbilirubinemia in the neonatal period secondary to congenital thrombotic thrombocytopenic purpura and ADAMTS13 deficiency. J Pediatr.Hematol.Oncol. 2004;26:535–538. doi: 10.1097/01.mph.0000135284.85292.2c. [DOI] [PubMed] [Google Scholar]
- 110.Snider CE, Moore JC, Warkentin TE, et al. Dissociation between the level of von Willebrand factor-cleaving protease activity and disease in a patient with congenital thrombotic thrombocytopenic purpura. Am.J.Hematol. 2004;77:387–390. doi: 10.1002/ajh.20221. [DOI] [PubMed] [Google Scholar]
- 111.Uchida T, Wada H, Mizutani M, et al. Identification of novel mutations in ADAMTS13 in an adult patient with congenital thrombotic thrombocytopenic purpura. Blood. 2004;104:2081–2083. doi: 10.1182/blood-2004-02-0715. [DOI] [PubMed] [Google Scholar]
- 112.Veyradier A, Lavergne JM, Ribba AS, et al. Ten candidate ADAMTS13 mutations in six French families with congenital thrombotic thrombocytopenic purpura (Upshaw-Schulman syndrome) J Thromb Haemost. 2004;2:424–429. doi: 10.1111/j.1538-7933.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 113.Liu F, Jin J, Dong NZ, Wang YG, Ruan CG. [Identification of two novel mutations in ADAMTS13 gene in a patient with hereditary thrombotic thrombocytopenic purpura] Zhonghua Xue.Ye.Xue.Za Zhi. 2005;26:521–524. [PubMed] [Google Scholar]
- 114.Noris M, Bucchioni S, Galbusera M, et al. Complement factor H mutation in familial thrombotic thrombocytopenic purpura with ADAMTS13 deficiency and renal involvement. J.Am.Soc.Nephrol. 2005;16:1177–1183. doi: 10.1681/ASN.2005010086. [DOI] [PubMed] [Google Scholar]
- 115.Donadelli R, Banterla F, Galbusera M, et al. In-vitro and in-vivo consequences of mutations in the von Willebrand factor cleaving protease ADAMTS13 in thrombotic thrombocytopenic purpura. Thromb.Haemost. 2006;96:454–464. [PubMed] [Google Scholar]
- 116.Shibagaki Y, Matsumoto M, Kokame K, et al. Novel compound heterozygote mutations (H234Q/R1206X) of the ADAMTS13 gene in an adult patient with Upshaw-Schulman syndrome showing predominant episodes of repeated acute renal failure. Nephrol.Dial.Transplant. 2006;21:1289–1292. doi: 10.1093/ndt/gfk072. [DOI] [PubMed] [Google Scholar]