Abstract
Neuronal network flexibility enables animals to respond appropriately to changes in their internal and external states. We are using the isolated crab stomatogastric nervous system to determine how extrinsic inputs contribute to network flexibility. The stomatogastric system includes the well-characterized gastric mill (chewing) and pyloric (filtering of chewed food) motor circuits in the stomatogastric ganglion. Projection neurons with somata in the commissural ganglia (CoGs) regulate these rhythms. Previous work characterized a unique gastric mill rhythm that occurred spontaneously in some preparations, but whose origin remained undetermined. This rhythm includes a distinct protractor phase activity pattern, during which all active gastric mill circuit and projection neurons fire in a pyloric rhythm-timed activity pattern instead of the tonic firing pattern exhibited by these neurons during previously studied gastric mill rhythms. Here we identify a new extrinsic input, the post-oesophageal commissure (POC) neurons, relatively brief stimulation (30 sec) of which triggers a long-lasting (tens of minutes) activation of this novel gastric mill rhythm at least in part via its lasting activation of CoG projection neurons, including the previously identified MCN1 and CPN2. Immunocytochemical and electrophysiological data suggest that the POC neurons excite MCN1 and CPN2 by release of the neuropeptide Cancer borealis tachykinin-related peptide Ia (CabTRP Ia). These data further suggest that the CoG arborization of the POC neurons comprises the previously identified anterior commissural organ (ACO), a CabTRP Ia-containing neurohemal organ. This endocrine pathway thus appears to also have paracrine actions that include activation of a novel and lasting gastric mill rhythm.
Keywords: Neuromodulation, Central Pattern Generator, Projection Neurons, Neuropeptide, Cancer borealis
Introduction
Neuromodulation enables single motor circuits to generate multiple distinct activity patterns by changing the intrinsic and synaptic properties of circuit neurons (Marder et al., 2005; LeBeau et al., 2005; Kiehn, 2006; Gordon and Whelan 2006; Tryba et al., 2006). Further flexibility in the output of these motor circuits is afforded by modulatory actions at the level of the projection neurons that drive circuit activity (Di Prisco et al., 2000; Beenhakker and Nusbaum, 2004; Blitz et al., 2004; McLean and Sillar, 2004; Brocard et al., 2005; Smetana et al., 2007). However, the extrinsic pathways that provide these modulatory influences on projection neurons are not well-documented in most systems.
We are using the stomatogastric nervous system (STNS) of the crab Cancer borealis to identify the extrinsic pathway responsible for the activation of a previously identified version of the gastric mill (chewing) rhythm (Wood et al., 2004). The stomatogastric nervous system is an extension of the decapod crustacean CNS that includes the unpaired stomatogastric (STG) and oesophageal (OG) ganglia plus the paired commissural ganglia (CoGs) (Nusbaum and Beenhakker, 2002; Marder and Bucher, 2007). Overlapping sets of the 26 neurons in the C. borealis STG contribute to the gastric mill and pyloric (filtering of chewed food) rhythms (Marder and Bucher, 2007). In C. borealis, these rhythms are regulated by input from no more than 20 projection neurons, most of which are present as single neurons within each CoG (Coleman et al., 1992; Nusbaum et al., 2001). In addition, extrinsic inputs that convey sensory and other information modify these rhythms by influencing circuit neurons and/or projection neurons (Meyrand et al., 1994; Combes et al., 1999; Christie et al., 2004; Beenhakker and Nusbaum 2004; Blitz et al., 2004).
Here we identify a novel extrinsic input to the STNS of C. borealis. This pathway, called the post-oesophageal commissure (POC) neurons, consists of approximately 100 bilaterally symmetric neurons that project through the post-oesophageal (poc) and circumoesophageal (coc) commissures to innervate the CoGs.
Extracellular poc stimulation drives the POC neurons to trigger a long-lasting activation of CoG projection neurons, which in turn drive the gastric mill rhythm. Two of these projection neurons are modulatory commissural neuron 1 (MCN1) and commissural projection neuron 2 (CPN2) (Coleman and Nusbaum, 1994; Norris et al., 1994). Interestingly, despite the likely participation of MCN1 and CPN2 in the POC-triggered gastric mill rhythm, the POC-triggered activity pattern of these projection neurons and the associated gastric mill rhythm are distinct from previous versions of this rhythm that are activated by these same two projection neurons (Beenhakker and Nusbaum, 2004; Blitz et al., 2004). Our data further suggest that the POC excitation of MCN1 and CPN2 is mediated by the neuropeptide transmitter Cancer borealis tachykinin-related peptide Ia (CabTRP Ia). The POC neurons also appear to be the source of the CabTRP Ia-containing anterior commissural organ (ACO), a dense neurohemal structure in the CoG neuropil (Messinger et al., 2005).
Some of this work was published previously in abstract form (White et al., 2005).
Materials and Methods
Animals
Male Jonah crabs (C. borealis Stimpson) were obtained from Commercial Lobster and Seafood Co., Boston, MA and the Marine Biological Laboratory, Woods Hole, MA. Before experimentation, crabs were housed in commercial tanks containing recirculating, filtered and aerated artificial seawater (10°C). Crabs were cold anesthetized by packing in ice for at least 30 minutes prior to dissection. The STNS was dissected as described previously (Blitz et al. 2004). Briefly, the foregut was first removed and pinned down in a Sylgard 170 (KR Anderson, Morgan Hill, CA, or World Precision, Sarasota, FL)-coated glass bowl in chilled C. borealis saline. The poc was bisected under visual guidance and the stomach was then bisected ventrally and pinned flat with the interior stomach wall against the Sylgard. The STNS, including all four ganglia (2 CoGs, OG, STG) plus their connecting and peripheral nerves (Fig. 1), was next dissected from the surface of the foregut and pinned in a Sylgard 184 (KR Anderson)-coated Petri dish. The foregut and nervous system were maintained in chilled (10–13°C) saline throughout the dissection and subsequent experiment.
Figure 1.
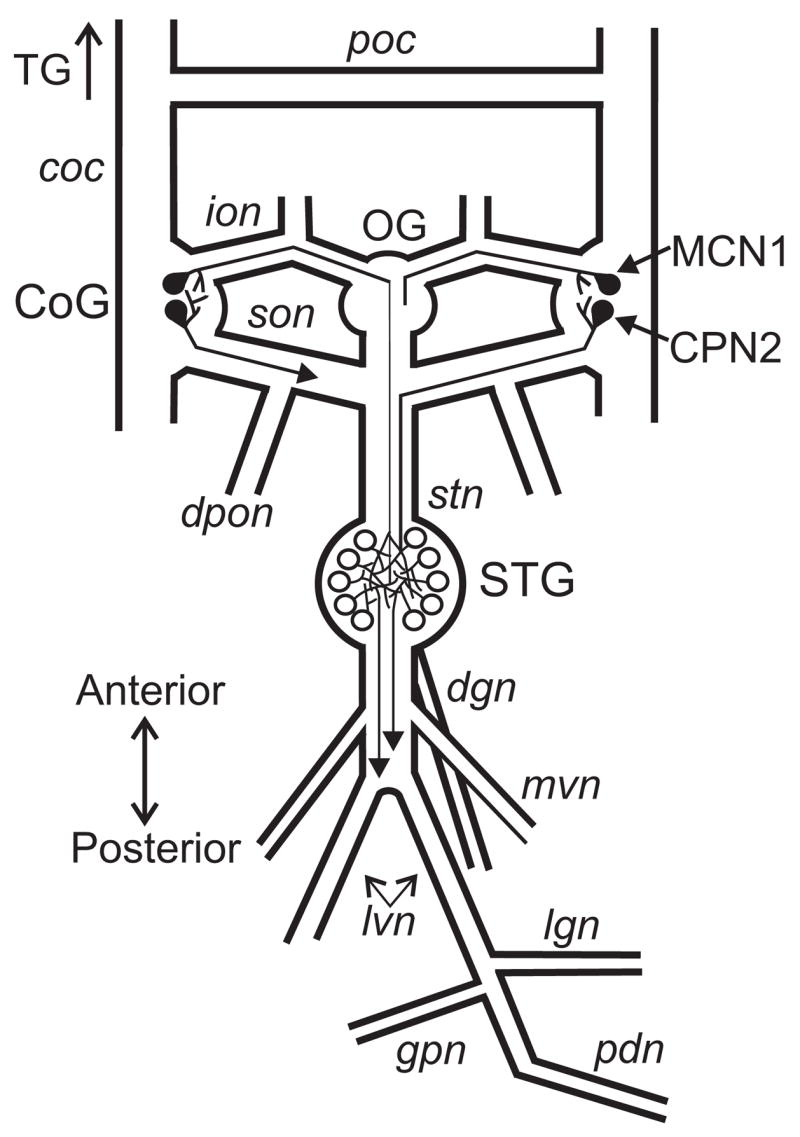
Schematic of the isolated stomatogastric nervous system, including the axon projections of MCN1 and CPN2 to the STG. Abbreviations: Ganglia- CoG, commissural ganglion; OG, oesophageal ganglion; STG, stomatogastric ganglion; TG, thoracic ganglion. Nerves- coc, circumoesophageal connective; dgn, dorsal gastric nerve; dpon, dorsal posterior oesophageal nerve; ion, inferior oesophageal nerve; lgn, lateral gastric nerve; lvn, lateral ventricular nerve; mvn, medial ventricular nerve; pdn, pyloric dilator nerve; poc, post-oesophageal commissure; son, superior oesophageal nerve. Neurons- CPN2, commissural projection neuron 2; MCN1, modulatory commissural neuron 1.
All C. borealis used for fiber counting, tracing the POC axons to the thoracic ganglion (TG) and axon diameter measurement were collected by hand at Mount Desert Island Biological Laboratory (Salisbury Cove, Maine, USA) and maintained in flow-through natural seawater tanks at ambient water temperature (10–14°C). For ease of dissection and immunoprocessing, these animals were smaller than those used for electrophysiological experiments. As above, for tissue collection these crabs were first anesthetized by packing in ice for at least 30 minutes. The dorsal carapace was then removed and the thoracic neuromeres, with the cocs and CoGs attached, were isolated by microdissection in chilled (approximately 10°C) C. borealis saline.
Solutions
Cancer borealis saline for dissections had the following composition (in mM): 440 NaCl, 26 MgCl2, 13 CaCl2, 11 KCl, 10 Trizma base, and 5 maleic acid (pH 7.4–7.6). During recording, 5 mM dextrose was added to the saline. In high-divalent cation saline (Hi-Di), MgCl2 and CaCl2 were raised to 130 mM and 65 mM, respectively. Phosphoramidon (Sigma, St. Louis, MO USA) and CabTRP Ia (Biotechnology Center, University of Wisconsin, Madison, WI USA) were stored as frozen aliquots and diluted in C. borealis saline immediately prior to use.
Electrophysiology
Extracellular recordings were made by isolating a section of nerve with petroleum jelly (Vaseline: Medical Accessories and Supply Headquarters, Alabaster, AL) and placing one stainless steel wire of a pair inside the Vaseline compartment and the other wire in the main bath compartment. These recordings were amplified in a 2-stage process (Stage 1: AM Systems Model 1700 AC Amplifier, Carlsborg, WA USA; Stage 2: Brownlee Precision Model 410 Amplifier, Santa Clara, CA USA). To facilitate intracellular recordings, ganglia were desheathed and viewed with light transmitted through a darkfield condenser (Nikon, Tokyo, Japan). Intracellular recordings were accomplished using borosilicate microelectrodes filled with 0.6 M K2SO4 plus 10 mM KCl (20–25 MΩ). Intracellular signals were amplified using Axoclamp 2B amplifiers (Molecular Devices, Sunnyvale, CA) and digitized at ~5 kHz using a Micro 1401 data acquisition interface and Spike2 software (Cambridge Electronic Design, Cambridge, England). Network and projection neurons were identified based on their activity patterns, synaptic connectivity and axonal projection patterns (Weimann et al., 1991; Norris et al., 1994; Coleman and Nusbaum 1994; Beenhakker and Nusbaum, 2004; Saideman et al., 2007a,b). In some experiments, the activity of the projection neuron CPN2 was monitored indirectly, via the presence of excitatory postsynaptic potentials (EPSPs) in the gastric mill (GM) protractor motor neuron (Norris et al., 1994).
Each half of the bisected poc was surrounded by a Vaseline well. Axons in the poc were stimulated extracellularly using a Grass S88 stimulator (AstroMed, Warwick, RI USA) and stimulus isolation unit (SIU5, AstroMed). The poc was stimulated tonically, using a range of voltages (4–15 V), at 15–30 Hz for 15–30 seconds. To activate the gastro-pyloric receptor 2 neuron (GPR2: Katz et al., 1989), the gastro-pyloric nerve (gpn) was stimulated tonically at 10 Hz for 4 seconds. The ventral cardiac neurons (VCNs: Beenhakker et al., 2004) were activated by stimulating the dorsal posterior oesophageal nerve (dpon) in a rhythmic pattern (burst duration: 6 sec, interburst freq.: 0.06 Hz, intraburst freq.: 15 Hz) (Beenhakker et al., 2004; Beenhakker and Nusbaum 2004). CabTRP Ia was pressure ejected (10−4 M, 6 – 10 psi, 0.5 – 10 sec) into the desheathed CoG neuropil using a Picospritzer II device (General Valve Corporation, Fairfield, NJ USA). The dorsal aspect of the CoG is covered with neuronal somata, and the neuropil is underneath these somata. Therefore, to focally apply CabTRP Ia into the CoG neuropil, we inserted the peptide-containing pipette through the soma layer and into the depth of the anterior neuropil (Blitz and Nusbaum, 1999). The endopeptidase inhibitor phosphoramidon (10−5 M) was superfused to the anterior portion of the nervous system, which was isolated from the STG by a Vaseline wall built across the recording dish. No data collection was made until phosphoramidon superfusion had occurred for at least 25 min.
Immunocytochemistry
Whole-mounts of the isolated STNS and the thoracic neuromeres with attached cocs and CoGs were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA USA) for 12–24 hours, rinsed at least 5 times, at one hour intervals, in phosphate (P) buffer (0.1 M) with 0.3 % Triton-X 100 (P-Triton) and then incubated for 24–72 hours with a monoclonal rat anti-Substance P antibody (1:300; Accurate Chemical and Scientific Corporation, Westbury, NY USA; Abcam Incorporated, Cambridge, Massachusetts, USA) that has been used previously on this system (Goldberg et al., 1988; Christie et al., 1997; Blitz et al., 1999; Messinger et al., 2005). The nervous system was then again rinsed in P-Triton, 5 times at one hour intervals, after which the STNS preparations were incubated with goat anti-rat Alexa Fluor 488 or 647 (1:300; Invitrogen Corporation, Carlsbad, CA USA) for 12–16 hours. In preparations where the thoracic nervous system was studied, the nervous system was incubated with donkey anti-rat IgG conjugated with either FITC or rhodamine Red-X (Jackson ImmunoResearch, West Grove, Pennsylvania, USA). In both cases, the preparations were then rinsed at least 5 times at one hour intervals with P buffer and then mounted in 80% glycerol/20% 20 mM sodium carbonate and cover-slipped. For the STNS preparations, fluorescence was visualized and photographed with a Leica DMRB microscope, a Leica DC 350 FS camera, and Image-Pro Express software (Leica, version 4.5.1.3) using a L4 or Y5 (Leica) filter set (Leica Microsystems Inc., Bannockburn, IL). The thoracic-CoG preparations were imaged using a Zeiss LSM 510 Meta confocal system (Carl Zeiss MicroImaging Inc., Thornwood, NY), equipped with a Zeiss Observer.Z1 inverted microscope and argon and HeNe lasers. Imaging was done using Zeiss EC plan-NEOFLUAR 10x/0.3 dry, Plan-Apochromat 20x/0.8 dry, EC plan-NEOFLUAR 40x/1.30 oil and Plan-Apochromat 63x/1.4 oil objective lenses, standard FITC and rhodamine filter sets, and manufacturer-supplied software.
Data Analysis
Data analysis was performed with custom written macros using Spike2 (‘The Crab Analyzer’, freely available at http://www.uni-ulm.de/~wstein/spike2/index.html). Gastric mill cycle period was measured as the duration from the onset of a lateral gastric (LG) neuron burst to the onset of the subsequent LG burst. An average of 10 consecutive cycles was obtained in each condition. Control MCN1 and CPN2 firing frequencies were measured during 30 continuous seconds prior to stimulation. MCN1 and CPN2 firing frequencies after stimulation were quantified during 10 consecutive protraction and retraction phases of the gastric mill rhythm in each preparation, as the average frequency across the entire protraction or retraction phase. MCN1 pyloric-timed activity was measured as the percentage of time it was active from the onset of a pyloric dilator (PD) neuron burst until the onset of the subsequent PD burst for the pyloric cycles occurring during 10 consecutive protraction and retraction phases in each preparation. Figures were made using Spike2, CorelDraw (Corel Corporation, Ottawa, Ontario, Canada) and Igor Pro (Wavemetrics, Portland, OR). Statistical analysis was performed with SigmaStat (Systat Software, San Jose, CA). The Paired Student’s t-test or Repeated Measures One-Way ANOVA followed by multiple comparisons using the Student-Newman-Keuls method were used as indicated. Significance was considered to be p<0.05. Data are expressed as mean ± s.e.m.
Results
The gastric mill rhythm is a two-phase motor pattern driven by descending input
The gastric mill rhythm (cycle period: 5–20 sec) drives the rhythmic protraction and retraction movements of the teeth in the gastric mill stomach compartment, thereby enabling the chewing of food (Heinzel, 1988; Heinzel et al., 1993). In C. borealis there are 8 different types of gastric mill neurons, 7 of which are motor neurons (Weimann et al., 1991; Saideman et al., 2007b; Stein et al., 2007). Four of these gastric mill neurons are protractor motor neurons, including the LG, GM, medial gastric (MG) and inferior cardiac (IC) neurons, although the IC and MG neurons can also exhibit retractor phase activity during some versions of the gastric mill rhythm (Beenhakker and Nusbaum, 2004; Blitz et al., 2004; Wood et al., 2004; Saideman et al., 2007b). There are also three retractor motor neurons, including the dorsal gastric (DG), anterior median (AM) and ventricular dilator (VD) motor neurons, plus interneuron 1 (Int1), which is also active during the retractor phase and is the sole interneuron in this circuit. There is a single neuron of each type per STG, except the GM neurons of which there are four functionally equivalent copies.
In the isolated STNS of C. borealis, some of the gastric mill neurons (Int1, MG, IC, VD) are spontaneously active in pyloric rhythm-time, even in the absence of the gastric mill rhythm (e.g. VD and IC in Fig. 2, left panel) (Weimann et al., 1991; Blitz and Nusbaum, 1997). The pyloric rhythm (cycle period 0.5 – 2 sec), which controls the filtering of chewed food in the posterior (pyloric) stomach compartment, is generated by a second motor circuit in the STG and is continuously active both in vitro and in vivo (Marder and Bucher, 2007).
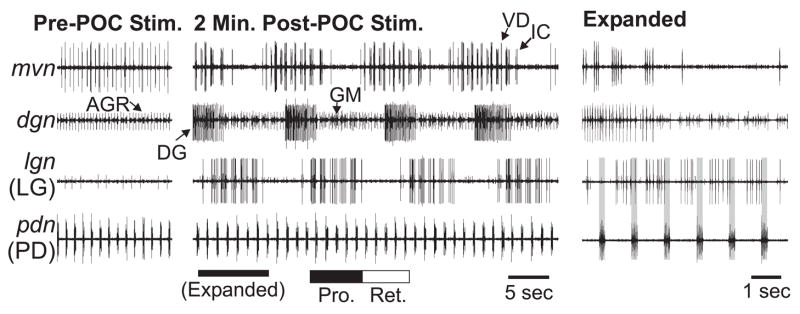
Figure 2.
The gastric mill rhythm is triggered by poc nerve stimulation. (Left) Prior to poc stimulation, there was an ongoing pyloric rhythm (mvn, pdn), but no gastric mill rhythm (dgn, lgn). The tonically active unit in the dgn corresponds to the activity of the anterior gastric receptor (AGR) neuron. AGR is a muscle tendon proprioceptor neuron that is spontaneously active in the isolated STNS (Combes et al., 1995; Smarandache and Stein, 2007). (Middle) Two minutes after tonic poc stimulation (15 Hz, 30 sec), the gastric mill rhythm was triggered, as is evident from the rhythmic bursting in the protractor LG and GM neurons that alternated with the retractor phase activity of the DG, VD and IC neurons. Note the pyloric-timed bursting in the LG neuron. (Right) This expanded section of the middle panel shows more explicitly that each protractor LG burst is time-locked to the pyloric rhythm. Each period of inactivity in LG starts with a pyloric dilator (PD) neuron burst (grey bars).
The gastric mill rhythm is usually silent, in the isolated STNS as well as in vivo, unless the projection neurons that drive it are activated (Fleischer 1981; Heinzel et al., 1993; Nusbaum et al., 2001; Beenhakker and Nusbaum, 2004; Blitz et al., 2004). Wood et al. (2004), however, characterized a version of the gastric mill rhythm that occurred in the absence of any experimental manipulation of projection neuron activity. This gastric mill rhythm was unusual in that all protraction phase neurons exhibited a pyloric rhythm-timed activity pattern, instead of the tonic firing pattern that they exhibit in all other characterized gastric mill rhythms in C. borealis (Coleman and Nusbaum, 1994; Beenhakker et al., 2004; Blitz et al., 2004; Christie et al., 2004; Saideman et al., 2007b). This spontaneously active gastric mill rhythm was driven largely by an unusually high level of spontaneous activity in the projection neuron MCN1. This MCN1 activity was pyloric rhythm-timed, and the resulting gastric mill rhythm was largely replicated by pyloric rhythm-timed extracellular stimulation of MCN1 (Wood et al., 2004).
POC stimulation triggers a long-lasting gastric mill rhythm
Stimulating the poc nerve (15 Hz tonic stimulation, 30 sec. duration) consistently triggered the gastric mill rhythm, beginning soon after the stimulation was terminated (n=39). In the example shown in Figure 2, this rhythm started approximately two minutes after the end of poc stimulation and, as is typical for gastric mill rhythms, there was rhythmic alternating bursting of the protractor (LG, GM neurons) and retractor (DG, VD) neurons. It is also noteworthy that, during these rhythms, the IC neuron was mostly active during the retractor phase instead of the protractor phase (Fig. 2). Across preparations, the poc-triggered gastric mill rhythm started approximately 1 minute after the end of poc stimulation (mean latency post-stimulation: 0.91 ± 0.05 min, n=39). These rhythms exhibited a cycle period of 13.1 ± 0.9 sec (n=20).
During each protractor phase, the active gastric mill neurons (i.e., LG, GM) exhibited pyloric rhythm-timed bursts (Fig. 2). These bursts alternated with activity in the pyloric pacemaker neurons (e.g. PD neuron in Fig. 2). In most previously characterized versions of the gastric mill rhythm, these gastric mill protractor neuron bursts exhibited a tonic firing pattern (Beenhakker and Nusbaum, 2004; Blitz et al., 2004; Christie et al., 2004; Saideman et al., 2007b). However, this same pyloric-timed burst pattern in the protractor LG neuron also occurred in the spontaneously active rhythm characterized by Wood et al. (2004).
The poc-triggered gastric mill rhythm was also long-lasting. After a 30 sec poc stimulation, the gastric mill rhythm tended to persist for many minutes, and sometimes for more than one hour (n=39) (Fig. 3). Specifically, in a few preparations this rhythm lasted for less than 5 minutes (n=4/39), but it often persisted for 5–20 minutes (n=22/39) or longer (n=13/39).
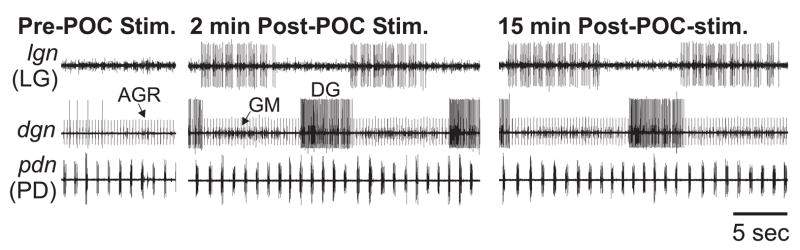
Figure 3.
The poc-triggered gastric mill rhythm is long-lasting. (Left) Before poc stimulation, there was an ongoing pyloric rhythm (pdn) but no gastric mill rhythm (lgn, dgn). (Middle) Two minutes after tonic poc stimulation (15 Hz, 30 sec), the gastric mill rhythm had been triggered and was ongoing. Note the pyloric-timed LG bursts. (Right) This rhythm persisted for more than 15 minutes after poc stimulation.
POC stimulation indirectly activates the gastric mill rhythm
Extrinsic inputs can alter STG circuit activity via synaptic actions on circuit neurons and/or descending projection neurons (Hooper and Moulins, 1990; Katz and Harris-Warrick, 1990; Meyrand et al., 1994; Combes et al., 1999; Beenhakker and Nusbaum 2004; Blitz et al., 2004). To determine whether the pathway(s) activated by poc stimulation influenced the gastric mill circuit directly or indirectly, we selectively superfused the CoGs with high divalent cation (Hi-Di: 5 X Ca2+/5 X Mg2+) saline while continuing to supply normal C. borealis saline to the STG. The Hi-Di saline raises action potential threshold and reduces the likelihood of polysynaptic transmission (Blitz and Nusbaum, 1999). This allowed us to reversibly block any poc synaptic actions on CoG projection neurons and thereby determine whether it activated the gastric mill rhythm via direct actions on STG neurons.
Stimulating the poc, when the CoGs were superfused with Hi-Di saline, never activated the gastric mill rhythm (n=6), even when the stimulation voltage was increased by 2 V (Fig. 4). To ensure that the inability of poc stimulation to activate the gastric mill rhythm was not a consequence of a dysfunctional gastric mill circuit, we used extracellular stimulation of the ion to drive this rhythm via selective activation of the projection neuron MCN1 (Bartos et al., 1999). Tonic MCN1 stimulation elicits a distinct gastric mill rhythm from the one triggered by poc stimulation, but both rhythms involve the same gastric mill circuit neurons (Coleman et al., 1995; Bartos et al., 1999; Wood et al., 2004; Saideman et al., 2007b; this paper). Extracellular MCN1 stimulation consistently elicited the gastric mill rhythm despite the presence of high-divalent cation saline to the CoGs (n=3, data not shown). Further, after washing out the Hi-Di saline, poc stimulation again triggered the gastric mill rhythm (n=5/6). Thus, axons in the poc appear to project into the CoGs to activate projection neurons and thereby indirectly activate the gastric mill rhythm. We have designated the poc pathway that triggers the gastric mill rhythm as the POC neurons (see below).
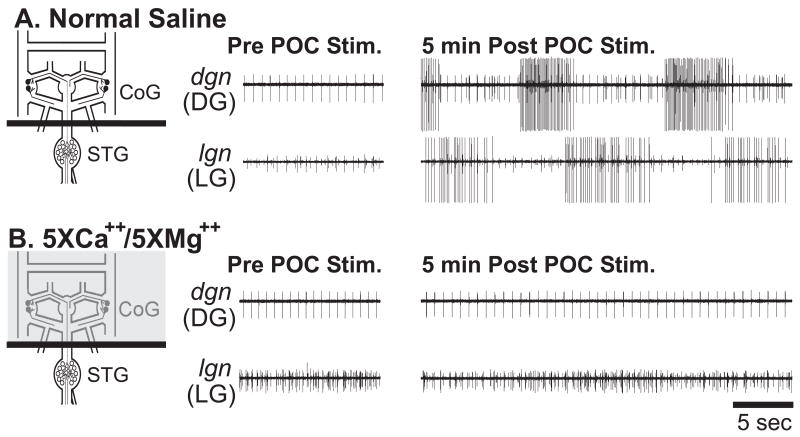
Figure 4.
The poc-triggered gastric mill rhythm requires the activation of CoG projection neurons. (A) During normal saline superfusion of the CoGs, tonic poc stimulation (15 Hz, 30 sec) triggered the gastric mill rhythm. (B) During superfusion of 5X Mg2+/5X Ca2+ saline to the CoGs, the same poc stimulation did not trigger the gastric mill rhythm.
The POC neurons excite the projection neurons MCN1 and CPN2
Two previously identified CoG projection neurons in C. borealis, MCN1 and CPN2, are necessary and sufficient for driving two previously characterized gastric mill rhythms, elicited by stimulation of a mechanosensory (VCN neurons) or proprioceptor (GPR neuron) pathway (Beenhakker and Nusbaum, 2004; Blitz et al., 2004). Further, the spontaneously active gastric mill rhythm studied by Wood et al. (2004) was largely mimicked by direct stimulation of MCN1. We therefore examined the activity of MCN1 and CPN2 before and after POC stimulation, and found that the stimulation consistently triggered a long lasting excitatory response in both projection neurons (n=39). This excitatory response included an increased firing rate and pyloric-timed activity (Fig. 5).
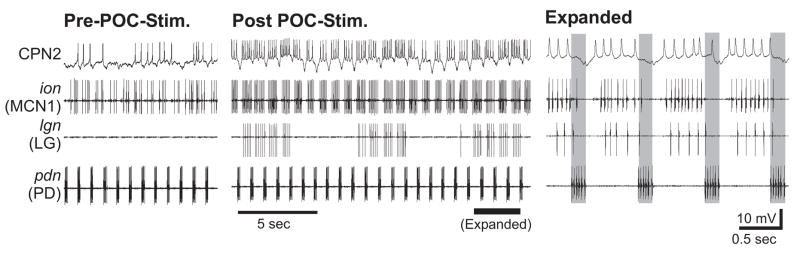
Figure 5.
POC stimulation triggers activation of the CoG projection neurons MCN1 and CPN2 as well as the gastric mill rhythm. (Left) Before stimulation, the projection neurons CPN2 and MCN1 were weakly active and there was an ongoing pyloric rhythm (pdn) but no gastric mill rhythm (lgn, dgn). (Middle) After poc stimulation (15 Hz, 30 sec), CPN2 and MCN1 were excited and the gastric mill rhythm was elicited. (Right) Expanded time scale from the middle panel showing that the activity of LG, MCN1 and CPN2 is interrupted in pyloric-time. Note that each such interruption occurs during activity of the pyloric pacemaker PD neuron (grey bars). Most hyperpolarized membrane potential: CPN2, −45 mV.
The POC-triggered excitation of MCN1 and CPN2 always coincided with the triggering of the gastric mill rhythm (n=39). After POC stimulation, the firing frequency of MCN1 was consistently higher than pre-stimulation (pre-POC stim.: 4.0 ± 0.5 Hz; post-POC stim.: protraction phase (LG burst), 14.5 ± 1.2 Hz, retraction phase (LG inter-burst), 14.7 ± 1.2 Hz, n=10; protraction and retraction significantly different from control, p<0.05, protraction not significantly different from retraction, p>0.05, Repeated Measures One-way ANOVA followed by Student-Newman-Keuls test of multiple comparisons). Similarly, CPN2 firing frequency was consistently increased after POC stimulation (pre-POC: 2.8 ± 1.1 Hz; post-POC: protraction, 18.2 ± 3.3 Hz; retraction, 15.6 ± 3.3 Hz, n=4; protraction and retraction significantly different from control, p<0.05, protraction not significantly different from retraction, p>0.05, Repeated Measures One-way ANOVA followed by Student-Newman-Keuls test of multiple comparisons).
During these POC-triggered gastric mill rhythms, MCN1 was active for ~65% of each pyloric cycle during protraction (MCN1 onset: 34.9 ± 2.9%, MCN1 offset: 100.0 ± 2.5%; onset and offset measured as a percentage of the PD neuron cycle period) and was active for ~58% of each pyloric cycle during retraction (MCN1 onset: 39.4 ± 3.1%, MCN1 offset: 98.3 ± 0.6%) (n=6). MCN1 was silent during each pyloric pacemaker neuron burst (pacemaker onset: 0%, pacemaker offset: 20.1 ± 0.4% during protraction, 20.2 ± 0.6% during retraction, n=6). Comparably, CPN2 was active for ~72% of each pyloric cycle during protraction (CPN2 onset: 30.3 ± 0.9%, CPN1 offset: 102.4 ± 0.8%) and was active for ~47% of each pyloric cycle during retraction (CPN2 onset: 42.3 ± 1.3%, CPN2 offset: 88.4 ± 7.2%) (n=3). As was the case for MCN1, CPN2 was not active during each pyloric pacemaker burst (pacemaker offset: 20.8 ± 0.4% during protraction, 20.7 ± 0.7% during retraction) (n=3). MCN1 and CPN2 were presumably silent during the pacemaker burst due to feedback inhibition in the CoG from the anterior burster (AB), the pyloric pacemaker interneuron (Coleman and Nusbaum, 1994; Norris et al., 1994; Wood et al., 2004).
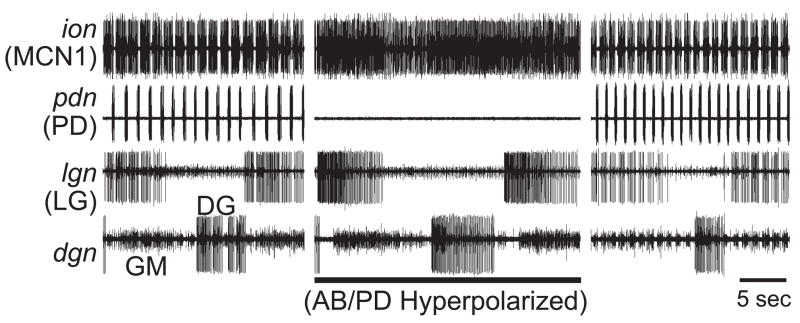
We tested the hypothesis that the AB neuron feedback to MCN1 and CPN2 in the CoG was responsible for the pyloric-timed activity pattern of these projection neurons during the POC-triggered gastric mill rhythm. Specifically, we used hyperpolarizing current injection into the pyloric pacemaker neurons to suppress their activity and, consequently, that of the pyloric rhythm. The pyloric pacemaker neurons are a group of electrically coupled neurons that include the single AB neuron plus the paired PD and lateral posterior gastric (LPG) neurons (Weimann et al., 1991; Weimann and Marder, 1994). When the pyloric rhythm was suppressed during the POC-triggered gastric mill rhythm, MCN1 and CPN2 activity switched from pyloric-timed to tonic (n= 4) (Fig. 6). At these times, the LG neuron activity pattern also switched from pyloric-timed to tonic, presumably because its activity was driven by these projection neurons (Beenhakker and Nusbaum, 2004). There is no direct synapse from the pyloric pacemaker neurons to LG (Bartos et al., 1999). In contrast to our findings, in the European lobster Homarus gammarus the pyloric-like activity of some CoG projection neurons can persist when the pyloric feedback is eliminated (Cardi and Nagy, 1994).
Figure 6.
The pyloric rhythm in the STG is responsible for the pyloric-timed activity of the CoG projection neuron MCN1 and the gastric mill neuron LG during the POC-triggered gastric mill rhythm. (Left) During the POC-triggered gastric mill rhythm, MCN1 exhibited pyloric-timed activity during both protraction and retraction, as did the protractor neuron LG. (Middle) When the pyloric rhythm was suppressed, by hyperpolarization of the pyloric pacemaker neurons, the POC-triggered gastric mill rhythm persisted but the MCN1 and LG neuron activity changed from pyloric-timed to tonic. (Right) After releasing the pyloric pacemaker neurons from hyperpolarization, the pyloric rhythm resumed and the neurons MCN1 and LG returned to exhibiting pyloric-timed activity.
In previously studied gastric mill rhythms (Bartos et al., 1999; Wood et al., 2004), the gastric mill cycle period was regulated by the pyloric rhythm. Specifically, suppressing the pyloric rhythm increased the gastric mill cycle period. This was due to both inter-circuit interactions within the STG and to the pyloric-timing of MCN1 activity (Wood et al., 2004). Thus, we tested whether the cycle period of the POC-triggered gastric mill rhythm was also regulated by pyloric rhythm. We found that the POC-triggered gastric mill cycle period was indeed increased when the pyloric rhythm was suppressed, from 12.3 ± 1.8 sec to 19.4 ± 2.7 sec (n=4; p<0.05, Paired t-test).
The POC neurons project through the medial aspect of the coc to innervate the CoGs
As a step towards localizing the POC neurons, we determined whether their axons preferentially projected through the lateral or medial aspect of the coc. We anticipated that the POC neurons projected through the medial coc, because most projections through the coc that innervate the CoG do so via the medial coc (Kirby and Nusbaum, 2007). To determine if this was indeed the case for the POC neurons, we first stimulated the poc with the entire coc intact, to ensure the ability of this pathway to trigger the gastric mill rhythm in these preparations (Fig. 7). We then selectively transected either the lateral (n=3) or medial (n=3) aspect of the coc, after which we again assessed the ability of poc stimulation to trigger the gastric mill rhythm (Fig. 7). There were no landmarks to enable precise transection of exactly one half of each coc. Therefore, these transections were done in a fashion to ensure the retention of the lateral-most or medial-most coc, with a variable degree of transection of the central aspect of this nerve from preparation to preparation.
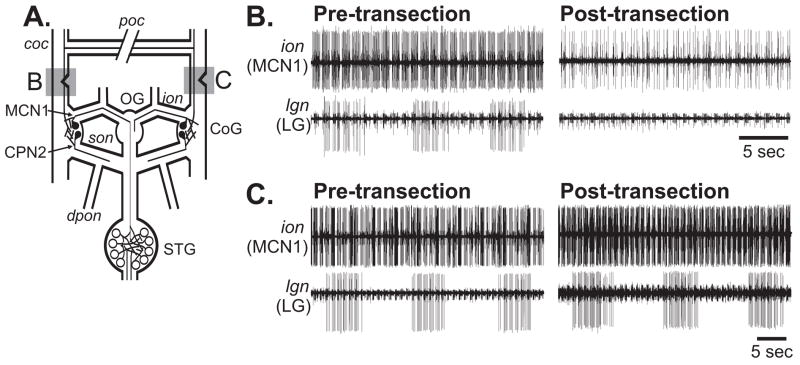
Figure 7.
The POC neurons project through the medial aspect of the coc to influence MCN1 and CPN2 in the CoG. (A) STNS schematic indicating the location and extent of the coc transections that occurred in Panels B and C (grey boxes). (B) Transecting the medial aspect of the coc eliminated the ability of poc stimulation to trigger the gastric mill rhythm. (Left) POC stimulation before medial coc transection triggered the gastric mill rhythm. (Right) POC stimulation after medial coc transection did not trigger the gastric mill rhythm. (C) Transecting the lateral aspect of the coc did not alter the ability of poc stimulation to trigger the gastric mill rhythm. POC stimulation before (Left) and after (Right) lateral coc transection triggered the gastric mill rhythm.
Poc stimulation never triggered the gastric mill rhythm after medial coc transection (n=3) (Fig. 7B). In contrast, poc stimulation consistently triggered the gastric mill rhythm in every preparation after the lateral coc was transected (n=3). The resulting motor pattern retained its characteristic pyloric-timed activity pattern during the protractor phase (Fig. 7C). In these latter experiments, the resulting gastric mill rhythm continued to persist for a long duration, ranging from 8–24 minutes (n=3).
To ensure that the CoG projection neurons and STG circuit neurons were still capable of generating the gastric mill rhythm after medial coc transection, we stimulated the VCN neurons (Beenhakker et al., 2004; Beenhakker and Nusbaum, 2004). The VCN-triggered gastric mill rhythm was readily elicited in each of the 3 preparations after the medial coc was transected (not shown).
The POC neurons appear to contain the peptide transmitter CabTRP Ia
There is a dense CabTRP Ia-immunoreactive (CabTRP Ia-IR) arborization within the anterior CoG neuropil, called the anterior commissural organ (ACO) (Fig. 8A) (Messinger et al., 2005). The ACO innervates each CoG via a population of small diameter axons that project as a bundle through the medial aspect of the anterior coc (Goldberg et al., 1988; Messinger, 2005). Based on the results of the coc transection experiments reported above, and the fact that MCN1 and CPN2 arborize in the anterior CoG neuropil (Coleman and Nusbaum, 1994; Norris et al., 1994), we examined whether the ACO axons projected through the poc and therefore might be the axons of the POC neurons.
Figure 8.
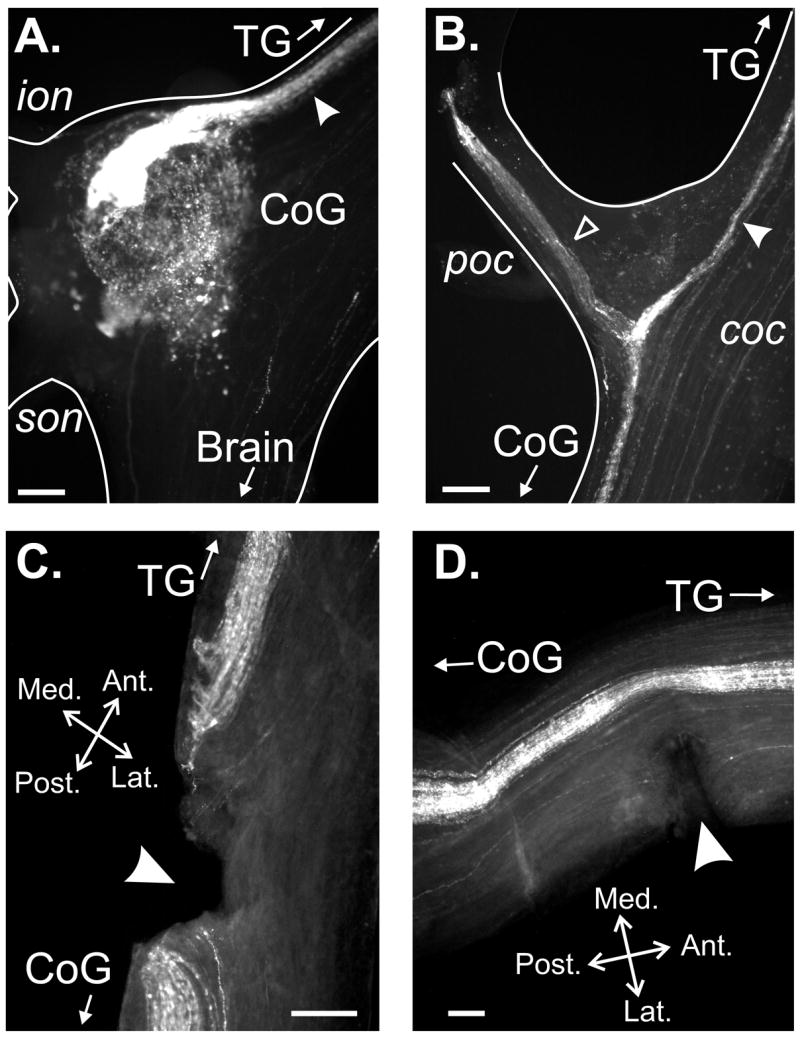
A CabTRP Ia-immunoreactive (IR) axon bundle projects through the poc and medial aspect of the anterior coc to form terminal arborizations in the CoG. (A) CabTRP Ia-IR occurred in a tightly associated axon bundle in the medial aspect of the anterior coc (arrowhead) that terminated as a dense arborization in the antero-medial CoG. There was also more diffuse CabTRP Ia-IR throughout the CoG neuropil and in a subset of CoG neuronal somata. (B) The CabTRP Ia-lR axon bundle in the medial aspect of the anterior coc (filled arrowhead) projected past the poc towards the TG, and also projected through the poc (open arrowhead). (C) CabTRP Ia-IR bundle was transected in a preparation in which the medial coc was transected (arrowhead). (D) CabTRP Ia-IR bundle was not transected in a preparation in which the lateral coc was transected (arrowhead). Spatial axes in (C) are for (A-C). All scale bars=150 μm.
Wholemount immunocytochemistry revealed that the ACO axon population did indeed project through the poc (Fig. 8B). Specifically, at the junction between the coc and poc, a fraction of the CabTRP Ia-IR axon bundle in the medial coc separated and projected through the poc, while the remainder projected posteriorly past the poc as a tight bundle along the medial coc and terminated as the ACO in the ipsilateral CoG (n=16) (Fig. 8B). This CabTRP Ia-IR fiber bundle projection continued anteriorly in the medial coc, past the poc, towards the TG (Fig. 8B) (n=16).
As further support that the POC neurons were likely to be the source of the ACO, we determined whether the CabTRP-IR bundle in the medial coc was transected or retained in each of the above coc transection experiments. We found that, in each experiment in which the medial coc was transected and poc stimulation no longer triggered the gastric mill rhythm, the CabTRP Ia-IR bundle had been transected (Fig. 8C; n=3). Conversely, the CabTRP Ia-IR bundle remained intact in preparations in which the lateral coc was transected and poc stimulation still triggered the gastric mill rhythm (Fig. 8D; n=3).
We also combined CabTRP immunocytochemistry and confocal microscopy to determine the number and distribution of axon diameters for the CabTRP-IR axons in the poc and medial coc bundle. In both the poc and coc just anterior to the CoG, the CabTRP Ia-IR axons were of small diameter (<1 μm) and often tightly fasciculated. Their relatively small diameter and tight fasciculation made it difficult to unambiguously determine the number of individual axons present. For example, in 5 separate preparations we obtained a distribution of CabTRP Ia-IR axon counts from the left coc (81, 83, 78, 108 and 93) and right coc (98, 88, 72, 67 and 88). In the same 5 preparations, the distribution of axon counts in the poc suggested a smaller number of CabTRP Ia-IR axons (53, 76, 68, 67 and 65). In no preparation was branching from the axon bundles seen within the cocs or poc.
In all preparations examined, the CabTRP Ia-IR bundle in the medial coc was traced to the junction of the coc with the TG (n=5). At this location, the POC axon bundle was less tightly fasciculated, often fanning out and covering a large portion of the nerve (not shown). In 5 separate preparations, we obtained similar axon counts to those from the coc near the CoG (left coc: 80, 74, 82, 88 and 67; right coc: 77, 93, 43, 64 and 86). Due to the density and intensity of CabTRP-IR within the thoracic ganglion, it was not possible to localize the destination of the POC axons within this ganglion. It is possible that CabTRP Ia-IR somata within the thoracic ganglion are the origin of the POC axons. However, no discrete clusters of 50–100 CabTRP Ia-IR somata were identified within this ganglion (data not shown).
The POC neurons appear to use the peptide transmitter CabTRP Ia
To determine whether ACO-released CabTRP Ia mediated the long-term actions of the POC neurons on MCN1 and/or CPN2, we examined whether focal application of CabTRP Ia mimicked the POC excitation of these projection neurons. In some of these experiments (e.g. Fig. 9), CPN2 activity was monitored via intracellular GM neuron recordings. CPN2 is the sole source of discrete excitatory postsynaptic potentials in GM (Norris et al., 1994).
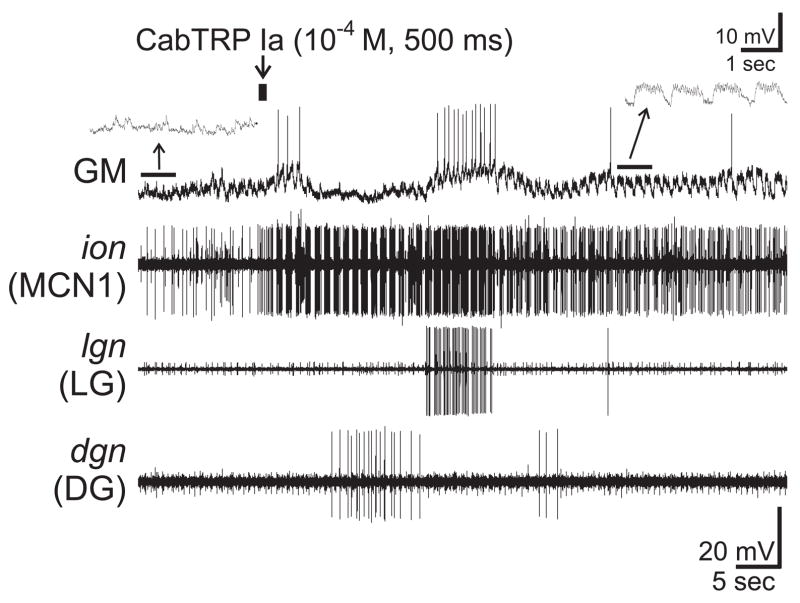
Figure 9.
Exogenous CabTRP Ia mimics the POC activation of MCN1 and CPN2. A brief (500 ms) puff of CabTRP Ia (10−4 M) into the antero-medial aspect of the CoG neuropil excited MCN1 and CPN2 (monitored as EPSPs in GM; see text), and subsequently activated LG and DG. Note that CabTRP Ia triggered pyloric-timed activity in MCN1, CPN2 and LG. Insets at an expanded time scale indicate that the GM membrane potential was not pyloric-timed before CabTRP Ia application but exhibited barrages of EPSPs that were interrupted in pyloric-time after this application. Most hyperpolarized membrane potential: GM, −67 mV.
Brief, focal application of CabTRP Ia (10−4 M: 500 msec) into the anterior CoG neuropil triggered increased activity in MCN1 and CPN2 (n=4) (Fig. 9). This increased activity was consistently pyloric-timed. In some preparations, the CabTRP Ia-triggered excitation of MCN1 and CPN2 led to the equivalent of a single gastric mill cycle, including an action potential burst in the retractor DG neuron preceding a burst in the protractor LG and GM neurons (Fig. 9).
To further assay whether CabTRP Ia mediated the actions of the POC neurons on MCN1 and/or CPN2, we determined whether suppressing the extracellular peptidase-mediated degradation of this peptide would prolong the POC influence on these projection neurons. To this end, we applied the endopeptidase inhibitor phosphoramidon (10−5 M), which effectively prolongs the actions of both focally applied and neuronally released CabTRP Ia (Wood and Nusbaum, 2000; Stein et al., 2007). Because the poc stimulus protocol used to trigger the gastric mill rhythm had such a long-lasting effect, we used briefer poc stimulations (15 Hz, 15 sec) to achieve relatively brief control responses. These control stimulations triggered increased activity in MCN1 and CPN2 as well as a relatively short-lasting gastric mill rhythm (duration: 0.5 –13 min, n=5) (Fig. 10A). For example, in Figure 10A the projection neuron activity was subsiding and the gastric mill rhythm had terminated by 90 sec post-POC stimulation (Fig. 10A). As shown in Figure 10A, the POC-triggered rhythm during phosphoramidon superfusion persisted for more than 90 sec post-stimulation. After washout of the phosphoramidon, the POC action on CPN2 and the gastric mill rhythm returned to pre-application levels (Fig. 10A). In all cases, when phosphoramidon (10−5 M) was superfused selectively to the CoGs, POC stimulation triggered a more prolonged excitation of MCN1 (not shown) and CPN2 and triggered a longer-lasting gastric mill rhythm (n=5).
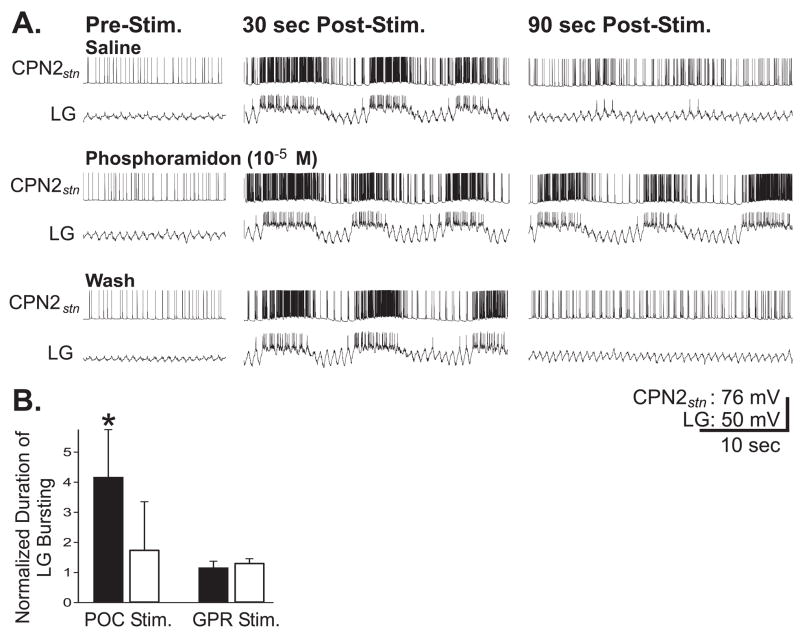
Figure 10.
Blocking extracellular peptidase-mediated degradation of CabTRP Ia prolongs the actions of the POC neurons. (A) Before, during and after superfusion of the endopeptidase inhibitor phosphoramidon (10−5 M) to the CoGs, CPN2 was weakly active before poc stimulation and LG was silent (left panel: top, middle, bottom). Thirty seconds after poc stimulation (15 Hz, 15 sec), the gastric mill rhythm was triggered (as indicated by the rhythmic LG bursting) and CPN2 activity was strengthened (middle panel: top, middle, bottom). Ninety seconds after poc stimulation, the gastric mill rhythm had terminated and CPN2 activity had subsided during saline superfusion both before and after phosphoramidon application (right panel: top, bottom). In contrast, ninety seconds after poc stimulation during phosphoramidon superfusion, CPN2 activity remained strong and the gastric mill rhythm persisted. (B) The duration of LG bursting after poc stimulation in phosphoramidon (10−5 M; black bar) and saline wash (white bar) was normalized relative to its bursting duration in saline before phosphoramidon application. There was a significant increase in the normalized duration of LG bursting after poc stimulation (black bar: p<0.05, n=5; statistical analysis was performed on the raw data) in the presence of phosphoramidon. In contrast, phosphoramidon (10−5 M; black bar) did not alter the normalized duration of POC-triggered LG bursting after stimulation of the proprioceptor sensory GPR neurons relative to pre-application controls. White bar represents the normalized duration of LG bursting when GPR was stimulated after washout of phosphoramidon. Most hyperpolarized membrane potentials: CPN2stn, −73mV; LG, −63 mV.
We quantified the influence of phosphoramidon on the duration of POC actions by measuring the duration of time during which the LG neuron generated rhythmic bursts after POC stimulation. The duration of LG bursting was reversibly increased, on average by approximately 4-fold, by phosphoramidon application (Fig. 10B) (Saline: 6.1 ± 1.9 min, Phosphoramidon: 22.5 ± 6.7 min, Wash: 12.5 ± 4.8 min) (n=5; p<0.05, Repeated Measures One-Way ANOVA followed by the Student-Newman-Keuls test of multiple comparisons).
To control for the specificity of phosphoramidon action, we examined the influence of phosphoramidon on the duration of LG bursting after stimulating the gastro-pyloric receptor neuron (GPR: Katz et al., 1989; Katz and Harris-Warrick, 1990). GPR stimulation excites MCN1 and CPN2 and thereby elicits the gastric mill rhythm (Blitz et al., 2004). GPR does not, however, contain CabTRP Ia, but instead contains the co-transmitters acetylcholine, serotonin and allatostatin (Katz and Harris-Warrick, 1990; Skiebe and Schneider 1994). Phosphoramidon (10−5 M) superfusion did not change the duration of LG bursting after GPR stimulation (Fig. 10B) (n=4, p>0.5 Repeated Measures One-Way ANOVA).
Discussion
We have identified an extrinsic input, the POC neurons, that triggers a long-lasting activation of identified CoG projection neurons and thereby initiates a distinct version of the gastric mill rhythm in the C. borealis STG. The POC axons project as a tightly associated bundle through the medial aspect of each coc, from the TG, to innervate the ipsilateral CoG. A subset of these axons also project through the poc, enabling them to innervate the contralateral CoG. The long-term activation of the CoG projection neurons MCN1 and CPN2 by POC stimulation is likely mediated by the peptide transmitter CabTRP Ia.
The POC neurons appear to be the source of the extensive CabTRP Ia-IR arborization in the anterior CoG neuropil (Goldberg et al., 1988). This arborization was recently characterized as a neurohemal organ, the ACO, which is well-situated to release CabTRP Ia into the hemolymph as a circulating hormone in the related species Cancer productus (Messinger et al., 2005). In that study, the ACO was also studied extensively for the presence of co-transmitters but none were identified. One function of circulating hormones, including CabTRP Ia, is to modulate the properties of muscles that mediate movements of the foregut (Jorge-Rivera and Marder 1996; Messinger et al., 2005). Therefore, POC-mediated release of CabTRP Ia may well coordinately trigger the gastric mill rhythm and modulate the response of gastric mill muscles to the incoming motor pattern. Recently, a second isoform of CabTRP (CabTRP II) was isolated from the STNS, including the CoGs (Stemmler et al., 2007). Both CabTRP isoforms are recognized by the same antibody and have similar actions on the pyloric rhythm (Stemmler et al., 2007). Thus, either or both CabTRP peptides may mediate the POC actions in this system.
The likelihood that the CabTRP Ia released from the ACO terminals locally excites MCN1 and CPN2 supports the hypothesis that this neuronal population has both paracrine and endocrine functions. Given the sensitivity of MCN1 and CPN2 to relatively brief POC stimulation, there may well be times when this pathway acts largely or exclusively as a local modulator of neuronal activity, while at other times its activation results in both paracrine and endocrine actions. Previous studies in other systems have established the ability of the same neurons to release signaling molecules that act both locally, in a paracrine fashion, and as circulating hormones (Mayeri 1979; Sigvardt et al., 1986; Jung and Scheller, 1991; Loechner and Kaczmarek, 1994; Ludwig and Pittman, 2003; Fort et al., 2004; Oliet et al., 2007).
We have not yet identified the location of the POC neuronal somata. These somata may well be located within the TG, in which the anterior coc terminates. The TG contributes to many aspects of behavior, insofar as the entire thoracic and abdominal nervous system in C. borealis is condensed into this single ganglion. However, the POC somata may instead be located within one or more peripheral nerves or related structures, as is common for muscle- and abdominal-stretch sensitive sensory neuron populations in decapod crustaceans (Alexandrowicz, 1951; Cattaert et al., 2002; Katz et al., 1989; Beenhakker et al., 2004). Whether these neurons originate in the TG or a peripheral structure, their point of origin appears likely to be outside the STNS. Thus, the POC neurons may help to coordinate the chewing of food with other behaviors, perhaps acting as a trigger for chewing in response to cues from other regions of the animal. In addition, these neurons may well contribute to the long-term maintenance of chewing in the intact crab and lobster insofar as the gastric mill rhythm can persist for hours after food is ingested (Fleischer 1981; Turrigiano and Selverston 1990). Similarly, there are long-lasting actions of the vertebrate tachykinin peptide, substance P, on rhythmic locomotor activity in the vertebrate CNS (Treptow et al., 1983; Parker and Grillner, 1999). Here we find that the tachykinin peptide CabTRP Ia triggers a long-term activation of projection neurons that in turn drive a persisting gastric mill rhythm. Short-duration sensory stimuli can also trigger long-term activation of descending reticulospinal neurons that drive locomotion in lamprey (Di Prisco et al. 1997).
The POC-elicited gastric mill rhythm is qualitatively different from gastric mill rhythms elicited by other extrinsic inputs in C. borealis. Specifically, the protraction phase activity pattern of MCN1, CPN2 and LG is pyloric-timed during the POC-triggered rhythm whereas these neurons exhibit tonic protraction phase activity during other gastric mill rhythms (Beenhakker and Nusbaum 2004; Blitz et al., 2004; Christie et al., 2004; Saideman et al., 2007b). The LG-innervated muscles mediate protraction of the lateral teeth within the gastric mill. Thus, the distinct LG neuron activity pattern during the POC-triggered gastric mill rhythm could result in a different mode of chewing relative to the previously characterized gastric mill rhythms. In fact, both smooth protraction and pyloric-timed movements of the lateral teeth occur during in vivo endoscopic recordings of these teeth movements in Cancer crabs (Heinzel et al., 1993). Future work will be needed to establish whether the pyloric-timed LG neuron pattern is retained at the level of the LG-innervated muscles during the POC-triggered rhythm.
The distinct activity pattern of MCN1 during the POC rhythm also has consequences for motor pattern generation and inter-circuit coordination. For example, the pyloric circuit feedback to MCN1 during the protractor phase of the spontaneous POC-like gastric mill rhythm enables the pyloric rhythm to regulate the speed and pattern of the gastric mill rhythm, as well as its coordination with the pyloric rhythm (Wood et al., 2004). This is also evident in the present study from the change in gastric mill cycle period when the pyloric rhythm was suppressed. This pyloric regulation of the gastric mill rhythm during the protractor phase, via feedback inhibition of MCN1 and CPN2, occurs only during the POC-type of gastric mill rhythm (Beenhakker and Nusbaum, 2004; Blitz et al., 2004; Christie et al., 2004). Previous work documented additional cellular and synaptic mechanisms underlying inter-circuit regulation during other versions of the gastric mill rhythm (Bartos and Nusbaum, 1997; Clemens et al., 1998; Bartos et al., 1999; Wood et al., 2004). Although coordination between different behaviors, such as locomotion and respiration, occurs in many animals (Bramble and Carrier 1983; Syed and Winlow 1991; Kawahara et al., 1989; Morin and Viala 2002; Saunders et al., 2004), the underlying cellular mechanisms remain to be determined in these systems.
It appears likely that the previously studied POC-like gastric mill rhythm by Wood et al. (2004) does represent POC-triggered rhythms, presumably resulting from POC activation that occurred during the dissection. In both cases there was a prominent activation of MCN1, and they further share the distinct pyloric-timed activity pattern during the protractor phase. CPN2 activity, however, was not studied in the earlier work (Wood et al., 2004). Wood et al. (2004) did establish that pyloric-timed MCN1 stimulation elicited a gastric mill rhythm that was comparable to the spontaneous POC-like rhythm. Given that MCN1 and CPN2 are necessary and sufficient to elicit the VCN- and GPR-elicited gastric mill rhythms (Blitz et al., 2004; Beenhakker and Nusbaum, 2004), it is likely that they play pivotal roles during the POC-triggered rhythm as well. Addressing this issue will provide insight into the extent to which this system uses convergent activation of the same projection neurons to elicit distinct activity patterns. This would contrast to the prevalent hypothesis in other model systems that the generation of distinct but related movements results from the activation of distinct but overlapping sets of projection neurons (Georgopoulos, 1995; Kristan and Shaw, 1997; Lewis and Kristan, 1998; Liu and Fetcho, 1999).
Acknowledgments
This work supported by grant NS42813 (M.P.N.) from the National Institute of Neurological Disorders and Stroke, and a Mount Desert Island Biological Laboratory New Investigator Award from the Salisbury Cove Research Fund provided through the Thomas H. Maren Foundation (A.E.C.).
List of Abbreviations
- AB
anterior burster
- ACO
anterior commissural organ
- AGR
anterior gastric receptor
- AM
anterior median
- CabTRP Ia
Cancer borealis tachykinin-related peptide Ia
- CabTRP Ia-IR
Cancer borealis tachykinin-related peptide Ia immunoreactivity
- coc
circumoesophageal commissure
- CoG
commissural ganglia
- CPN2
commissural projection neuron 2
- DG
dorsal gastric
- dgn
dorsal gastric nerve
- dpon
dorsal posterior nerve
- EPSPs
excitatory postsynaptic potentials
- GM
gastric mill
- gpn
gastro-pyloric nerve
- GPR
gastro-pyloric receptor cell
- IC
inferior cardiac
- Int1
interneuron 1
- ion
inferior oesophageal nerve
- LG
lateral gastric
- lgn
lateral gastric nerve
- LPG
lateral posterior gastric
- lvn
lateral ventricular nerve
- MCN1
modulatory commissural neuron 1
- MG
medial gastric
- mvn
medial ventricular nerve
- OG
oesophageal
- PD
pyloric dilator
- pdn
pyloric dilator nerve
- poc/POC
post-oesophageal commissure
- son
superior oesophageal nerve
- STG
stomatogastric ganglion
- STNS
stomatogastric nervous system
- TG
thoracic ganglion
- VCNs
ventral cardiac neurons
- VD
ventricular dilator
References
- Alexandrowicz JS. Muscle receptor organs in the abdomen of Homarus vulgaris and Palinurus vulgaris. Quart J Micr Sci. 1951;92:163–199. [Google Scholar]
- Bartos M, Nusbaum MP. Intercircuit control of motor pattern modulation by presynaptic inhibition. J Neurosci. 1997;17:2247–2256. doi: 10.1523/JNEUROSCI.17-07-02247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Manor Y, Nadim F, Marder E, Nusbaum MP. Coordination of fast and slow rhythmic neuronal circuits. J Neurosci. 1999;19:6650–6660. doi: 10.1523/JNEUROSCI.19-15-06650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Nusbaum MP. Mechanosensory activation of a motor circuit by coactivation of two projection neurons. J Neurosci. 2004;24:6741–6750. doi: 10.1523/JNEUROSCI.1682-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Blitz DM, Nusbaum MP. Long-lasting activation of rhythmic neuronal activity by a novel mechanosensory system in the crustacean stomatogastric nervous system. J Neurophysiol. 2004;91:78–91. doi: 10.1152/jn.00741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Motor pattern selection via inhibition of parallel pathways. J Neurosci. 1997;17:4965–4975. doi: 10.1523/JNEUROSCI.17-13-04965.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci. 1999;19:6774–678. doi: 10.1523/JNEUROSCI.19-16-06774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci. 1999;19:5449–5463. doi: 10.1523/JNEUROSCI.19-13-05449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Beenhakker MP, Nusbaum MP. Different sensory systems share projection neurons but elicit distinct motor patterns. J Neurosci. 2004;24:11381–11390. doi: 10.1523/JNEUROSCI.3219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramble DM, Carrier DR. Running and breathing in mammals. Science. 1983;219:251–256. doi: 10.1126/science.6849136. [DOI] [PubMed] [Google Scholar]
- Brocard F, Bardy C, Dubuc R. Modulatory effect of substance P to the brain stem locomotor command in lampreys. J Neurophysiol. 2005;93:2127–2141. doi: 10.1152/jn.00401.2004. [DOI] [PubMed] [Google Scholar]
- Cardi P, Nagy F. A rhythmic modulatory gating system in the stomatogastric nervous system of Homarus gammarus. III. Rhythmic control of the pyloric CPG. J Neurophysiol. 1994;71:2503–2516. doi: 10.1152/jn.1994.71.6.2503. [DOI] [PubMed] [Google Scholar]
- Cattaert D, Le Bon M, Le Ray D. Efferent controls in crustacean mechanoreceptors. Microsc Res Tech. 2002;58:312–324. doi: 10.1002/jemt.10139. [DOI] [PubMed] [Google Scholar]
- Christie AE, Lundquist CT, Nassel DR, Nusbaum MP. Two novel tachykinin-related peptides from the nervous system of the crab Cancer borealis. J Exp Biol. 1997;200:2279–2294. doi: 10.1242/jeb.200.17.2279. [DOI] [PubMed] [Google Scholar]
- Christie AE, Stein W, Quinlan JE, Beenhakker MP, Marder E, Nusbaum MP. Actions of a histaminergic/peptidergic projection neuron on rhythmic motor patterns in the stomatogastric nervous system of the crab Cancer borealis. J Comp Neurol. 2004;469:153–169. doi: 10.1002/cne.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Combes D, Meyrand P, Simmers J. Long-term expression of two interacting motor pattern-generating networks in the stomatogastric system of freely behaving lobster. J Neurophysiol. 1998;79:1396–1408. doi: 10.1152/jn.1998.79.3.1396. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Nusbaum MP. Functional consequences of compartmentalization of synaptic input. J Neurosci. 1994;11:6544–6552. doi: 10.1523/JNEUROSCI.14-11-06544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Meyrand P, Nusbaum MP. A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature. 1995;378:502–505. doi: 10.1038/378502a0. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Nusbaum MP, Cournil I, Claiborne BJ. Distribution of modulatory inputs to the stomatogastric ganglion of the crab, Cancer borealis. J Comp Neurol. 1992;325:581–594. doi: 10.1002/cne.903250410. [DOI] [PubMed] [Google Scholar]
- Combes D, Simmers AJ, Moulins M. Structural and functional characterization of a muscle tendon proprioceptor in lobster. J Comp Neurol. 1995;363:221–234. doi: 10.1002/cne.903630205. [DOI] [PubMed] [Google Scholar]
- Combes D, Meyrand P, Simmers J. Dynamic restructuring of a rhythmic motor program by a single mechanoreceptor neuron in lobster. J Neurosci. 1999;19:3620–3628. doi: 10.1523/JNEUROSCI.19-09-03620.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Prisco GV, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science. 1997;278:1122–1125. doi: 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- Di Prisco GV, Pearlstein E, Le Ray D, Robitaille R, Dubuc R. A cellular mechanism for the transformation of a sensory input into a motor command. J Neurosci. 2000;20:8169–8176. doi: 10.1523/JNEUROSCI.20-21-08169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer AG. The effect of eyestalk hormones on the gastric mill in the intact lobster Panulirus interruptus. J Comp Physiol. 1981;141:363–368. [Google Scholar]
- Fort TJ, Brezina V, Miller MW. Modulation of an integrated central pattern generator-effector system: dopaminergic regulation of cardiac activity in the blue crab Callinectes sapidus. J Neurophysiol. 2004;92:3455–3470. doi: 10.1152/jn.00550.2004. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP. Current issues in directional motor control. Trends Neurosci. 1995;18:506–510. doi: 10.1016/0166-2236(95)92775-l. [DOI] [PubMed] [Google Scholar]
- Goldberg D, Nusbaum MP, Marder E. Substance P-like immunoreactivity in the stomatogastric nervous systems of the crab Cancer borealis and the lobsters Panulirus interruptus and Homarus americanus. Cell Tissue Res. 1988;252:515–522. doi: 10.1007/BF00216638. [DOI] [PubMed] [Google Scholar]
- Gordon IT, Whelan PJ. Deciphering the organization and modulation of spinal locomotor central pattern generators. J Exp Biol. 2006;209:2007–2014. doi: 10.1242/jeb.02213. [DOI] [PubMed] [Google Scholar]
- Heinzel HG. Gastric mill activity in the lobster. I. Spontaneous modes of chewing. J Neurophysiol. 1988;59:528–550. doi: 10.1152/jn.1988.59.2.528. [DOI] [PubMed] [Google Scholar]
- Heinzel HG, Weimann JM, Marder E. The behavioral repertoire of the gastric mill in the crab, Cancer pagurus: an in situ endoscopic and electrophysiological examination. J Neurosci. 1993;13:1793–1803. doi: 10.1523/JNEUROSCI.13-04-01793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper SL, Moulins M. Cellular and synaptic mechanisms responsible for a long-lasting restructuring of the lobster pyloric network. J Neurophysiol. 1990;64:1574–1589. doi: 10.1152/jn.1990.64.5.1574. [DOI] [PubMed] [Google Scholar]
- Jorge-Rivera JC, Marder E. TNRNFLRFamide and SDRNFLRFamide modulate muscles of the stomatogastric system of the crab Cancer borealis. J Comp Physiol [A] 1996;179:741–751. doi: 10.1007/BF00207353. [DOI] [PubMed] [Google Scholar]
- Jung LJ, Scheller RH. Peptide processing and targeting in the neuronal secretory pathway. Science. 1991;251:1330–1335. doi: 10.1126/science.2003219. [DOI] [PubMed] [Google Scholar]
- Katz PS, Eigg MH, Harris-Warrick RM. Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. I. Identification and characterization of the gastropyloric receptor cells. J Neurophysiol. 1989;62:558–570. doi: 10.1152/jn.1989.62.2.558. [DOI] [PubMed] [Google Scholar]
- Katz PS, Harris-Warrick RM. Neuromodulation of the crab pyloric central pattern generator by serotonergic/cholinergic proprioceptive afferents. J Neurosci. 1990;10:1495–1512. doi: 10.1523/JNEUROSCI.10-05-01495.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K, Kumagai S, Nakazono Y, Myamoto Y. Coupling between respiratory and stepping rhythms during locomotion in decerebrate cats. J Appl Physiol. 1989;67:110–115. doi: 10.1152/jappl.1989.67.1.110. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kirby MS, Nusbaum MP. Central nervous system projections to and from the commissural ganglion of the crab Cancer borealis. Cell Tissue Res. 2007;328:625–637. doi: 10.1007/s00441-007-0398-2. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Shaw BK. Population coding and behavioral choice. Curr Opin Neurobiol. 1997;7:826–831. doi: 10.1016/s0959-4388(97)80142-0. [DOI] [PubMed] [Google Scholar]
- LeBeau FE, El Manira A, Grillner S. Tuning the network: modulation of neuronal microcircuits in the spinal cord and hippocampus. Trends Neurosci. 2005;28:552–561. doi: 10.1016/j.tins.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Kristan WB. A neuronal network for computing population vectors in the leech. Nature. 1998;391:76–79. doi: 10.1038/34172. [DOI] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Loechner KJ, Kaczmarek LK. Autoactive peptides act at three distinct receptors to depolarize the bag cell neurons of Aplysia. J Neurophysiol. 1994;71:195–203. doi: 10.1152/jn.1994.71.1.195. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol. 2005;15:R685–R699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Mayeri E. Local hormonal modulation of neural activity in Aplysia. Fed Proc. 1979;38:2103–2108. [PubMed] [Google Scholar]
- McLean DL, Sillar KT. Metamodulation of a spinal locomotor network by nitric oxide. J Neurosci. 2004;24:9561–9571. doi: 10.1523/JNEUROSCI.1817-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger DI, Kutz KK, Le T, Verley DR, Hsu YW, Ngo CT, Cain SD, Birmingham JT, Li L, Christie AE. Identification and characterization of a tachykinin-containing neuroendocrine organ in the commissural ganglion of the crab Cancer productus. J Exp Biol. 2005;208:3303–3319. doi: 10.1242/jeb.01787. [DOI] [PubMed] [Google Scholar]
- Meyrand P, Simmers J, Moulins M. Dynamic construction of a neural network from multiple pattern generators in the lobster stomatogastric nervous system. J Neurosci. 1994;14:630–644. doi: 10.1523/JNEUROSCI.14-02-00630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin D, Viala D. Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs. J Neurosci. 2002;22:4756–4765. doi: 10.1523/JNEUROSCI.22-11-04756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris BJ, Coleman MJ, Nusbaum MP. Recruitment of a projection neuron determines gastric mill motor pattern selection in the stomatogastric nervous system of the crab, Cancer borealis. J Neurophysiol. 1994;72:1451–1463. doi: 10.1152/jn.1994.72.4.1451. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Baimoukhametova DV, Piet R, Bains JS. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–1333. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Grillner S. Long-lasting substance-P-mediated modulation of NMDA-induced rhythmic activity in the lamprey locomotor network involves separate RNA- and protein-synthesis-dependent stages. Eur J Neurosci. 1999;11:1515–1522. doi: 10.1046/j.1460-9568.1999.00565.x. [DOI] [PubMed] [Google Scholar]
- Saideman SR, Ma M, Kutz-Naber KK, Cook A, Torfs P, Schoofs L, Li L, Nusbaum MP. Modulation of rhythmic motor activity by pyrokinin peptides. J Neurophysiol. 2007a;97:579–595. doi: 10.1152/jn.00772.2006. [DOI] [PubMed] [Google Scholar]
- Saideman SR, Blitz DM, Nusbaum MP. Convergent motor patterns from divergent circuits. J Neurosci. 2007b;27:6664–6674. doi: 10.1523/JNEUROSCI.0315-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders SW, Rath D, Hodges PW. Postural and respiratory activation of the trunk muscles changes with mode and speed of locomotion. Gait Posture. 2004;20:280–290. doi: 10.1016/j.gaitpost.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Sigvardt KA, Rothman BS, Brown RO, Mayeri E. The bag cells of Aplysia as a multitransmitter system: identification of alpha bag cell peptide as a second neurotransmitter. J Neurosci. 1986;6:803–813. doi: 10.1523/JNEUROSCI.06-03-00803.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiebe P, Schneider H. Allatostatin peptides in the crab stomatogastric nervous system: inhibition of the pyloric motor pattern and distribution of allatostatin-like immunoreactivity. J Exp Biol. 1994;194:195–208. doi: 10.1242/jeb.194.1.195. [DOI] [PubMed] [Google Scholar]
- Smarandache CR, Stein W. Sensory-induced modification of two motor patterns in the crab, Cancer pagurus. J Exp Biol. 2007;210:2912–2922. doi: 10.1242/jeb.006874. [DOI] [PubMed] [Google Scholar]
- Smetana RW, Alford S, Dubuc R. Muscarinic receptor activation elicits sustained, recurring depolarizations in reticulospinal neurons. J Neurophysiol. 2007;97:3181–3192. doi: 10.1152/jn.00954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci. 2007;26:1148–1165. doi: 10.1111/j.1460-9568.2007.05744.x. [DOI] [PubMed] [Google Scholar]
- Stemmler EA, Peguero B, Bruns EA, Dickinson PS, Christie AE. Identification, physiological actions, and distribution of TPSGFLGMRamide: a novel tachykinin-related peptide from the midgut and stomatogastric nervous system of Cancer crabs. J Neurochem. 2007;101:1351–1366. doi: 10.1111/j.1471-4159.2007.04520.x. [DOI] [PubMed] [Google Scholar]
- Syed NI, Winlow W. Coordination of locomotor and cardiorespiratory networks of Lymnaea stagnalis by a pair of identified interneurones. J Exp Biol. 1991;158:37–62. doi: 10.1242/jeb.158.1.37. [DOI] [PubMed] [Google Scholar]
- Treptow K, Oehme P, Gäbler E, Bienert M. Modulation of locomotor activity by substance P in rats. Regul Pept. 1983;5:343–351. doi: 10.1016/0167-0115(83)90292-6. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Selverston AI. A cholecystokinin-like hormone activates a feeding-related neural circuit in lobster. Science. 1990;344:866–868. doi: 10.1038/344866a0. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Marder E. Switching neurons are integral members of multiple oscillatory networks. Curr Biol. 1994;4:896–902. doi: 10.1016/s0960-9822(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Meyrand P, Marder E. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J Neurophysiol. 1991;65:111–122. doi: 10.1152/jn.1991.65.1.111. [DOI] [PubMed] [Google Scholar]
- White RS, Nadim F, Nusbaum MP. Neuroscience Meeting Planner Program No. 722.52. Washington D.C: Society for Neuroscience Online; 2005. Activation of a peripheral modulatory system elicits a distinct gastric mill rhythm. [Google Scholar]
- Wood DE, Nusbaum MP. Extracellular peptidase activity tunes motor pattern modulation. J Neurosci. 2000;22:4185–4195. doi: 10.1523/JNEUROSCI.22-10-04185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DE, Manor Y, Nadim F, Nusbaum MP. Intercircuit control via rhythmic regulation of projection neuron activity. J Neurosci. 2004;24:7455–7563. doi: 10.1523/JNEUROSCI.1840-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]